Abstract
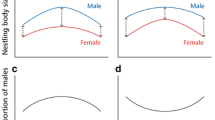
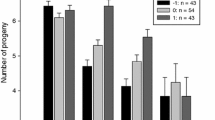
The sex-dependent effect of environmental conditions on nestlings has been extensively studied in size dimorphic birds. Whether males or females are more sensitive to poor conditions is not yet clear; however, the degree of sexual size-dimorphism, brood size and their interactions seem to influence the pattern. Much less is known about sex-dependent environmental sensitivity in size-monomorphic species, even though it may result in biased sex allocation. We altered the rearing conditions by brood size manipulation in the size-monomorphic collared flycatcher and then examined the sex-specific development of the nestlings. In all analyses, we controlled for the effect of paternity, because one may expect extra-pair young to be of better genetic quality and perform better at least under poor conditions. However, this was not the case, because we did not find any difference in growth rate or fledging size between extra- and within-pair young. We found that male nestlings had the potential for faster growth under favourable conditions, but suffered more under poor conditions. We found no sex × environment interaction for fledging size probably because the growth curves level off before fledging, and the disadvantaged nestlings can catch up with their siblings. The larger sensitivity of males does not explain the previously found seasonal shift in brood sex ratios and contradicts previous findings in another size-monomorphic species where females were more sensitive. This suggests that even in size-monomorphic species, no general rule exists, which determines the more sensitive sex.


Similar content being viewed by others
References
Alonso-Alvarez C, Bertrand S, Faivres B, Sorci G (2007) Increased susceptibility to oxidative damage as a cost of accelerated growth in the zebra finches. Funct Ecol 21:873–879
Birkhead TR, Fletcher F, Pellatt EJ (1999) Nestling diet, secondary sexual traits and fitness in the zebra finch. Proc R Soc Lond B 266:385–390
Blount JD, Metcalfe NB, Arnold KE, Surai PF, Devevey GL, Monaghan P (2003) Neonatal nutrition, adult antioxidant defences and sexual attractiveness in the zebra finch. Proc R Soc Lond B 270:1691–1696
Bradbury RB, Blakey JK (1998) Diet, maternal condition, and offspring sex ratio in the zebra finch, Poephila guttata. Proc R Soc Lond B 265:895–899
Brommer JE, Karell P, Pihlaja T, Painter JN, Primmer CR, Pietiäinen H (2003) Ural owl sex allocation and parental investment under poor food conditions. Oecologia 137:140–147
Cordero PJ, Viñuela J, Aparicio JM, Veiga JP (2001) Seasonal variation in sex ratio and sexual egg dimorphism favouring daughters in first clutches of the spotless starling. J Evol Biol 14:829–834
Daan S, Dijkstra C, Weissing FJ (1996) An evolutionary explanation for seasonal trends in avian sex ratios. Behav Ecol 7:426–450
David P, Bjorksten T, Fowler K, Pomiankowski A (2000) Condition dependent signalling of genetic variation in stalked-eyed flies. Nature 406:186–188
de Kogel CH (1997) Long term effects of brood size manipulation on morphological development and sex-specific mortality of offspring. J Anim Ecol 66:167–178
Dijkstra C, Daan S, Buker JB (1990) Adaptive seasonal variation in the sex ratio of kestrel broods. Funct Ecol 4:143–147
Dubiec A, Cichoń M, Deptuch K (2006) Sex-specific development of cell-mediated immunity under experimentally altered rearing conditions in blue tit nestlings. Proc R Soc Lond B 273:1759–1764
Fridolfsson A-K, Ellegren H (1999) A simple and universal method for molecular sexing of non-ratite birds. J Avian Biol 30:116–121
Garvin JC, Abroe B, Pedersen MC, Dunn PO, Whittingham LA (2006) Immune response of nestling warblers varies with extra-pair paternity and temperature. Mol Ecol 15:3833–3840
Hargitai R, Török J, Tóth L, Hegyi G, Rosivall B, Szigeti B, Szöllősi E (2005) Effects of environmental conditions and parental quality on the inter- and intraclutch egg-size variation in the collared flycatcher (Ficedula albicollis). Auk 122:509–522
Hasselquist D, Kempenaers B (2002) Parental care and adaptive brood sex ratio manipulation in birds. Phil Trans R Soc B 357:363–372
Ilmonen P, Hasselquist D, Langefors Å, Wiehn J (2003) Stress, immunocompetence and leucocyte profiles of pied flycatchers in relation to brood size manipulation. Oecologia 136:148–154
Jones KS, Nakagawa S, Sheldon BC (2009) Environmental sensitivity in relation to size and sex in birds: meta-regression analysis. Am Nat 174:122–133
