Abstract
Correlations between brood sex ratios (BSRs) and parental or environmental quality have been found in many species. This phenomenon is called sex ratio adjustment, and is expected to evolve if certain factors affect the fitness return from the offspring in a sex-dependent way. However, it is seldom studied whether biased sex ratios are indeed adaptive. We manipulated BSRs in a cross-fostering experiment, and investigated parental costs in terms of feeding rate and survival in the collared flycatcher (Ficedula albicollis). In our population, male nestlings can grow faster under good conditions, but are more sensitive to adverse conditions. Assuming that the sensitivity of the males results from their larger energy requirement, we predicted increased costs in broods with male-biased experimental BSR. Assuming that BSR adjustment is adaptive and related to parental care giving capacity, we expected higher feeding and survival rate by parents that originally had more sons, and predicted that low quality parents are less able to adjust their feeding rates to the needs of their foster broods or pay higher survival cost. However, we found that the manipulated BSR and its interaction with original BSR affected neither the feeding rate nor the survival of the parents. Only male feeding rate was correlated with original BSR, however, contrary to our prediction: males with female-biased original BSR fed their foster chicks more frequently. Our results, with those of a previous report about the effects of the experiment on nestlings, do not support that the observed BSRs are adaptive in our population.
Significance statement
Many hypotheses propose that higher vertebrates adaptively adjust the primary sex ratio of their offspring to individual or environmental quality. While the potential adaptive value of the observed patterns is regularly discussed, studies that specifically test the adaptivity of sex ratio adjustment are very scarce and correlative. Using a special cross-fostering experiment, we investigated whether original brood sex ratios are related to the rearing capacity of the parents, and experimental sex ratios are related to the rearing costs in terms of feeding effort or survival. We found no effect of experimentally altered brood sex ratios on either parental feeding effort or survival. Furthermore, contrary to the adaptive scenario, males that had female-biased broods originally had higher feeding rates. So far, we have found no evidence that the sex ratio adjustment is adaptive in the collared flycatcher.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The relative fitness benefit of producing male and female offspring may change with parental quality or environmental conditions, because these factors may have sex-specific effects on offspring survival, reproductive success or rearing costs. If so, it is expected to be advantageous for the parents to adjust the sex (ratio) of their offspring to the trait(s) in question (for a review, see Cockburn et al. 2002; Szász et al. 2012). This phenomenon is called sex ratio adjustment or sex ratio manipulation.
Several hypotheses have been raised in relation to the evolution of sex ratio adjustment. Perhaps the best-known is the Trivers–Willard hypothesis (Trivers and Willard 1973), which argues that in species where maternal body condition has a stronger effect on the future reproductive success of male offspring than on the future reproductive success of female offspring, mothers that are able to adjust offspring sex ratio to their own body condition, have an evolutionary advantage. If the ability to adjust the sex ratio has evolved, females in good condition are expected to produce more male offspring, while females in poor condition are expected to produce more female offspring. The same logic can be extended for example to male attractiveness, i.e. females mated to an attractive male should produce more male offspring because those will inherit their father’s attractiveness and will be more successful than their sisters (“attractiveness hypothesis” (Burley 1981, 1986)).
While the previous hypotheses are based on sex-specific reproductive success of the offspring, the “cost of reproduction hypothesis” focuses on the sex-specific rearing cost, and argues that females in poor condition are unable to bear the cost of producing the energetically costlier sex, thus should adjust the sex ratio of their broods accordingly (Myers 1978). Sex ratio manipulation may evolve also as a consequence of sex differences in environmental sensitivity. The so-called “sensitivity hypothesis” suggests that under less favourable conditions females should bias their offspring sex ratio toward the less sensitive sex to avoid reduced offspring development and survival (Szász et al. 2012).
Despite several hypotheses on adaptive sex ratio adjustment and the well-documented occurrences of brood sex ratio biases in wild populations (Cockburn et al. 2002; Szász et al. 2012, 2019a), studies that investigated the adaptive value of the phenomenon are scarce and most of them are correlative (e.g. Gomendio et al. 1990; Appleby et al. 1997). However, if we compare some fitness components of male and female-biased broods in correlative studies, it is impossible to disentangle genetic effects, early maternal effects and the effects of offspring sex (ratio). To evaluate the effect of sex (ratio) per se, cross-fostering experiments are needed. Because the effects of rearing sex-biased broods may depend on whether the parents originally produced male- or female-biased broods, it is important to investigate not only the effect of manipulated brood sex ratio, but also the interactive effect of original and manipulated brood sex ratio. To our knowledge, this has been investigated only in two species. In tammar wallabies (Macropus eugenii), offspring reared by females that originally produced male offspring performed better independently of the sex of the foster offspring, and offspring mass at weaning (or one year later) was not influenced by the sex of the original or foster offspring (Robert et al. 2010). However, the interaction of the sex of the original and foster offspring had a significant effect on the probability of the mother giving birth in the year following the experiment (Schwanz and Robert 2016). Females that cared for the sex opposite of what they had originally produced were less likely to give birth in the next year, and this suggests that sex ratio adjustment in this species is adaptive. The other species where the interaction of the sex of the original and foster offspring was studied is the collared flycatcher (Ficedula albicollis, Szász et al. 2023).
