Abstract
Across the animal kingdom, males advertise their quality to potential mates. Males of low reproductive quality, such as those that are sick, may be excluded from mating. In eusocial species, there is some evidence that reproductive females gauge the quality of their mates. However, males often spend much more time with non-reproductive females when being raised or when returning from unsuccessful mating flights. Do non-reproductive workers evaluate the quality of male reproductives? Here we address this question using male honey bees (Apis mellifera), called drones, as a model. We generated immune-challenged drones by injecting them with lipopolysaccharide and tested: 1) do workers evict immune-challenged drones from their colony, 2) do cuticular hydrocarbon (CHC) profiles, body size, or mass change when drones are immune-challenged, and 3) are these changes used by workers to exclude low quality males from the colony? We found that an immune challenge causes changes in CHC profiles of drones and reduces their body mass. Workers selectively evict small and immune-challenged drones who, themselves, do not self-evict. This work demonstrates that some eusocial males undergo an additional layer of scrutiny prior to mating mediated by the non-reproductive worker caste.
Significance statement
Males of some species must advertise their quality to mates but, in the case of eusocial species, must they also advertise their quality to nestmates? By manipulating honey bee male quality, we found that small and immune-challenged drones are evicted from colonies overnight. Workers may not use a drone’s cuticular hydrocarbon profile to make this assessment. This is a new example of social immunity expressed against adult males and an example of worker involvement in reproductive decisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
From sage grouse and salamanders to crickets and crustaceans, animals have evolved methods to advertise their reproductive quality to potential mates (Andersson 1982; Ward 1984; Gibson and Bradbury 1985; Green 1991; Johnstone 1995). These signals may indicate that an animal has had higher-quality nutrition or can provide a nest. They may also indicate when an animal is of lower quality. For instance, male field crickets can develop malformed sound-producing organs if they experience immune stress during maturation, leading to a loss in calling ability. If a female cricket chooses to mate with a male producing more frequent calls, she is thereby choosing a higher quality male (Jacot et al. 2005).
In eusocial species, an advertisement of reproductive quality may not only be received by potential mates, but also nestmates. Social species regularly interact with related nestmates and can glean information about their health statuses. For example, social insect workers are able to detect when a nestmate is sick or injured and change how they interact with that individual (Marikovsky 1962; Oi and Pereira 1993; Boecking and Spivak 1999; Cremer et al. 2007; Aubert and Richard 2008). In eusocial species, it is especially important for non-reproductive workers to be able to assess the quality of their reproductive nestmates as their fitness is derived primarily from the output of the reproductive castes. There is ample evidence that workers gauge the quality of their reproductive females (often called queens), and they have been known to kill and replace queens who are injured, poisoned (Sandrock et al. 2014), or of declining fecundity (Pettis et al. 1997). Fire ant workers, for example, kill queens whose fecundity lessens (Fletcher and Blum 1983) and harvester ant workers kill queens who have been wounded (Rissing and Pollock 1987). Even eusocial mammals have been observed ousting ‘weak’ queens (Faulkes et al. 1991).
Ant workers have been shown to control what males may access their queens (Sunamura et al. 2011; Helft et al. 2015; Vidal et al. 2021). However, less is known about how colonies evaluate their own males. This lack of information is surprising not only because males are a source of direct fitness for eusocial workers but also because they are energetically costly to rear and maintain (Haydak 1970; Hrassnigg and Crailsheim 2005). In eusocial insects, once male eggs hatch, workers must provide food and care until mating occurs (Paxton 2005). The pre-reproductive period can be exceptionally long: in carpenter ants (Camponotus), male larvae are cared for throughout winter (Cannon 1990). In honey bees, males (called drones) are reared for three days longer than workers and require four times more food (Winston 1987). In honey bees, the expense does not end when mating flights begin. Drones die upon successfully mating, but after unsuccessful flights drones return to their natal nests to ‘fuel up’ on nectar (Reyes et al. 2019). They do this for approximately 20–30 days until they mate or die (Reyes et al. 2019). Males typically do not take part in any colony function as they lack the behavioral repertoire or even the anatomy to contribute to most colony functions (Wilson 1971; Starr 1985), although they may contribute to thermoregulation (Kovac et al. 2009). Colonies typically produce drones only when they have sufficient food resources and a large enough worker population to care for them (Smith et al. 2014) because of the substantial investment of resources they require, and the presence adult drones stifles the production of new drones (Rinderer et al.1985). The number of drones a colony will support, therefore, is limited, and workers must determine which drones to keep. To avoid wasting resources on drones unlikely to successfully mate, we hypothesized that workers preferentially house and care for high-quality drones.
Here, we explore whether and how workers evaluate drone quality with a focus on two potential signals of quality: size and cuticular hydrocarbon (CHC) profile. Body mass in honey bee drones is a predictor of reproductive quality (Metz and Tarpy 2019) and is reduced by immune response (Jones et al. 2018) and therefore seemed a viable metric for workers to evaluate. The other potential signal we investigated was the CHC profile. This layer of waxy hydrocarbons on the cuticles of many insects is an important component of nestmate recognition (Cervo et al. 2002; Dani et al. 2005; Smith et al. 2009; Cappa et al. 2016) and fertility signaling in social insects (Steiger et al. 2007; Smith et al. 2009; Liebig et al. 2009). In honey bees, CHC profiles shift when a worker is infected with a disease (Dani et al. 2005; Richard et al. 2008; Cappa et al. 2016; Geffre et al. 2020). When the CHC profile of an immune-challenged worker is applied to a healthy worker, her nestmates treat her as if she is sick (Richard et al. 2008; Conroy and Holman 2022). Honey bee workers use CHC profiles to detect non-nestmate workers (Dani et al. 2005; Pradella et al. 2015; Cappa et al. 2016) and at least one study has shown that workers are able to detect non-nestmate drones (Kirchner and Gadagkar 1994), but the role of the CHC profile was not determined. We investigated whether workers use CHC profile to evaluate the health status of drones as they do with other workers.
