Abstract
The evolutionary relationships between ecology, cognition, and neurobiology remain elusive, despite important contributions from functional studies and comparative analyses. Recently, Heliconius butterflies and their Heliconiini allies have emerged as a promising system for investigating the evolution and ecology of cognition. In Heliconius, regions of the brain involved in learning and memory, called the mushroom bodies, have quadrupled in size and contain up to 8 times more neurons than closely related genera. This expansion, largely driven by increased dedication to processing visual input, occurred relatively recently (~12–18 Ma) and coincides with the evolution of a novel foraging behaviour — trapline foraging between pollen resources, which provide an adult source of amino acids. Behavioural experiments show that, relative to other Heliconiini, Heliconius exhibit superior visual long-term memory and non-elemental learning, behaviours which have putative relevance for visual learning during traplining, while exhibiting no differences in shape learning or reversal learning. These cognitive differences are also associated with changes in the plastic response of the mushroom body to learning and experience. Heliconius thus constitute a clear example of a suite of neural adaptations that coincides with a novel behaviour reliant on distinct cognitive shifts. We highlight the Heliconiini as a well-positioned, developing case study in cognitive ecology and evolution, where there is the possibility of synthesising comparative neuroanatomical, developmental and behavioural data with extensive genomic resources. This would provide a rich dataset linking genes, brains, behaviour, and ecology, and offer key insights into the mechanisms and selective pressures shaping the evolution of interspecific cognitive variation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The evolution of integrative brain regions and challenges in comparative cognition
The processes shaping interspecific variation in cognitive abilities, and the neural bases underlying these behavioural differences, continue to be core questions in evolutionary biology and animal behaviour. Relative brain size has long been viewed as representative of cognitive capacity (Jerison 1973, 1985) and indeed it has been putatively linked to variation in certain cognitive traits in select groups (Reader and Laland 2002; Dunbar and Shultz 2007; Benson-Amram et al. 2016). However, the use of whole brain measurements as a proxy for general “intelligence” has been critiqued (Healy and Rowe 2007; Chittka and Niven 2009; Logan et al. 2018), as it can obscure important interspecific differences in brain connectivity and composition (for examples of this variation see Barton and Harvey 2000; Farris and Schulmeister 2011; Herculano-Houzel et al. 2015; Olkowicz et al. 2016). Several strands of research have instead focused on the expansion of integrative brain centres, which receive and process input from primary sensory neuropils. Such expansions have occurred independently in a diverse range of lineages including, for example, the primate neocortex (Barton and Harvey 2000) and cerebellum (Barton and Venditti 2014), the cerebella of actively electrosensing fish (Sukhum et al. 2018; Schumacher and Carlson 2022), the hippocampus in food storing birds (Krebs et al. 1989; Healy and Krebs 1992), and the mushroom bodies in several insect groups (Farris 2013). Focusing on specific brain regions can provide more precise functional predictions of size variation. Indeed, integrative brain regions are generally accepted as playing critical roles in learning and memory or pattern extraction (Mumford 1992; Liu et al. 1999; Norman 2010; Hawkins and Ahmad 2016). However, the selective pressures driving their elaboration and behavioural consequences of these adaptations can be difficult to pinpoint (Farris 2015).
Functional studies, predominantly focusing on single, model species, have greatly furthered our knowledge of how these integrative brain regions receive and process information (Rolls 1989; Belle and Heisenberg 1994; Hampton and Shettleworth 1996; Mizunami et al. 1998). However, unravelling the evolutionary processes shaping nervous systems, including selection pressures, and developmental and functional constraints, requires this work to be combined with comparative studies across divergent populations or species. Phylogenetic comparative analyses have revealed ways brain composition can vary with ecology across selected taxa (Lefebvre et al. 2004; Sol et al. 2010; DeCasien et al. 2017; Schumacher and Carlson 2022). These studies demonstrate how ecological interactions among different behavioural, life history, and climatic traits can act in complex ways to shape investment in brain structure across large phylogenetic distances. For example, there is evidence for both diet and sociality as possible drivers of neocortex expansion in primates (Clutton-Brock and Harvey 1980; Dunbar 1992; DeCasien et al. 2017); however, their relative importance continues to be debated (Dunbar and Shultz 2017; Grabowski et al. 2023). A current weakness in the field of cognitive evolution is the relative paucity of studies incorporating more precise neuroanatomical and behavioural data in a common comparative framework, with an attempt to link changes in specific brain structures to distinct cognitive shifts. A classic example of this kind of integrative work concerns the relationship between hippocampus expansion, food-storing behaviour and enhanced spatial learning ability across birds species (Shettleworth 2003), or between populations of species that vary in the availability and reliability of food sources (e.g. Pravosudov and Clayton 2002). In many cases, a major obstacle for comparative studies is ensuring that the neural, behavioural, and ecological data collected is indeed comparable (Logan et al. 2018). The use of highly ecologically or phylogenetically divergent clades can make it difficult to precisely identify the selective pressures involved in particular traits, and comparing species with deep phylogenetic splits can present a challenging context when accounting for confounding variables. Comparative behavioural studies can also ignore important ecological differences between species, casting doubt over the biological relevance of the assessed cognitive tasks (Pritchard et al. 2016; Logan et al. 2018). Many of the issues above can be avoided by looking to adaptive radiations which result in behaviourally and neuroanatomical diverse, but closely related, groups of species, thereby providing solid foundations for comparative work. As a richly speciose and diverse group (Tihelka et al. 2021), insects naturally provide many such radiations, and their experimental tractability allows for the collection of large, multi-species datasets. Investigating the evolution of the insect mushroom body, an evolutionarily labile region of the brain with a key role in learning and memory (Farris and Van Dyke 2015), can thus help to answer many outstanding questions in cognitive evolution.