Empirical data from different populations of collared flycatchers have shown correlations between brood sex ratio and various variables, such as secondary sexual characters (wing patch size: Bowers et al. 2013; forehead patch size: Ellegren et al. 1996), size (tarsus length: Szász et al. 2014), laying date (Rosivall et al. 2004; Bowers et al. 2013; Szász et al. 2014), and parental personality (male aggressiveness: Szász et al. 2014). In addition, male nestlings of this species are more sensitive to early adverse conditions than female nestlings (Rosivall et al. 2010; Szász et al. 2017). Namely, male nestlings, which have higher growth rates under favourable conditions, suffered a more severe reduction in growth under poor conditions (i.e. in experimentally enlarged broods) than female nestlings (Rosivall et al. 2010), suggesting that male offspring have larger energy requirements. Moreover, brood enlargement had long-term consequences for male, but not for female recruits (Szász et al. 2017). Males from enlarged broods had shorter lifespans and this had a negative effect also on reproductive output. As a consequence, in our previous study, we predicted that parents with better offspring rearing capacities produce more male offspring (as forecasted by the sensitivity hypotheses), broods with experimentally male-biased sex ratios have larger energy requirements and therefore perform worse, and offspring reared in male-biased broods by parents that originally had female-biased broods (i.e. parents presumed to be of lower quality) will suffer more. However, we found that original brood sex ratio, experimental brood sex ratio and their interactions had no effect on nestling growth, survival and recruitment (Szász et al. 2023). This may suggest that sex ratio adjustment is not adaptive in our collared flycatcher population. However, it is also possible that we have not seen the effects of brood sex ratios on offspring performance, because the parents adjusted their feeding rate to the sex ratio of the brood, and thus the parents paid the costs of biased sex ratios, not the offspring. Results concerning parental feeding rates and brood sex ratios are quite mixed. While some papers found no correlation between the two variables (e.g. Leonard et al. 1994; Kieffer and Ritchison 2012), many studies found positive correlations between feeding rate and the within-brood proportion of the sex with the larger energy requirement (e.g. Yasukawa et al. 1990; Nishiumi et al. 1996; Westerdahl et al. 2000; Green 2002; Suorsa et al. 2003; Khwaja et al. 2017).
In this paper, we aimed to investigate the adaptive value of brood sex ratio adjustment by analysing the relationship between feeding rate and brood sex ratios in a videorecorded subset of the broods that were cross-fostered by Szász et al. (2023). Assuming that sex ratio adjustment is adaptive, we expected that parents with low capacity of parental effort produced female-biased broods, because males are more sensitive to early environmental conditions (Rosivall et al. 2010; Szász et al. 2017), and therefore predicted a positive correlation between feeding rate of the parents at the experimental broods and their original brood sex ratio (i.e. proportion of male nestlings in the brood). Owing to the male nestlings’ higher growth capacity under good conditions and their larger sensitivity to adverse conditions (Rosivall et al. 2010; Szász et al. 2017), we assumed that they have larger energy requirements, and predicted that parents rearing male-biased broods have larger feeding effort. Furthermore, we expected that parents with low capacity of parental effort (that also produce female-biased brood) are less able to adjust their feeding rates to the needs of their foster broods; therefore, we predicted that original and experimental sex ratios have an interactive effect on feeding rate. As parental investment may affect parental survival probabilities (Cichoń et al. 1998; Santos and Nakagawa 2012), we also examined the effect of the experiment on parental survival. We expected a negative effect of male biased broods on parental survival that is less pronounced in the broods of parents that originally produced male-biased broods. Because we wanted to separate genetic and early maternal effects from the effects of brood sex ratio, we used a cross-fostering design where each parent reared only foreign nestlings that originated from two different broods (Fig. 1, see more details in the methods).
Methods
Study site and population
Our study site was an artificial nest box plot in a continuous, oak-dominated woodland in the Pilis-Visegrádi Mountains (47° 43′ N, 19° 01′ E), Hungary. Collared flycatchers are insectivorous, hole-breeding, primarily socially monogamous passerines with normally one clutch per breeding season. They spend the winter in the Sub-Saharan Africa, and return to our breeding area in the middle of April. Only females incubate their typically five to seven eggs, but both parents feed their nestlings. Females often start the incubation before clutch completion, thus hatching asynchrony is common. Most nestlings fledge when they are 15–16 days old.
Field methods and brood sex ratio manipulation
During the breeding seasons of 2017 and 2018, we visited the nests daily around the presumed time of hatching. Two days after hatching of the first chick of the brood, we cross-fostered nestlings between trios of nests with the same hatching date. The three broods were either identical in brood size or the difference was not more than one offspring. After the cross-fostering, all parents got the same number of nestlings as originally hatched, and all nestlings were unrelated to the foster parents, coming from two different broods approximately in an equal proportion (Fig. 1). Cross-fostering was done randomly with respect to the sex of the nestlings. Consequently, brood sex ratio but not brood size was manipulated. During cross-fostering, the nestlings were weighed to the nearest 0.1 g with a Pesola spring balance. Offspring were marked individually by removing tufts of down on their head and back. All offspring were ringed at the age of 6–8 days. A small blood sample was taken from each nestling by brachial vein puncture on the eighth day post-hatching. Unhatched eggs and tissue samples from dead offspring were also collected for molecular sexing. Parents were captured, ringed and measured when nestlings were 9–11 days old.