Do non-reproductive workers evaluate the quality of male reproductives? We generated immune-challenged drones by injecting them with lipopolysaccharide and tested: 1) do workers evict immune-challenged drones from their colony, 2) does the cuticular hydrocarbon (CHC) profile, body size, or mass change when drones are immune-challenged, and 3) are these changes used by workers to exclude low quality males from the colony?
Materials and methods
Drone collection, handling, and lipopolysaccharide inoculation
All colonies were maintained at Purdue University's Research Apiary in West Lafayette, IN, USA and experiments were conducted between May and September 2023. The apiary houses approximately fifty honey bee colonies of mixed genetic backgrounds sourced from across the United States. All drones from our experiments were collected from colonies either from within a colony or as they returned from mating flights. Drones were collected directly into screen-topped 1L glass jars. We used glass jars to prevent plastic contamination when extracting cuticular hydrocarbons (see Cuticular hydrocarbon profile analysis and transfer below). Once collected, individual drones were randomly assigned to a treatment group and painted with a corresponding enamel paint mark on their thorax.
We immune-challenged drones by pricking them under an abdominal tergite with a size 0 stainless steel entomological pin that had been flame-sterilized then dipped in a 0.5 mg/mL solution of lipopolysaccharide (LPS; Escherichia coli serotype O55:B5, Sigma-Aldrich). The 0.5 mg/mL LPS was made using Ringer’s solution (128 mM NaCl, 18 mM CaCl2, 1.3 mM KCl, 2.3 mM NaHCO3, 1 L dH2O) (Laughton et al. 2011). Control drones were handled in the same way as treated drones but left unpricked. After handling and treatment, drones were kept in 1L glass jars with up to 30 individuals of the same treatment group and fed diluted Pro-Sweet™ (Mann Lake Ltd., Hackensack, MN, USA) at a 60% dilution with water ad libitum. All jars were kept in a Percival I36NL incubator (Percival Scientific, Perry, IA, USA) set at 34° C for up to 24 h.
Verifying LPS inoculation causes an immune response in drones
The honey bee immune system detects the outer membrane components of bacteria (e.g. lipopolysaccharide, LPS) and reacts by expressing immune effector proteins, such as defensin2 (Yang and Cox-Foster 2005; Evans 2006; Richard et al. 2008; Laughton et al. 2011; Harpur et al. 2014). LPS can cause an immune challenge in the absence of a pathogen. In honey bees, pathogens have been shown to manipulate honey bee CHC profiles and behavior (Geffre et al. 2020). To verify that the LPS inoculation causes an immune response in drones, we measured the expression of defensin2 in control drones and treatment drones four and 24-h after being inoculated (N = 4/group). All drones were flash-frozen prior to RNA extraction. We used a Zymo Direct-zol™ RNA Miniprep kit to extract RNA from their thoraces. We then performed RT-qPCR using a Luna® Universal One-Step RT-qPCR kit. We used primers previously used to measure immune response in honey bees and eIF3-S8 as a control (Richard et al. 2008).
The primers used were as follows: Def2-F: CAGAATTGATGGATTCCAACGA3; Def2-R: CGCACGTTACCCTTCGATGT; eIFS8-F: TGAGTGTCTGCTATGGATTGCAA; eIFS8-R: TCGCGGCTCGTGGTAAA. The relative expression of defensin2 for each sample was calculated using the delta-delta Ct method (Livak and Schmittgen 2001) and the resulting data were log transformed prior to statistical analysis to normalize residuals (see Statistics section below).
Estimating mass and body size
We measured the thorax width of 122 control or immune-challenged drones after 24 h. Thorax width was measured at the widest point to the nearest hundredth of a millimeter with ToolShop™ electronic digital calipers. We estimated the dry mass to the nearest 0.1 mg of 109 control or immune-challenged drones 24 h post-injection. We first freeze-dried all samples using a Harvest Right™ Scientific In-home Freeze Dryer on the lowest setting for one week, long enough for mass to stabilize and all moisture to be lost from the samples.
Drone eviction assays
To test if low-quality (immune-challenged) drones are evicted from colonies at a higher rate than control drones, we followed a previously-established eviction assay (Currie and Jay 1988) modified to fit a three frame nucleus colony (mini-colony throughout). Drones are less likely to be accepted when placed into queenright colonies with fewer resources (Free and Williams 1975; Currie and Jay 1988; Boes 2010). We therefore used queenright mini colonies of ~1000 workers that were restricted to a maximum of two frames of resources (honey and pollen) to increase the likelihood of eviction.
For eviction assays, treatment and control drones were kept in the incubator in glass jars for four hours. After four hours, immobile or dead drones were removed, and the remaining ones were introduced into one of three mini colonies. Introductions took place in the evenings between 20:00 and 21:00 local time to reduce the chances of drones leaving the colony on an additional mating flight (Currie and Jay 1988). To introduce drones, we removed the lid of each colony and replaced it with a modified ‘drone includer’ (Currie and Jay 1988): a wooden box with 1.27 cm hardware cloth attached to the bottom to slow the drones from entering all at once. Each jar containing drones was then poured gently into the includer. We placed the colony lid on top of the includer to allow drones to enter the colony. After all drones had moved down through the hardware cloth into the colony, the drone includer was removed and the colony was closed. Treatment and control drones were introduced simultaneously in roughly equal numbers depending on availability, with a total of 68 to 89 drones being introduced each time. In total, 300 control drones and 271 immune challenged drones were introduced over 7 unique introduction events between June 7 and June 19, 2023. Colonies were revisited in the morning before drone flights began. We collected and froze all marked drones. Drones collected from inside the colony were recorded as “retained”; drones found outside the hive were categorized as “evicted”. Drones that were not recovered in the morning were assumed to be evicted. Blinding of the human collector was not necessary due to the objective counting of present or absent drones. All drones were collected and kept frozen at -80° C for later use.
We also tested if immune-challenged drones would be more likely to self-evict. Honey bee workers have been shown to altruistically self-evict when ill to prevent disease spread (Rueppell et al. 2010), so to see if drones display the same behavior, we introduced control (n = 46) and immune-challenged (n = 48) drones following the same procedure, above, to a colony with two frames of food, one empty frame of wax, and no workers.