Kilner R (1998) Primary and secondary sex ratio manipulation by zebra finches. Anim Behav 56:155–164
Krebs EA, Green DJ, Double MC, Griffiths R (2002) Laying date and laying sequence influence the sex ratio of crimson rosella broods. Behav Ecol Sociobiol 51:447–454
Martins TLF (2004) Sex specific growth rates in zebra finch nestlings: a possible mechanism for sex ratio adjustment. Behav Ecol 15:174–180
Metcalfe NB, Monaghan P (2001) Compensation for a bad start: grow now, pay later? Trends Ecol Evol 16:254–260
Michaud T, Leonard M (2000) The role of development, parental behavior, and nestmate competition in fledging of nestling tree swallows. Auk 117:996–1002
Nager RG, Monaghan P, Griffiths R, Houston DC, Dawson R (1999) Experimental demonstration that offspring sex ratio varies with maternal condition. Proc Natl Acad Sci USA 96:570–573
Nager RG, Monaghan P, Houston DC, Genovart M (2000) Parental condition, brood sex ratio and differential young survival: an experimental study in gulls (Larus fuscus). Behav Ecol Sociobiol 48:452–457
Naguib M, Riebel K, Marzal A, Gil D (2004) Nestling immunocompetence and testosterone covary with brood size in songbird. Proc R Soc Lond B 271:833–838
Nilsson J-Å, Svensson M (1996) Sibling competition affects nestling growth strategies in marsh tits. J Anim Ecol 65:825–836
Oddie K (2000) Size matters: competition between male and female great tit offspring. J Anim Ecol 69:903–912
Råberg L, Stjernman M, Nilsson J-Å (2005) Sex and environmental sensitivity in blue tit nestlings. Oecologia 145:496–503
Rosivall B, Török J, Hasselquist D, Bensch S (2004) Brood sex ratio adjustment in collared flycatchers (Ficedula albicollis): results differ between populations. Behav Ecol Sociobiol 56:346–351
Rosivall B, Szöllősi E, Török J (2005) Maternal compensation for hatching asynchrony in the collared flycatcher Ficedula albicollis. J Avian Biol 36:531–537
Rosivall B, Szöllősi E, Hasselquist D, Török J (2009) Effects of extrapair paternity and sex on nestling growth and condition in the collared flycatcher, Ficedula albicollis. Anim Behav 77:611–617
Rowland E, Love OP, Verspoor JJ, Sheldon L, Williams TD (2007) Manipulating rearing conditions reveals developmental sensitivity in the smaller sex of a passerine bird, the European starling Sturnus vulgaris. J Avian Biol 38:612–618
Sheldon BC, Merilä J, Lindgren G, Ellegren H (1998) Gender and environmental sensitivity in nestling collared flycatchers. Ecology 79:1939–1948
Szöllősi E, Rosivall B, Török J (2007) Is hatching asynchrony beneficial for the brood? Behav Ecol 18:420–426
Török J, Tóth L (1990) Costs and benefits of reproduction of the collared flycatcher, Ficedula albicollis. In: Blondel J, Gosler A, Lebreton J-D, McCleery R (eds) Population biology of passerine birds. Springer Verlag, Berlin, pp 305–319
Török J, Hegyi G, Tóth L, Könczey R (2004) Unpredictable food supply modifies costs of reproduction and hampers individual optimization. Oecologia 141:432–443
Tschirren B, Fitze PS, Richner H (2003) Sexual dimorphism in succeptability to parasites and cell-mediated immunity in great tit nestlings. J Anim Ecol 72:839–845
Velando A (2002) Experimental manipulation of maternal efforts produces differential effects in sons and daughters: implications for adaptive sex ratios in the blue-footed booby. Behav Ecol 13:443–449
Verhulst S, Holveck M-J, Riebel K (2006) Long-term effects of manipulated natal brood size on metabolic rate in zebra finches. Biol Lett 2:478–480
Acknowledgements
We thank Gergely Hegyi, Rita Hargitai and Márton Herényi for their help in the field. The study was supported by the Hungarian State Eötvös Fellowship to B.R., the Hungarian Scientific Research Fund grants (OTKA T049650, F68295, PD75481), a Hungarian National Office for Research and Technology grant (OMFB-1513/2006), grants from the Swedish Research Council (VR), the Swedish Research Council for Environment, Agricultural Science and Spatial Planning (Formas), the Carl Trygger Foundation, Eötvös Loránd University, Lund University and Pilis Park Forestry.
Ethical notes
The authors declare that the experiment complies with the current laws of Hungary.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Leonard
Rights and permissions
About this article
Cite this article
Rosivall, B., Szöllősi, E., Hasselquist, D. et al. Males are sensitive — sex-dependent effect of rearing conditions on nestling growth. Behav Ecol Sociobiol 64, 1555–1562 (2010). https://doi.org/10.1007/s00265-010-0969-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-010-0969-1