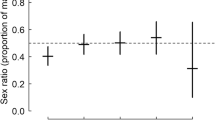
After the field season, we performed molecular sex determinations using the F2550 and the R2718 primers (Fridolfsson and Ellegren 1999) and the protocol described in Rosivall et al. (2004). The change in sex ratio of the broods at the time of video recordings compared to the original brood sex ratios varied between -0.5 and + 0.67 (see Fig. S1 in Supplementary Information).
Videotaping and video analysis
Feeding activity was monitored during the peak of the feeding period, 8 or 9 days after hatching (with one exception, where video was recorded when nestlings were 10 days old) between 7:00 am and 2:00 pm using video-cameras that were set up on a tripod ca. 10–15 m away from the nest boxes. Video recordings were later analysed using Media Player Classic Home Cinema (MPC-HC, version 1.7.13). To minimize observer bias, blinded methods were used when behavioural data were recorded and analyzed. Collared flycatcher males and females are easy to distinguish by feather colouration, so male and female feeding rates (number of feedings per hour) were calculated separately. We defined feeding events as the bird going into the nest box or leaning in the nest box from the hole, then leaving without prey. Although parents were only minimally influenced by the human disturbance and resumed their feeding behaviour just minutes after the recordings were initiated, we started the video analyses when both parents had already resumed feeding. Furthermore, video length and number of feeding events were also corrected for the duration of accidental disturbance caused by human (e.g. louder footsteps or talking in the surrounding area) or potential nest predator (e.g. woodpecker activities) by excluding the time of the disturbance until both parents resumed feeding the nestlings again. The net length of each video recording (i.e. length after correction) was at least 90 min (mean ± SD = 96.89 ± 20.14).
Statistical analyses
Due to logistic constraints not all of the experimental broods were videotaped. We included 44 broods (27 in 2017 and 17 broods in 2018) in our statistical analyses, where both parents fed the nestlings, and the original primary sex ratio and brood size, as well as brood size and sex ratio at the time of video recording were known. At 38 broods the male was monogamous, 5 broods were the primary brood of a polygynous male. The mean male feeding rates apparently did not differ between the two groups (monogamous males: mean ± SD = 23.76 ± 7.35; polygynous males at primary nests: mean ± SD = 23.01 ± 4.21). At one brood, the social male was not captured, therefore its breeding status was unknown. However, its feeding rate was in the mid-range, and the exclusion of this brood from our analyses did not affect our results qualitatively, so we included this sample in our final analyses.
Because of logistic constraints (e.g. limited number of cameras), age of the nestlings at the time of recording and the start of the recording within days varied among broods. Therefore, before our analyses, we examined whether these factors were related to feeding rates. When analysing the effect of age, we combined the single brood recorded on day 10 post-hatch with the broods recorded on day 9 and found no effect of offspring’s age during the video recording (8 vs. 9/10 days) on parental provisioning rate (Mann–Whitney U test for males: U = 96.00, Z = 0.616, P = 0.538; females: U = 64.00, Z = 1.710, P = 0.087). Whether recordings were started before or after 10 am (i.e. first or second recording session of the day) did not affect the feeding activity of the parents (t test for males: t42 = -0.026, P = 0.980; females: t42 = 0.739, P = 0.464). As nestling age and recording time had no effect on parental feeding behaviour, we did not control for these factors in the statistical analyses.
Since parents with more offspring need to feed the brood more often (e.g. Stoehr et al. 2000; Kiss et al. 2013; Griffioen et al. 2019), the effect of brood size has to be taken into account when other predictors of parental care are studied. Brood size varied between five and seven in the dataset. Because it was five in only four broods, we used brood size as a two-level categorical predictor (brood size five and six vs. brood size seven).
We constructed general linear models using the feeding rates of male and female foster parents as response variables, while our predictors were the original and experimental (at the time of video recording) brood sex ratio, and the interaction of these sex ratios. Other parameters, such as partner’s feeding rate, brood size, year, the interactions of year with other variables, and the 3-way interaction of year, original and experimental sex ratios were included in the models to control for the potential effects of these variables on feeding rates. Before fitting GLMs, we checked the independence of the predictor variables by pairwise Pearson correlations, t tests and χ2-tests (all P > 0.382), in addition, in the main effect model all VIF values (function vif in car package, Fox and Weisberg 2019) were smaller than 1.633, so multicollinearity was not an issue.
After running the full model, we performed a backward stepwise model simplification, eliminating non-significant (P > 0.05) terms step by step from the model, starting with the interactions and the terms with the highest P value. In each analysis of full and final models, model residuals were normally distributed (Kolmogorov–Smirnov test: all P > 0.20). For more reliable parameter estimates we re-entered non-significant variables to the final model one by one (Hegyi and Laczi 2015), and presented the results accordingly (function Anova in car package, Fox and Weisberg 2019).