Cuticular hydrocarbon profile analysis and transfer
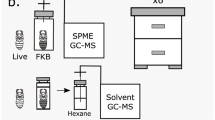
Cuticular chemicals were extracted from a drone by immersing it in 500 uL of HPLC-grade hexane contained in a glass vial with a TFE cap and gently agitating the vial by hand for two minutes at which time the drone was removed. Vials were labeled and stored at 4° C until analyzed. We analyzed 15 control drones and 23 immune-challenged drones.
Extracts were analyzed by coupled gas chromatography-mass spectrometry (GC–MS) with electron impact ionization (70 eV) using an Agilent 6890N gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) equipped with a DB-5MS capillary column (30 m × 0.25 mm × 0.25 um film, Agilent 19091 J-433) in splitless mode with helium as the carrier gas and interfaced to an Agilent 5975 mass selective detector. We followed the protocols set forth in Vernier et al. 2019 with minor changes. 1 uL of extract was injected into the heated GC injection port (250 °C). After a 1-min hold at 60 °C, the oven temperature was ramped at 5 °C/minute with a 10-min hold. Linear and branched chain hydrocarbons were identified by comparing their retention times and mass spectra with those of standards, or by interpretation of the mass spectra in combination with Kovats retention indices (El-Sayed 2023). The abundance of each compound was calculated as a percentage of the total corrected peak area of all hydrocarbons that were consistently present in the total ion chromatograms (ChemStation, Version B.03.01; Hewlett-Packard Corp.). We removed any peak not found in at least 20% of our samples, leaving 18 total peaks which we focused on across all samples for analyses below.
To determine if workers use CHC profiles to assess immune status of a drone, we used the same methods described above to extract CHCs from untreated (control) and LPS-treated drones 24 h post-injection. Following, the vials containing the extract were left uncapped in the fume hood until the solvent evaporated off, leaving the concentrated CHCs behind. Previous studies in honey bee workers transferred the CHC profile of immune-challenged workers to control workers and observed differences in nestmate interactions (Richard et al. 2008). We attempted to transfer the CHC profile of immune-challenged drones to control drones first by applying it with hexane (Richard et al. 2008; Del Piccolo et al. 2010). We discovered that drones could not tolerate any amount of hexane applied to their cuticles: application resulted in ejaculation and death. We instead opted to add 20 uL of ddH2O to the concentrated hexane, mix by vortexing, and then introduce individual marked control drones into single vials for 10 min. Variations of this method have been used successfully in several insect species (Dani et al. 2005; Roux et al. 2009; Conroy and Holman 2022) to transfer hydrocarbons between individuals. We coated 62 control drones in the cuticular extract of immune-challenged drones and 60 in the cuticular extract of control drones. After ten minutes, all 122 drones were removed and introduced to a host colony, described above, to quantify eviction rates. We also collected the extract from one control drone coated in immune-challenged-drone extract and subjected it to GCMS as described above to confirm successful transfer.
Statistics
For all analysis, we used R version 4.1.2 (R Core Team 2020). We tested differences in means among groups using one- or multi-way ANOVA after testing data for underlying assumptions of normality and equality of variance. We used two-tailed tests except where stated in results (e.g. we predicted lower mass for immune-challenged drones; other specific hypotheses listed in results). We kept experimental colonies homogenized: they were maintained with the same number of frames, approximately the same number of workers, and were queenright throughout. They were also kept near each other to reduce variability in removal rates that might arise as a result of colony location. Still, we tested for colony effects in initial ANOVA models for eviction and found none (F2,10 = 1.3; P = 0.31). We therefore included colony only as a random effect throughout. To test for differences in weight and treatment status between evicted and retained drones, we used a mixed effect generalized linear model with trials as random factor and eviction status treated as a binomial (glmer; family = binomial; link = logit). We found no evidence of overdispersion in the resulting model: the ratio of the Pearson residuals and the residual degrees of freedom (0.64) was not greater than one (χ2 = 66; P = 0.99). To test for differences in CHC profile components, we ran individual t-tests across each of the 19 peaks between immune-challenged and control drones. We then corrected for multiple tests using Storey’s Q (Storey 2003) at a threshold of q < 0.05.
Results
LPS injection in drones causes an immune response. Immune-challenged drones lose mass and experience a shift in CHC profile
We tested if LPS elicited an immune response by quantifying the expression of defensin2, an immune effector, in control and LPS-injected drones at four- and 24-h post-injection. We found that LPS causes a significant immune response in drones over the course of 24 h; (ANOVA F2,7 = 25; P = 0.0006; Fig. 1A). LPS inoculation also causes early mortality in drones: we found significantly more drones dead in jars (13.9%) at 24 h post-injection than control drones (1.7%; F1,14 = 11.2; P = 0.0048).
Lipopolysaccharide (LPS) injection causes an immune challenge in drones. A LPS injection increases the expression of the immune effector gene defensin2. Drones were collected from the field and pricked with an LPS-contaminated, sterile, insect pin under their abdominal tergite (Immune Challenged) or handled and not pricked (Control). All drones were placed in 1L glass mason jars containing sugar syrup and left for 24 h (Control and 24 h Immune Challenge) or four hours. We found significant differences in the expression (delta-delta CT) of defensin2 across conditions with expression being significantly highest at 24 h (ANOVA). B After 24 h, control and immune-challenged drones were flash frozen to estimate dry body mass (left: ANOVA F1,107 = 5.5; P = 0.02) and thorax width (right: ANOVA; P > 0.2). C Two representative chromatograms showing the peaks identified in control (top; blue) and immune-challenged (bottom; orange) drones. Insert is PCA showing separation of CHC profiles across a panel of drones. D The eleven numbered peaks in C were found to be significantly different between immune-challenged and control drones
We also tested if an immune challenge affected the dry mass and size (thorax width) of drones by assessing freeze-dried control and LPS-injected drones. We found that dry mass was significantly lower in immune-challenged drones when compared to control drones (F1,107 = 5.5; P = 0.02; Fig. 1B). Thorax width did not vary significantly between immune-challenged and control drones (F1,120 = 0.31; P = 0.58; Fig. 1B). When immune-challenged, worker honey bees experience a shift in their CHC profiles such that there are significant differences in specific CHC compounds between ‘sick’ and healthy workers (Richard et al. 2008). We quantified the CHC profile of control drones and LPS-injected drones and robustly identified 18 peaks in both immune-challenged and control drones across samples, of which 11 varied significantly between treatments (Fig. 1C, D). Retention times and proposed identities of the peaks that change with immune challenge are presented in Table 1.