In 10 of the broods the original clutch size was not identical to the brood size at video recording because of infertile or unhatched egg or embryo or nestling mortality (the difference was not larger than one). Therefore, we investigated the effects of sex ratios on feeding rates both with and without these broods.
We also analysed whether original or experimental sex ratios (or their interaction) were related to parental survival. Breeding birds in our population are systematically monitored in every year and we try to capture the parents at each nest. In this study, we determined survival based on whether or not the parent was recaptured in any of the years following the experiment (2018–2023). Collared flycatchers show a high degree of breeding site fidelity (the distance between the nest boxes used in subsequent year is 129 and 248 m on average in males and non-experimental females, respectively; Könczey et al. 1992). Moreover, in our full 2002–2023 dataset, we found no parent with more than 5 years between subsequent breeding events (HG et al. unpublished data). So, we are confident that the parents we have not yet recaptured will not return in the following years, and our estimate of survival is reliable. In our analyses of parental survival we controlled for brood size, age of the parent and year. Two-way interactions of the predictors with year were also included with the exception of the age × year interaction, which could not be tested due to complete separation of age categories by the response variable in 2018 in females. Since for those parents that were ringed as adults, the exact age was unknown, we used the minimum age of the parents. We constructed a binomial regression model with logit link function for females and males separately. In the case of females, we ran models with our full dataset (n = 44). For the males, the sample size was 43 in the analysis, because one individual was not captured after the videorecording (see above); hence, its identity was unknown. The fit of each full and final models were validated visually by diagnostic plots based on quantile residuals generated by R package DHARMa (using function simulate Residuals, Hartig 2022), and the plots did not show problems. The predictors in the regression model were independent, as all VIF values (function vif in car package, Fox and Weisberg 2019) were smaller than 1.203. After the backward-stepwise elimination of the non-significant terms, none of the variables were retained in the model. Therefore, non-significant terms were re-entered to the null model, and we presented the resulting P values.
For the analyses we used R 4.2.2 (R Core Team 2022) and STATISTICA 6.1 (StatSoft, Inc. 2003).
Results
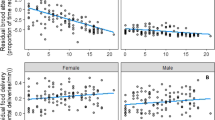
When we analysed the full dataset (N = 44), male feeding rate was negatively correlated with the original brood sex ratio (defined as the proportion of male nestlings) (Table 1, Fig. 2a), which indicates that males that originally had female-biased broods fed the foster nestlings more frequently. Female feeding rate was not affected by original sex ratio (Table 1, Fig. 2b). Neither experimental sex ratio, nor the interaction of original and experimental sex ratios had an effect on male and female feeding rates (Table 1, Fig. 3). However, the feeding frequencies of both sexes increased significantly with an increase in brood size (Table 1). Other background variables and interactions were not related to parental feeding rates.
The trends were similar in the restricted dataset (i.e. containing only those broods where the original clutch size was identical to the brood size at video recording, N = 34), but the relationships became less or non-significant (see Table S1 in Supplementary Information). In particular, using this restricted dataset, original sex ratio was only marginally significantly related to male feeding rate (F1,31 = 3.96, P = 0.055), while the relationship between brood size and female feeding rate became non-significant (F1,32 = 2.12, P = 0.156). The relationship between male feeding rate and brood size remained significant (F1,31 = 7.79, P = 0.009). We also have to note that using full models instead of backward stepwise model simplification, the results concerning original sex ratio and male feeding rate was not significant (F1,31 = 0.291, P = 0.593). There were no qualitative changes concerning the rest of the variables. Whether the difference between the two approaches is the result of increased type I error rate in the case of backward stepwise model simplification, or increased type II error rate in the case of the full model (Hegyi and Laczi 2015), cannot be determined.
In the case of females, the survival rate after the experimental manipulation was approximately 65%, while for males, this value appeared to be around 51%. Whether a parent survived after the manipulation was not related to any of the examined variables or interactions (Table 2).
Discussion
Whether sex ratio adjustment is indeed adaptive (as suggested by the prevailing hypotheses; see introduction) has been investigated in only a few correlative studies (e.g. Gomendio et al. 1990; Appleby et al. 1997), and experimental studies on this issue have so far been limited to two species, the tammar wallaby (Schwanz and Robert 2016) and the collared flycatcher (Szász et al. 2023). Schwanz and Robert (2016) found that the interaction of original and experimental sex ratio had a significant effect on the probability of the tammar wallaby mothers breeding in the following year, suggesting that the observed sex ratios are adaptive. The study on collared flycatchers found no support for adaptive sex allocation (Szász et al. 2023). Specifically, our previous study indicated that the growth, survival and recruitment rates of the nestlings were not affected by the original and experimental sex ratio (Szász et al. 2023). We hypothesized that the effect of experimental manipulation on nestlings may have been absent, because parents adjusted their investment to the altered need of the experimental broods (and thereby paid the costs of the manipulation). However, contrary to our expectations, neither experimental brood sex ratio, nor the interaction of the original and experimental sex ratio had an effect on parental feeding frequencies or survival. Furthermore, original sex ratio was correlated only with male provisioning, but in the opposite direction than we predicted, as males with originally female-biased broods delivered food at a higher rate.
Overall, these results suggest that not the parents with better feeding abilities produced the male-biased (presumably needier) broods. Furthermore, biased experimental brood sex ratios inferred costs neither to the parents (measured in feeding rate and survival; this study) nor to the offspring (measured in growth, survival and recruitment rates; Szász et al. 2023). Consequently, we found no support for the cost of reproduction or sensitivity hypotheses. We cannot exclude the possibility that the higher feeding rate provided by the fathers that originally had female-biased broods has more positive effect on the future reproductive success of female offspring than that of male offspring. However, this is unlikely, because of the higher variation in male reproductive success due to occasional polygyny and frequent extra-pair copulations in our population (Garamszegi et al. 2004; Rosivall et al. 2009; Herényi et al. 2012). To conclude, we have found no evidence that the observed sex ratio patterns are adaptive.
In spite of the apparent sex-dependent environmental sensitivity of collared flycatcher nestlings to rearing conditions (Rosivall et al. 2010; Szász et al. 2017), there was no association between experimental sex ratio and either offspring performance (Szász et al. 2023) or parental effort and survival (this study). These results may raise the question, whether the energy requirements of the two sexes differ (as we assumed), or the larger negative effect of unfavourable environmental conditions on male nestlings’ performance is rather explained by sex differences in energy allocation. Namely, in some species, sons and daughters seem to differ in how they allocate resources under poor conditions between growth and e.g. immune function (see e.g.Dubiec et al. 2006; Bowers et al. 2015); thus slower growth of one sex may be the consequence of preferential investment in immune function and not larger energy requirement of this sex. However, the energy allocation scenario seems to be an unlikely explanation for sex-biased growth patterns in our study species for two reasons. First, experimental brood size manipulation appears to have lifelong sex-dependent consequences, as male collared flycatchers reared under unfavourable conditions realize shorter breeding lifespans in our study population (Szász et al. 2017). Second, under natural conditions no sex differences were found either in body size or in the immunocompetence of the nestlings in another collared flycatcher population (Wilk et al. 2007).
Further explanation for the lack of expected effects on feeding rate could be that the total energy requirement of the whole nestling period differs between male and female nestlings, but at the given age, when the video recordings were performed, the difference was very minor and hard to detect. However, the fact that parental survival was unrelated to experimental sex ratio makes this explanation unlikely. An alternative explanation for the lack of effect on feeding rate may be that in response to the change in energy requirement of the broods, parents altered the quality, not the quantity of the delivered food. Unfortunately, we did not have the opportunity to investigate food quality (e.g. prey type, food size) during the feeding events.
Previous research from a Czech population of collared flycatchers (Bowers et al. 2013) found an association between offspring weight at the age of 13 days and original brood sex ratio, and concluded that parents with better parental abilities produce male-biased broods. In accordance with sex allocation theories, we expected the same pattern regarding feeding rate: namely that better quality parents can provide more frequent feedings and have relatively more male nestlings. However, original sex ratio was not related to female feeding rate and was correlated with males provisioning rate in the opposite direction than we predicted – males with originally female-biased broods fed the nestlings more often. One may argue that the results could be explained by the differential allocation hypothesis, which suggests that a relatively low-quality individual should allocate greater investment in their current reproductive event in order to obtain and/or maintain a pair-bond with a high-quality mate (Burley 1981, 1986). Indeed, negative correlations between male quality and feeding rate have been found in a few species (Ramos et al. 2015; Segura and Mahler 2019). If partners of males of low genetic quality produce more female offspring at the same time (as suggested by the sex allocation theory), a negative correlation between feeding rate and brood sex ratio may occur. Although, in our study population the examined paternal morphological traits were not related to feeding rates (Kiss et al. 2013; Laczi et al. 2017), we cannot exclude the possibility that some other indicators of individual quality are associated negatively with parental care.
Another explanation for the negative correlation between original sex ratio and male parental investment is a relationship between males’ personality and these two variables in a way that males with more female offspring have a personality associated with higher feeding activity. Though it has been suggested that more aggressive males feed their chicks less often (e.g. Wischhoff et al. 2018), males feeding rate did not correlate with their level of aggressiveness in our study population (Szász et al. 2019b), which does not preclude the possibility that feeding rates may be related to personality traits that have not been investigated yet.
Only one of the background variables, namely the brood size was related to parental feeding rate, as provisioning rate of both females and males were higher when rearing more nestlings. An earlier study in our population also found that female feeding rate was positively correlated with brood size (Kiss et al. 2013). And indeed, the association between the feeding rates of one or both parents and the number of their offspring can be considered a general pattern, not surprisingly, as parents react to offspring begging calls indicating the demands of the nestlings (Grieco 2001 and references therein).