Immune-challenged and small drones are evicted at a higher rate than healthy drones
Having demonstrated that LPS causes an immune response in drones, we next wanted to test if workers are able to detect and evict immune-challenged drones. We allowed colonies to evaluate both LPS-injected and control drones overnight and collected the retained and evicted drones in the morning. We predicted that colonies would evict a higher proportion of low-quality (immune-challenged) drones compared to controls across colonies. Across trials, we found significantly more immune-challenged drones were evicted than control drones (ANOVA; F1,12 = 5.3; one-tailed P = 0.019; Fig. 2A).
Low-quality drones are evicted from colonies more frequently. A Over one week, 300 control (blue) and 271 immune-challenged (orange) drones were introduced at sunset into one of three mini-colonies (see methods), represented here as a circle, triangle, or square. White and gray background stripes indicate separate introduction events. Across all trials, we found that significantly more immune-challenged drones were evicted than control drones (Fisher Exact; P = 0.019). B We found that both immune-challenged and evicted drones (regardless of immune status) were significantly smaller than control drones or retained drones. C Immune-challenged drones and control drones self-evict from worker-less colonies at the same rate (Fisher Exact; P > 0.12). Ratio in each bar indicates number evicted over the total introduced and one subtracted from this value is plotted
We predicted that evicted drones, irrespective of immune status, would be smaller in body weight than drones allowed to remain in the colony. We found that immune challenge resulted in smaller drones overall (F1,105 = 8.3; P = 0.004; Fig. 2B). We also find that evicted drones, regardless of immune status, were significantly smaller than drones allowed to remain in the colony (GLM; P = 0.00000161; Fig. 2B).
Workers are required for the eviction of immune-challenged drones, or immune-challenged drones may not self-evict
The experiments above demonstrated that immune-challenged drones are more likely to be found outside the colony entrance than healthy drones. This finding could be the result of either worker-led eviction or drones altruistically self-evicting. We tested this by introducing control and immune-challenged drones to a colony without workers present (see methods). We found no significant differences in the proportion of control and LPS-injected drones remaining inside the colony (or found outside the colony) overnight (Fisher Exact Test; P > 0.05; Fig. 2C).
Workers may not use CHCs to identify immune-challenged, low-quality, drones
After transferring the CHC extract from an immune-challenged drone to a control drone, we extracted this chimeric CHC profile and ran the sample through the GCMS to confirm that our treatment was effective enough to alter the overall CHC profile of treated drones. We observed a shift in CHC profile in the CHC-transferred samples such that CHC-transferred drones were intermediate to immune-challenged and control drones (Fig. 3A). We found no significant difference in eviction rates between drones coated in cuticular extract from control or immune-challenged drones (Fisher Exact Test; P = 0.81; Fig. 3B).
Workers may not use a drone’s CHC profile to assess its quality. A Three representative chromatograms showing the peaks identified in control (top; blue) and immune-challenged (middle; orange) and CHC-transferred drones (bottom; purple). B We transferred the CHC profile of immune-challenged drones to control drones (N = 62; purple) and introduced these and control drones (N = 60; blue) to micro-colonies at sunset (see methods). We found no significant differences in eviction rates between each group (Fisher Exact; P > 0.8)
Discussion
Generally, male animals advertise their quality to conspecifics (Johnstone 1995). It can be obvious to those being presented to when a male is sick and should be avoided, leading to unsuccessful mating attempts and reduced fitness (Jacot et al. 2004, 2005). We have found that, in honey bees, drones are evaluated by non-reproductive workers and, if expressing symptoms of an illness or reduced quality, are evicted. Our findings demonstrate an additional layer of social immunity in honey bees and an additional layer of assessment that eusocial species may apply to their reproductive castes. On the former, honey bees and other eusocial species modulate interactions among themselves based on their immune status (Richard et al. 2008; Baracchi et al. 2012; Cappa et al. 2016; Jones et al. 2018). This has been termed ‘social immunity’ (Cremer et al. 2007) and can be an effective form of reducing the spread of disease in a colony (Stroeymeyt et al. 2018). Worker honey bees will evict their sick female nestmates, and sick workers will also altruistically self-evict (Rueppell et al. 2010; Conroy and Holman 2022).
To our knowledge, ours is the first report of workers expressing any form of social immunity towards adult drones. Drones are cited as a source of disease and pests entering colonies, especially in commercial settings where they can drift (Currie 1987; Traver and Fell 2011; Yañez et al. 2012; Peck and Seeley 2019). We found that eviction requires the presence of workers, suggesting that drones do not self-evict when immune-challenged. This contrasts with evidence that workers will abandon their colony if sick and that doing so quantifiably reduces disease load (Rueppell et al. 2010). Altruism in male honey bees may not be expected. Relatedness between drones and their female nestmates is lower, on average, than relatedness among workers, and drones are short-lived so perhaps there is little pressure for altruistic self-eviction to evolve. Looking more broadly across social Hymenoptera, altruism in males is rare (Beani et al. 2014). Alternatively, the presence of nestmates is required to trigger an altruistic response from drones, or comb without workers does not register to drones as a colony. Perhaps even the risk of drones spreading disease is lower than that of workers, or drones use workers to prevent spread. When in a colony, drones are typically found on honey sources and do not interact with the queen (Free 1957; Ohtani 1974; Neubauer et al. 2023); they may use older foragers as a buffer against spreading disease within a colony. More work could explore interactions between immune-challenged drones and workers in a smaller, observational study (e.g. Conroy and Holman 2022) to additionally explore how (and if) immune-challenged drones change their behavior and/or if workers interact with them differently. This finding may have relevance to other eusocial species. Certainly, we predict similar behavior across the genus Apis where all males are reared en masse and return to their natal colony after unsuccessful mating attempts (Hagan et al. 2023), but we also may expect similar behavior to have evolved independently across other eusocial species with long pre-reproductive and reproductive periods in males.
Immune challenge causes a CHC profile shift in drones, but is it used as a signal by workers?
Social immunity and reproductive quality assessments depend on workers being able to interpret an honest signal of immunity or quality in drones. Many insects use the CHC profile as a source of information about other insects they may interact with (Dani et al. 2005; Steiger et al. 2007; Smith et al. 2009; Liebig et al. 2009; Cappa et al. 2016). In eusocial insects, the CHC profile broadcasts information that workers use. As just a few examples, the CHC profile is used to discriminate nestmates from non-nestmates (Cervo et al. 2002; Dani et al. 2005), signal larval sex (Sasaki et al. 2004), identify the social role of nestmates (Smith et al. 2009; Del Piccolo et al. 2010), and identify dead or infected larvae or adults for eviction (Spivak and Reuter 1998). When a honey bee worker is immune challenged, her CHC profile shifts (Cappa et al. 2016). Workers respond to LPS-injected nestmates by increasing aggression and evictions (Richard et al. 2008). In at least one case, the viral pathogen Israeli Acute Paralysis Virus (IAPV), immune-related CHC shifts increase colony acceptance of infected workers and may contribute to disease spread (Geffre et al. 2020). We found that drones, like workers, have a significant shift in their CHC profile when immune-challenged.
Following previous works in bees (Dani et al. 2005; Conroy and Holman 2022) and other social insects (Roux et al. 2009), we transferred CHC profiles of immune-challenged individuals to untreated controls. We found drones to be intolerant of hexane applied in any amount to their body and therefore could not wipe off their CHC profile prior to CHC transfer. To our knowledge, removing the CHC profile prior to transfer is not done in honey bees and the resulting CHC profile after transfer is therefore chimeric in ours and previous studies (Dani et al. 2005; Richard et al. 2008; Conroy and Holman 2022). Therefore, the interpretation from previous studies could be that workers do not tolerate aberrant CHC profiles on other workers. We only applied CHCs to control drones; it is possible that adding CHCs from healthy drones could “rescue” immune-challenged drones from eviction. However, applying the CHCs from an immune-challenged worker to a healthy one is sufficient to trigger eviction in workers (Richard et al. 2008; Conroy and Holman 2022) and our experiment shows the same does not follow for drones. Following this logic, we suggest that workers are indifferent to the CHC profile of drones. This seems apparent from the natural history of honey bees: workers strongly prevent entry from unrelated workers (Cappa et al. 2016) while as many as 50% of drones in a colony may be foreign (Currie and Jay 1988; Reyes et al. 2019). Previous work has demonstrated that workers can detect foreign drones (Kirchner and Gadagkar 1994) but did not ascertain eviction. For drones, the CHC profile may not be a signal used by workers and/or may not affect eviction decisions.
What is the signal workers use to detect and evict low-quality drones? We propose that it may be mass. Body mass is predictive of drone reproductive quality: heavier drones have more and healthier sperm (Schlüns et al. 2003, 2004; Yániz et al. 2020; Quartuccio et al. 2020). Previous work has demonstrated that workers alter their behavior towards smaller drones (Slone et al. 2012; Goins and Schneider 2013) compared worker-drone interactions for drones reared in worker comb (thus, significantly smaller in size and mass) to ‘normal’ drones. They found that workers engaged in trophallaxis and shook drones equally regardless of size, but smaller drones received more grooming, more aggressive interactions, and more eviction attempts. The authors noted they could not observe evictions as drones could not leave the colony they were placed in. Our results show that, regardless of immune status, smaller drones are more likely to be evicted. We also show that in addition to size reduction due to experimental rearing conditions that restrict growth, naturally occurring size variation is sufficient to trigger eviction. As noted above, workers ‘vibrate’ drones in the colony, a signal thought to encourage drones to beg for food and move more (Boucher and Schneider 2009). Vibration may also be a means to assess a drone’s mass and therefore reproductive quality. The present study was not designed to explicitly test this hypothesis. It could be empirically tested by adding weights to drones, as has been done to other bees (Hayworth et al. 2009), and quantifying eviction rates and aggressive worker behaviors toward heavy and light drones. It is also possible that smaller drones are easier to evict, and future behavioral studies will be necessary to determine whether size affects the amount of aggression received or the ability to resist aggression.
On variation in colony choosiness
Drone eviction by workers varies with environmental conditions inside and outside of the colony (Free and Williams 1975; Currie and Jay 1988; Boes 2010). Drones are resource-intensive to rear and maintain for a colony (Winston 1987) so when colony resources are low, eviction becomes more likely. As an example, in autumn, drones are evicted during periods of poor food availability (Free and Williams 1975), and by winter all drones are evicted as the colony enters a state of dormancy (Morse et al. 1967). Drone production depends on the climate but typically occurs when colonies are bringing in resources (Free and Williams 1975; Boes 2010). Colony size also contributes to drone eviction rates: large colonies can host more drones than smaller ones (Free and Williams 1975). Queenlessness is an additional contributor to drone acceptance: queenless colonies accept more drones than queenright colonies (Currie and Jay 1988).
In our study, we maintained colonies that would be more likely to evict drones. We maintained small, queen-right colonies that were allowed to produce brood with only a few food frames available. Therefore, our findings are likely to be limited to times when colonies must be more discriminating about which drones enter. A large, healthy colony in peak season may accept any drone regardless of quality or immune status. Similarly, such a colony may only be choosy when it has reached its drone caring capacity. While we have demonstrated that workers are choosy of the drones they allow to enter, additional work is needed to understand how variation in colony and worker choosiness arises.
Conclusions
We found that an immune challenge causes changes in CHC profiles of drones and reduces body mass. Workers selectively evict small and immune-challenged drones who, themselves, do not self-evict. While CHC profiles are relevant to worker-worker communication, they may not indicate a male’s quality to his female nestmates. This work demonstrates that eusocial males undergo an additional layer of scrutiny prior to mating, one mediated by the non-reproductive worker caste. The discriminated signal remains elusive, but we hypothesize it may be mass.