Our result together with that of a previous report about the effects of the sex ratio manipulation experiment on nestling growth, survival and recruitment (Szász et al. 2023) found no evidence that rearing a male-biased brood imposes an additional cost on the parents or offspring. Furthermore, despite of the higher environmental sensitivity of males in this species, the extent of parental workload did not correlate positively with the original brood sex ratio. So, we can conclude that so far our results do not support that the observed sex ratio patterns are adaptive in our population. However, it would be very important to conduct similar studies on other species too, because experimental investigation of the adaptive value of sex allocation are essential to understand this phenomenon, and the rearing costs and the potential fitness benefits of the manipulation may remarkably differ between species making generalization difficult.
Data availability
All data analysed in the current study are available in the repository of Eötvös Loránd University at https://edit.elte.hu/xmlui/handle/10831/108597.
References
Appleby BM, Petty SJ, Blakey JK, Rainey P, Macdonald DW (1997) Does variation of sex ratio enhance reproductive success of offspring in tawny owls (Strix aluco)? Proc R Soc Lond B 264:1111–1116https://doi.org/10.1098/rspb.1997.0153
ASAB Ethical Committee/ABS Animal Care Committee (2024) Guidelines for the ethical treatment of nonhuman animals in behavioural research and teaching. Anim Behav 207:I–XI. https://doi.org/10.1016/S0003-3472(23)00317-2
Bowers EK, Munclinger P, Bures S, Kučerová L, Nádvirník P, Krist M (2013) Cross-fostering eggs reveals that female collared flycatchers adjust clutch sex ratios according to parental ability to invest in offspring. Mol Ecol 22:215–228. https://doi.org/10.1111/mec.12106
Bowers EK, Thompson CF, Sakaluk SK (2015) Persistent sex-by-environment effects on offspring fitness and sex-ratio adjustment in a wild bird population. J Anim Ecol 84:473–486. https://doi.org/10.1111/1365-2656.12294
Burley N (1981) Sex ratio manipulation and selection for attractiveness. Science 211:721–722. https://doi.org/10.1126/science.211.4483.721
Burley N (1986) Sexual selection for aesthetic traits in species with biparental care. Am Nat 127:415–445. https://doi.org/10.1086/284493
Cichoń M, Olejniczak P, Gustafsson L (1998) The effect of body condition on the cost of reproduction in female collared flycatchers Ficedula albicollis. Ibis 140:128–130. https://doi.org/10.1111/j.1474-919x.1998.tb04549.x
Cockburn A, Legge S, Double MC (2002) Sex ratios in birds and mammals: can the hypotheses be disentangled? In: Hardy I (ed) Sex ratios: concepts and research methods. Cambridge University Press, Cambridge, UK, pp 266–286
Dubiec A, Cichon M, Deptuch K (2006) Sex-specific development of cell-mediated immunity under experimentally altered rearing conditions in blue tit nestlings. Proc R Soc Lond B 273:1759–1764.https://doi.org/10.1098/rspb.2006.3510
Ellegren H, Gustafsson L, Sheldon BC (1996) Sex ratio adjustment in relation to paternal attractiveness in a wild bird population. P Natl Acad Sci USA 93:11723–11728.https://doi.org/10.1073/pnas.93.21.11723
Fox J, Weisberg S (2019) An {R} companion to applied regression, 3rd edn. Sage, Thousand Oaks. https://socialsciences.mcmaster.ca/jfox/Books/Companion/
Fridolfsson A-K, Ellegren H (1999) A simple and universal method for molecular sexing of non-ratite birds. J Avian Biol 30:116–121. https://doi.org/10.1163/003925995X00233
Garamszegi LZ, Török J, Michl G, Møller AP (2004) Female survival, lifetime reproductive success and mating status in a passerine bird. Oecologia 138:48–56. https://doi.org/10.1007/s00442-003-1408-z
Gomendio M, Clutton-Brock TH, Albon SD, Guinness FE, Simpson MJ (1990) Mammalian sex ratios and variation in costs of rearing sons and daughters. Nature 343:261–263. https://doi.org/10.1038/343261a0
Green DJ (2002) Pair bond duration influences paternal provisioning and the primary sex ratio of brown thornbill broods. Anim Behav 64:791–800. https://doi.org/10.1006/anbe.2002.1970
Grieco F (2001) Short-term regulation of food-provisioning rate and effect on prey size in blue tits, Parus caeruleus. Anim Behav 62:107–116. https://doi.org/10.1006/anbe.2001.1736
Griffioen M, Müller W, Iserbyt A (2019) A fixed agreement – consequences of brood size manipulation on alternation in blue tits. PeerJ 7:e6826. https://doi.org/10.7717/peerj.6826
Hartig F (2022) DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.4.6. https://CRAN.R-project.org/package=DHARMa
Hegyi G, Laczi M (2015) Using full models, stepwise regression and model selection in ecological data sets: Monte Carlo simulations. Ann Zool Fenn 52:257–279. https://doi.org/10.5735/086.052.0502
Herényi M, Hegyi G, Garamszegi LZ, Hargitai R, Michl G, Rosivall B, Török J (2012) Lifetime offspring production in relation to breeding lifespan, attractiveness, and mating status in male collared flycatchers. Oecologia 170:935–942. https://doi.org/10.1007/s00442-012-2362-4
Khwaja N, Preston SAJ, Hatchwell BJ, Briskie JV, Winney IS, Savage JL (2017) Flexibility but no coordination of visits in provisioning riflemen. Anim Behav 125:25–31. https://doi.org/10.1016/j.anbehav.2016.12.021
Kieffer BE, Ritchison G (2012) Effect of nestling sex ratio on the provisioning behavior of Sialia sialis (Eastern bluebird). Northeast Nat 19:685–690. https://doi.org/10.1656/045.019.0412
Kiss D, Hegyi G, Török J, Rosivall B (2013) The relationship between maternal ornamentation and feeding rate is explained by intrinsic nestling quality. Behav Ecol Sociobiol 67:185–192. https://doi.org/10.1007/s00265-012-1437-x
Könczey R, Török J, Tóth L (1992) Költéssiker és költési területhűség az örvös légykapónál (Ficedula albicollis). Állat Közlem 78:69–76
Laczi M, Kötél D, Török J, Hegyi G (2017) Mutual plumage ornamentation and biparental care: consequences for success in different environments. Behav Ecol 28:1359–1368. https://doi.org/10.1093/beheco/arx099
Leonard ML, Teather KL, Horn AG, Koenig WD, Dickinson JL (1994) Provisioning in western bluebirds is not related to offspring sex. Behav Ecol 5:455–459. https://doi.org/10.1093/beheco/5.4.455
Myers JH (1978) Sex ratio adjustment under food stress: maximization of quality or numbers of offspring? Am Nat 112:381–388. https://doi.org/10.1086/283280
Nishiumi I, Yamagishi S, Maekawa H, Shimoda C (1996) Paternal expenditure is related to brood sex ratio in polygynous great reed warblers. Behav Ecol Sociobiol 39:211–217. https://doi.org/10.1007/s002650050283
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Ramos M, Macedo RH, Diniz P (2015) Attractive males are less than adequate dads in a multimodal signalling passerine. Anim Behav 102:109–117. https://doi.org/10.1016/j.anbehav.2015.01.006
Robert KA, Schwanz LE, Mills HR (2010) Offspring sex varies with maternal investment ability: empirical demonstration based on cross-fostering. Biol Lett 6:242–245. https://doi.org/10.1098/rsbl.2009.0774
Rosivall B, Hasselquist D, Szöllősi E, Török J (2009) Effects of extrapair paternity and sex on nestling growth and condition in the collared flycatcher, Ficedula albicollis. Anim Behav 77:611–617. https://doi.org/10.1016/j.anbehav.2008.11.009
Rosivall B, Szöllősi E, Hasselquist D, Török J (2010) Males are sensitive - sex-dependent effect of rearing conditions on nestling growth. Behav Ecol Sociobiol 64:1555–1562. https://doi.org/10.1007/s00265-010-0969-1
Rosivall B, Török J, Hasselquist D, Bensch S (2004) Brood sex ratio adjustment in collared flycatchers (Ficedula albicollis): results differ between populations. Behav Ecol Sociobiol 56:346–351. https://doi.org/10.1007/s00265-004-0796-3
Santos ESA, Nakagawa S (2012) The costs of parental care: a meta-analysis of the trade-off between parental effort and survival in birds. J Evol Biol 25:1911–1917. https://doi.org/10.1111/j.1420-9101.2012.02569.x
Schwanz LE, Robert KA (2016) Costs of rearing the wrong sex: cross-fostering to manipulate offspring sex in tammar wallabies. PLoS ONE 11:e0146011. https://doi.org/10.1371/journal.pone.0146011
Segura LN, Mahler B (2019) Male red-crested cardinal plumage coloration is associated with parental abilities and breeding performance. Sci Rep 9:10958. https://doi.org/10.1038/s41598-019-47498-6
StatSoft Inc (2003) STATISTICA (data analysis software system), version 6.1. www.statsoft.com
Stoehr AM, McGraw KJ, Nolan PK, Hill GE (2000) Parental care in relation to brood size in the house finch. J Field Ornithol 72:412–418. https://doi.org/10.1648/0273-8570-72.3.412
Suorsa P, Helle H, Huhta E, Jäntti A, Nikula A, Hakkarainen H (2003) Forest fragmentation is associated with primary brood sex ratio in the treecreeper (Certhia familiaris). Proc R Soc Lond B 270:2215–2222https://doi.org/10.1098/rspb.2003.2490
Szász E, Kiss D, Rosivall B (2012) Sex ratio adjustment in birds. Ornis Hung 20:26–36. https://doi.org/10.2478/orhu-2013-0002
Szász E, Garamszegi LZ, Hegyi G, Szöllősi E, Markó G, Török J, Rosivall B (2014) Agressive behavior of the male parent predicts brood sex ratio in a songbird. Naturwissenschaften 101:653–660. https://doi.org/10.1007/s00114-014-1204-0
Szász E, Szöllősi E, Hegyi G, Török J, Rosivall B (2017) Rearing conditions have long-term sex-specific fitness consequences in the collared flycatcher. Behav Ecol 28:717–723. https://doi.org/10.1093/beheco/arx018
Szász E, Garamszegi LZ, Rosivall B (2019a) What is behind the variation in mate quality dependent sex ratio adjustment? – A meta-analysis. Oikos 128:1–12. https://doi.org/10.1111/oik.05157