Data availability
The datasets generated and analyzed during the study are available through DataDryad: https://doi.org/10.5061/dryad.4f4qrfjjw
References
Andersson M (1982) Sexual selection, natural selection and quality advertisement. Biol J Lin Soc 17:375–393. https://doi.org/10.1111/j.1095-8312.1982.tb02028.x
Aubert A, Richard F-J (2008) Social management of LPS-induced inflammation in Formica polyctena ants. Brain Behav Immun 22:833–837. https://doi.org/10.1016/j.bbi.2008.01.010
Baracchi D, Fadda A, Turillazzi S (2012) Evidence for antiseptic behaviour towards sick adult bees in honey bee colonies. J Insect Physiol 58:1589–1596. https://doi.org/10.1016/j.jinsphys.2012.09.014
Beani L, Dessì-Fulgheri F, Cappa F, Toth A (2014) The trap of sex in social insects: From the female to the male perspective. Neurosci Biobehav Rev 46:519–533. https://doi.org/10.1016/j.neubiorev.2014.09.014
Boecking O, Spivak M (1999) Behavioral defenses of honey bees against Varroa jacobsoni Oud. Apidologie 30:141–158. https://doi.org/10.1051/apido:19990205
Boes KE (2010) Honeybee colony drone production and maintenance in accordance with environmental factors: an interplay of queen and worker decisions. Insect Soc 57:1–9. https://doi.org/10.1007/s00040-009-0046-9
Boucher M, Schneider SS (2009) Communication signals used in worker–drone interactions in the honeybee, Apis mellifera. Anim Behav 78:247–254. https://doi.org/10.1016/j.anbehav.2009.04.019
Cannon, CA (1990) Demography, cold hardiness, and nutrient reserves of overwintering nests of the carpenter ant Camponotus pennsylvanicus (De Geer) (Hymenoptera: Formicidae). Doctoral dissertation, Virginia Tech
Cappa F, Bruschini C, Protti I et al (2016) Bee guards detect foreign foragers with cuticular chemical profiles altered by phoretic varroa mites. J Apic Res 55:268–277. https://doi.org/10.1080/00218839.2016.1229886
Cervo R, Dani FR, Zanetti P et al (2002) Chemical nestmate recognition in a stenogastrine wasp, Liostenogaster flavolineata (Hymenoptera Vespidae). Ethol Ecol Evol 14:351–363
Conroy TE, Holman L (2022) Social immunity in the honey bee: do LPS-challenged workers enter enforced or self-imposed exile? Behav Ecol Sociobiol 76:32. https://doi.org/10.1007/s00265-022-03139-z
Cremer S, Armitage SAO, Schmid-Hempel P (2007) Social immunity. Curr Biol 17:R693–R702. https://doi.org/10.1016/j.cub.2007.06.008
Currie RW (1987) The biology and behaviour of drones. Bee World 68:129–143. https://doi.org/10.1080/0005772X.1987.11098922
Currie RW, Jay SC (1988) Factors affecting the acceptance of foreign drones into honey bee (Apis mellifera l.) colonies. Apidologie 19:231–240. https://doi.org/10.1051/apido:19880302
Dani FR, Jones GR, Corsi S et al (2005) Nestmate recognition cues in the honey bee: differential importance of cuticular alkanes and alkenes. Chem Senses 30:477–489. https://doi.org/10.1093/chemse/bji040
Del Piccolo F, Nazzi F, Della Vedova G, Milani N (2010) Selection of Apis mellifera workers by the parasitic mite Varroa destructor using host cuticular hydrocarbons. Parasitology 137:967–973. https://doi.org/10.1017/S0031182009991867
El-Sayed A (2023) The Pherobase: Database of pheromones and semiochemicals. In: The Pherobase: Database of Pheromones and Semiochemicals. https://www.pherobase.com/. Accessed 25 Oct 2023
Evans JD (2006) Beepath: an ordered quantitative-PCR array for exploring honey bee immunity and disease. J Invertebr Pathol 93:135–139. https://doi.org/10.1016/j.jip.2006.04.004
Faulkes CG, Abbott DH, Jarvis JU (1991) Social suppression of reproduction in male naked mole-rats, Heterocephalus glaber. J Reprod Fertil 91:593–604. https://doi.org/10.1530/jrf.0.0910593
Fletcher DJ, Blum MS (1983) Regulation of queen number by workers in colonies of social insects. Science 219:312–314. https://doi.org/10.1126/science.219.4582.312
Free JB (1957) The food of adult drone honeybees (Apis mellifera). Br J Anim Behav 5:7–11. https://doi.org/10.1016/S0950-5601(57)80038-0
Free JB, Williams IH (1975) Factors determining the rearing and rejection of drones by the honeybee colony. Anim Behav 23:650–675. https://doi.org/10.1016/0003-3472(75)90143-8
Geffre AC, Gernat T, Harwood GP et al (2020) Honey bee virus causes context-dependent changes in host social behavior. Proc Natl Acad Sci 117:10406–10413. https://doi.org/10.1073/pnas.2002268117
Gibson RM, Bradbury JW (1985) Sexual selection in lekking sage grouse: phenotypic correlates of male mating success. Behav Ecol Sociobiol 18:117–123. https://doi.org/10.1007/BF00299040
Goins A, Schneider SS (2013) Drone “quality” and caste interactions in the honey bee, Apis mellifera L. Insect Soc 60:453–461. https://doi.org/10.1007/s00040-013-0310-x
Green AJ (1991) Large male crests, an honest indicator of condition, are preferred by female smooth, newts, Triturus vulgaris (Salamandridae) at the spermatophore transfer stage. Anim Behav 41:367–369. https://doi.org/10.1016/S0003-3472(05)80489-0
Hagan T, Lim J, Gloag R (2023) Drones do not drift between nests in a wild population of Apis cerana. Insects 14:323. https://doi.org/10.3390/insects14040323
Harpur BA, Chernyshova A, Soltani A et al (2014) No Genetic tradeoffs between hygienic behaviour and individual innate immunity in the honey bee. Apis Mellifera Plos One 9:e104214. https://doi.org/10.1371/journal.pone.0104214
Haydak MH (1970) Honey bee nutrition. Annu Rev Entomol 15:143–156. https://doi.org/10.1146/annurev.en.15.010170.001043
Hayworth MK, Johnson NG, Wilhelm ME et al (2009) Added weights lead to reduced flight behavior and mating success in polyandrous honey bee queens (Apis mellifera). Ethology 115:698–706. https://doi.org/10.1111/j.1439-0310.2009.01655.x
Helft F, Monnin T, Doums C (2015) First evidence of inclusive sexual selection in the ant cataglyphis cursor: worker aggressions differentially affect male access to virgin queens. Ethology 121:641–650. https://doi.org/10.1111/eth.12376
Hrassnigg N, Crailsheim K (2005) Differences in drone and worker physiology in honeybees (Apis mellifera) 36. https://doi.org/10.1051/apido:2005015
Jacot A, Scheuber H, Brinkhof MWG (2004) Costs of an induced immune response os sexual display and longevity in field crickets. Evolution 58:2280–2286. https://doi.org/10.1111/j.0014-3820.2004.tb01603.x
Jacot A, Scheuber H, Kurtz J, Brinkhof MWG (2005) Juvenile immune status affects the expression of a sexually selected trait in field crickets. J Evol Biol 18:1060–1068. https://doi.org/10.1111/j.1420-9101.2005.00899.x
Johnstone RA (1995) Sexual selection, honest advertisement and the handicap principle: reviewing the evidence. Biol Rev 70:1–65. https://doi.org/10.1111/j.1469-185X.1995.tb01439.x
Jones B, Shipley E, Arnold KE (2018) Social immunity in honeybees—Density dependence, diet, and body mass trade-offs. Ecol Evol 8:4852–4859. https://doi.org/10.1002/ece3.4011
Kirchner WH, Gadagkar R (1994) Discrimination of nestmate workers and drones in honeybees. Ins Soc 41:335–338. https://doi.org/10.1007/BF01242306
Kovac H, Stabentheiner A, Brodschneider R (2009) Contribution of honeybee drones of different age to colonial thermoregulation. Apidologie 40:82–95. https://doi.org/10.1051/apido/2008069
Laughton AM, Boots M, Siva-Jothy MT (2011) The ontogeny of immunity in the honey bee, Apis mellifera L. following an immune challenge. J Insect Physiol 57:1023–1032. https://doi.org/10.1016/j.jinsphys.2011.04.020
Liebig J, Eliyahu D, Brent CS (2009) Cuticular hydrocarbon profiles indicate reproductive status in the termite Zootermopsis nevadensis. Behav Ecol Sociobiol 63:1799–1807. https://doi.org/10.1007/s00265-009-0807-5
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Marikovsky PI (1962) On some features of behavior of the antsFormica rufa L. infected with fungous disease. Ins Soc 9:173–179. https://doi.org/10.1007/BF02224263
Metz BN, Tarpy DR (2019) Reproductive senescence in drones of the honey bee (Apis mellifera). Insects 2075–4450(10):11–21. https://doi.org/10.3390/insects10010011
Morse RA, Strang GE, Nowakowski J (1967) Fall death rates of drone honey bees. J Econ Entomol 60:1198–1202. https://doi.org/10.1093/jee/60.5.1198
Neubauer LC, Davidson JD, Wild B et al (2023) Honey bee drones are synchronously hyperactive inside the nest. Anim Behav 203:207–223. https://doi.org/10.1016/j.anbehav.2023.05.018
Ohtani T (1974) Behavior repertoire of adult drone honeybee within observation hives. J Fac Sci Hokkaido Univ VI Zool 19:709–721. Available at: https://eprints.lib.hokudai.ac.jp/dspace/bitstream/2115/27583/1/19(3)_P706-721.pdf
Oi DH, Pereira RM (1993) Ant Behavior and Microbial Pathogens (Hymenoptera: Formicidae). Fla Entomol 76:63–74. https://doi.org/10.2307/3496014
Paxton RJ (2005) Male mating behaviour and mating systems of bees: an overview. Apidologie 36:145–156. https://doi.org/10.1051/apido:2005007
Peck DT, Seeley TD (2019) Mite bombs or robber lures? The roles of drifting and robbing in Varroa destructor transmission from collapsing honey bee colonies to their neighbors. PLoS ONE 14:e0218392. https://doi.org/10.1371/journal.pone.0218392
Pettis JS, Higo HA, Pankiw T, Winston ML (1997) Queen rearing suppression in the honey bee - evidence for a fecundity signal. Insectes Soc 44:311–322. https://doi.org/10.1007/s000400050053
Pradella D, Martin SJ, Dani FR (2015) Using Errors by Guard Honeybees (Apis mellifera) to Gain New Insights into Nestmate Recognition Signals. Chem Senses 40:649–653. https://doi.org/10.1093/chemse/bjv053
Quartuccio M, Cristarella S, Scrofani A et al (2020) The sperm of Apis mellifera siciliana and Apis mellifera ligustica: A preliminary and comparative note. J Apic Res 59:1011–1016. https://doi.org/10.1080/00218839.2020.1752465
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Reyes M, Crauser D, Prado A, Le Conte Y (2019) Flight activity of honey bee (Apis mellifera) drones. Apidologie 50:669–680. https://doi.org/10.1007/s13592-019-00677-w
Richard F-J, Aubert A, Grozinger C (2008) Modulation of social interactions by immune stimulation in honey bee, Apis mellifera, workers. BMC Biol 6:50. https://doi.org/10.1186/1741-7007-6-50
Rinderer TE, Hellmich RL, Danka RG, Collins AM (1985) Male reproductive parasitism: a factor in the africanization of european honey-bee populations. Science 228:1119–1121. https://doi.org/10.1126/science.228.4703.1119
Rissing SW, Pollock GB (1987) Queen aggression, pleometrotic advantage and brood raiding in the ant Veromessor pergandei (Hymenoptera: Formicidae). Anim Behav 35:975–981. https://doi.org/10.1016/S0003-3472(87)80154-9
Roux O, Martin J-M, Ghomsi NT, Dejean A (2009) A non-lethal water-based removal-reapplication technique for behavioral analysis of cuticular compounds of ants. J Chem Ecol 35:904–912. https://doi.org/10.1007/s10886-009-9673-x