Szász E, Markó G, Hegyi G, Török J, Garamszegi LZ, Rosivall B (2019b) Nest-site defence aggression during courtship does not predict nestling provisioning in male collared flycatchers. Behav Ecol Sociobiol 73:62. https://doi.org/10.1007/s00265-019-2672-1
Szász E, Sarkadi F, Szöllősi E, Kopena R, Török J, Rosivall B (2023) Are brood sex ratios adaptive?—The effect of experimentally altered brood sex ratio on nestling growth, mortality and recruitment. J Evol Biol 36:156–168. https://doi.org/10.1111/jeb.14118
Trivers RL, Willard DE (1973) Natural selection of parental ability to vary the sex ratio of offspring. Science 179:90–92. https://doi.org/10.1126/science.179.4068.90
Westerdahl H, Bensch S, Hansson B, Hasselquist D, von Schantz T (2000) Brood sex ratios, female harem status and resources for nestling provisioning in the great reed warbler (Acrocephalus arundinaceus). Behav Ecol Sociobiol 47:312–318. https://doi.org/10.1007/s002650050671
Wilk T, Dubiec A, Cichoń M (2007) Seasonal decline in cell-mediated immunity of collared flycatcher Ficedula albicollis nestlings: does the sex of offspring matter? J Ornithol 148:199–205. https://doi.org/10.1007/s10336-006-0121-1
Wischhoff U, Marques-Santos F, Manica LT, Roper JJ, Rodrigues M (2018) Parenting styles in white-rumped swallows (Tachycineta leucorrhoa) show a trade-off between nest defense and chick feeding. Ethology 124:623–632. https://doi.org/10.1111/eth.12770
Yasukawa K, McClure JL, Boley RA, Zanocco J (1990) Provisioning of nestlings by male and female red-winged blackbirds, Agelaius phoeniceus. Anim Behav 40:153–166. https://doi.org/10.1016/S0003-3472(05)80675-X
Acknowledgements
We thank Gergely Hegyi, Márton Herényi, Mónika Jablonszky, Dóra Kötél, Katalin Krenhardt, Miklós Laczi, Gábor Markó, Gergely Nagy, Éva Vaskuti, and Sándor Zsebők for their help in the field. We are thankful to the Pilis Park Forestry for their support. We thank Marty Leonard and two anonymous reviewers for the helpful comments on an earlier version of the manuscript.
Funding
Open access funding provided by Eötvös Loránd University. This study was supported by research grants from the Hungarian National Research, Development and Innovation Office (grant number K120249, FK127917), János Bolyai research scholarships (grant number BO/663/17, BO/163/22) and New National Excellence Program grant from the Hungarian Ministry of Human Capacities (grant number ÚNKP/19–4-ELTE-779, ÚNKP/22–5-ELTE-1151) to BR and ESzö.
Author information
Authors and Affiliations
Contributions
Conceptualization: BR; Data curation: HG (lead), FS (lead), BR (supporting), RK (supporting), ESzá (supporting); Formal analysis: HG (lead), BR (supporting); Funding acquisition: BR (lead), ESzö (supporting); Investigation: HG (equal), RK (equal), BR (equal), FS (equal), ESzö (equal), ESzá (equal), JT (equal); Methodology: BR (equal), ESzö (equal), ESzá (equal); Supervision: BR; Visualization: HG; Writing – original draft: HG; Writing – review and editing: HG (lead), BR (lead), RK (equal), FS (equal), ESzö (equal), ESzá (equal), JT (equal).
Corresponding author
Ethics declarations
Ethics approval
Trapping, ringing and blood sampling of birds were conducted according to protocols established during the long-term monitoring of the study population of collared flycatchers since the early 1980s. Adult birds were trapped with spring traps attached to the nest box entrance when the chicks were already endothermic and did not require brooding. The birds were released immediately after measurements and resumed breeding activities soon thereafter. During the cross-fostering, nestlings were transferred in soft cotton bags placed in boxes heated with reusable pocket warmer packs. The cross-fostering apparently had no adverse effect on the nestlings, because fledging success in the experimental nests was similar to that in unmanipulated nests. To reduce disturbance, we used external video cameras set up approx. 10–15 m away from the recorded nest box. The nestlings continued to receive food shortly after the start of the video recording (X ± SD = 109 ± 82 s). The use of animals adheres to the guidelines set forth by the Animal Behavior Society/Association for the Study of Animal Behaviour (2024). This study received prior approval from the National Scientific Ethical Committee on Animal Experimentation and the Department of Environment and Nature Protection of the Hungarian Government Office (case numbers PE/KTF/11978- 6/2015, PEI/001/1054–6/2015, PE-06/KTF/3331–4/2018, PE/EA/77–8/2018).
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by M. Leonard.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gyarmathy, H., Kopena, R., Sarkadi, F. et al. Are brood sex ratios adaptive? – The effect of experimentally altered brood sex ratios on parental feeding behaviour. Behav Ecol Sociobiol 78, 74 (2024). https://doi.org/10.1007/s00265-024-03490-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-024-03490-3