Rueppell O, Hayworth MK, Ross NP (2010) Altruistic self-removal of health-compromised honey bee workers from their hive. J Evol Biol 23:1538–1546. https://doi.org/10.1111/j.1420-9101.2010.02022.x
Sandrock C, Tanadini M, Tanadini LG et al (2014) Impact of chronic neonicotinoid exposure on honeybee colony performance and queen supersedure. PLoS ONE 9:e103592. https://doi.org/10.1371/journal.pone.0103592
Sasaki K, Kitamura H, Obara Y (2004) Discrimination of larval sex and timing of male brood elimination by workers in honeybees (Apis mellifera L.). Appl Entomol Zool 39:393–399. https://doi.org/10.1303/aez.2004.393
Schlüns H, Schlüns EA, Van Praagh J, Moritz RFA (2003) Sperm numbers in drone honeybees (Apis mellifera ) depend on body size. Apidologie 34:577–584. https://doi.org/10.1051/apido:2003051
Schlüns H, Koeniger G, Koeniger N, Moritz RFA (2004) Sperm utilization pattern in the honeybee (Apis mellifera). Behav Ecol Sociobiol 56:458–463
Slone JD, Stout TL, Huang ZY, Schneider SS (2012) The influence of drone physical condition on the likelihood of receiving vibration signals from worker honey bees, Apis mellifera. Insect Soc 59:101–107. https://doi.org/10.1007/s00040-011-0195-5
Smith AA, Hölldober B, Liebig J (2009) Cuticular hydrocarbons reliably identify cheaters and allow enforcement of altruism in a social insect. Curr Biol 19:78–81. https://doi.org/10.1016/j.cub.2008.11.059
Smith ML, Ostwald MM, Loftus JC, Seeley TD (2014) A critical number of workers in a honeybee colony triggers investment in reproduction. Naturwissenschaften 101:783–790. https://doi.org/10.1007/s00114-014-1215-x
Spivak M, Reuter GS (1998) Performance of hygienic honey bee colonies in a commercial apiary. Apidologie 29:291–302. https://doi.org/10.1051/apido:19980308
Starr C (1985) Enabling mechanisms in the origin of sociality in the hymenoptera—The sting’s the thing. Ann Entomol Soc Am 78:836–840. https://doi.org/10.1093/aesa/78.6.836
Steiger S, Peschke K, Francke W, Müller JK (2007) The smell of parents: breeding status influences cuticular hydrocarbon pattern in the burying beetle Nicrophorus vespilloides. Proceedings of the Royal Society b: Biological Sciences 274:2211–2220. https://doi.org/10.1098/rspb.2007.0656
Storey JD (2003) The positive false discovery rate: A bayesian interpretation and the q-Value. Ann Stat 31:2013–2035. https://doi.org/10.1214/aos/1074290335
Stroeymeyt N, Grasse AV, Crespi A et al (2018) Social network plasticity decreases disease transmission in a eusocial insect. Science 362:941–945. https://doi.org/10.1126/science.aat4793
Sunamura E, Hoshizaki S, Sakamoto H et al (2011) Workers select mates for queens: a possible mechanism of gene flow restriction between supercolonies of the invasive Argentine ant. Naturwissenschaften 98:361–368. https://doi.org/10.1007/s00114-011-0778-z
Traver BE, Fell RD (2011) Nosema ceranae in drone honey bees (Apis mellifera). J Invertebr Pathol 107:234–236. https://doi.org/10.1016/j.jip.2011.05.016
Vernier CL, Krupp JJ, Katelyn M et al (2019) The cuticular hydrocarbon profiles of honey bee workers develop via a socially-modulated innate process. eLife 8. https://doi.org/10.7554/eLife.41855
Vidal M, Königseder F, Giehr J et al (2021) Worker ants promote outbreeding by transporting young queens to alien nests. Commun Biol 4:1–8. https://doi.org/10.1038/s42003-021-02016-1
Ward PI (1984) The effects of size on the mating decisions of Gammarus pulex (Crustacea, Amphipoda). Z Tierpsychol 64:174–184. https://doi.org/10.1111/j.1439-0310.1984.tb00358.x
Wilson EO (1971) The Insect Societies. Belknap Press, Cambridge, MA
Winston ML (1987) The biology of the honey bee. Harvard University Press, Cambridge, Mass
Yañez O, Jaffé R, Jarosch A et al (2012) Deformed wing virus and drone mating flights in the honey bee (Apis mellifera): implications for sexual transmission of a major honey bee virus. Apidologie 43:17–30. https://doi.org/10.1007/s13592-011-0088-7
Yang X, Cox-Foster DL (2005) Impact of an ectoparasite on the immunity and pathology of an invertebrate: Evidence for host immunosuppression and viral amplification. Proc Natl Acad Sci 102:7470–7475. https://doi.org/10.1073/pnas.0501860102
Yániz JL, Silvestre MA, Santolaria P (2020) Sperm quality assessment in honey bee drones. Biology (basel) 9:174. https://doi.org/10.3390/biology9070174
Acknowledgements
The authors would like to thank all the members of the Harpur and Ginzel labs at Purdue for their input and add another heap of thanks to Dr. Ben Taylor for his thorough and thoughtful comments on an earlier draft. We thank Krispn Given for his help in creating the mini colonies used in this study. The final manuscript was improved with the kind comments of three anonymous reviewers.
Funding
This study was funded in part by Purdue SCARF (Summer College of Agriculture Research Fellowship; IRG) program, Project Apis M. (BAH), and USDA AFRI CARE (BAH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare.
Additional information
Communicated by O. Rueppell
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gilchrist, I.R., Nixon, J.M., Shultz, R.R. et al. To house or oust: Honey bee (Apis mellifera) colonies can evaluate and evict drones of low quality. Behav Ecol Sociobiol 78, 47 (2024). https://doi.org/10.1007/s00265-024-03461-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-024-03461-8