Insect mushroom bodies and Heliconius as a model in cognitive evolution
The insect mushroom bodies are bilaterally-symmetrical regions of high synaptic density (“neuropils”) in the central brain. They predominantly receive visual and/or olfactory information from primary sensory centres and play an important role in learning and memory (Farris and Van Dyke 2015). The behavioural functions of the mushroom bodies vary substantially between taxa, particularly in the relative importance of the visual and olfactory modalities (Farris and Van Dyke 2015). For example, in Drosophila, the mushroom bodies are important for olfactory learning (Heisenberg et al. 1985; Belle and Heisenberg 1994), but the calyces receive comparatively less visual input (Li et al. 2020) than other groups, including the apocritan Hymenoptera (Mobbs 1982) and some Lepidoptera (Kinoshita et al. 2015; Couto et al. 2020, 2023), in which a large proportion of the calyx is innervated by projection neurons from visual centres. Importantly, the mushroom bodies show marked expansion in at least four independent insect lineages, including Hymenoptera (Farris and Schulmeister 2011), cockroaches (Neder 1959), herbivorous scarab beetles (Farris and Roberts 2005), and Heliconius butterflies (Sivinski 1989; Montgomery et al. 2016; Couto et al. 2023). Functional studies of the mushroom body, predominantly conducted on single species, have shaped our understanding of its involvement in visual and olfactory associative learning and memory (Belle and Heisenberg 1994; Zars 2000; Pascual and Préat 2001; Vogt et al. 2014; Li et al. 2017), reversal learning (Devaud et al. 2007), non-elemental learning (Devaud et al. 2015), and spatial navigation (Mizunami et al. 1998; Kamhi et al. 2020; Buehlmann et al. 2020). However, few studies have directly investigated how behaviour and cognition vary with mushroom body structure between closely-related species (van Dijk et al. 2017; Gordon et al. 2018). A comparative study of Hymenoptera, the most widely studied example of mushroom body elaboration, shows that, there, the expansion coincided with the emergence of parasitoidism, suggesting it may have been driven by the cognitive demands of spatial memory of host locations, rather than social cognition (Farris and Schulmeister 2011). However, this expansion event likely occurred approximately 250 million years ago (Farris and Schulmeister 2011; Peters et al. 2017) and this large phylogenetic distance, and accompanying ecological and life history differences, makes it difficult to directly test hypothesised links between cognitive ecology and mushroom body expansion through comparative behavioural experiments due to the potential confounding factors resulting from comparing such distantly related taxa.
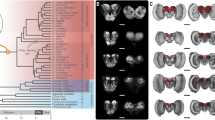
Here, we review the highly diverse neotropical butterfly tribe Heliconiini as a promising case study in neural elaboration and associated behavioural innovations. A new dataset covering 30 species of Heliconius, the most diverse genus of this tribe, and 11 closely related Heliconiini (Fig. 1a) reveals repeated shifts in mushroom body size, resulting in the genus Heliconius having mushroom bodies, 2 to 4 times larger than in other Heliconiini lineages, relative to the rest of the brain (Figure 1b–e; Couto et al. 2023). The mushroom bodies of Heliconius also show evidence of visual specialisation, as a far higher proportion of the calyx receives visual, as opposed to olfactory, innervation than in other Heliconiini (Fig. 1c). Mushroom body expansion in Heliconius is also primarily a function of an increased number of Kenyon cells, the intrinsic neurons of the mushroom body (Fig. 1f; Couto et al. 2023). Indeed, uncorrected for overall brain size, the Heliconiini mushroom bodies vary by over 20-fold in volume, and by at least eightfold in Kenyon cell number (Fig. 1f; Couto et al. 2023). Comparable volumetric expansions are not detected in other neuropils, including the antennal lobes and multiple visual neuropils, suggesting targeted selection on mushroom body function (Couto et al. 2023), although downstream processes remain to be investigated.
Mushroom body expansion and visual specialisation in Heliconius (adapted from Couto et al. 2023). a Dated Heliconiini phylogeny with colours (yellow circles = posterior probability) corresponding to b phylogenetic shifts in the scaling relationship between the volume of the mushroom body and the rest of the central brain. Solid points = species means; faded points = individuals. Estimated ancestral states for each group shown by density maps. c Controlling for the volume of the olfactory region of the mushroom body calyx, Heliconius show an upshift in the volume of the visual calyx relative to other Heliconiini. d, e Comparative mushroom body size in Heliconius hecale and Agraulis vanillae. d 3D-reconstructions of the whole brain with anterior (left) and posterior (right) views showing the mushroom bodies (red) (scale bars = 500 μm). e 3D-reconstructions of the mushroom body, showing the calyx (dark red) and peduncles and lobes (light red) (scale bars = 250 μm). f Total number of Kenyon cells in six species representing major Heliconiini lineages
Unlike Hymenoptera, mushroom body expansion in Heliconius occurred relatively recently (~12–18 Ma) (Fig. 1a) (Kozak et al. 2015; Cicconardi et al. 2023), and the Heliconiini share a generally conserved ecology, including overlapping habitat and host plant preferences, body size and juvenile life history (Young and Montgomery 2020). Heliconiini are also tractable behavioural subjects across a range of assays, including visual, olfactory, and spatial learning tasks (Fig. 2a; Dell’Aglio et al. 2016, 2022; Toure et al. 2020; Moura et al. 2023b; Couto et al. 2023; Young et al. 2023c). Finally, there is a rich history of ecological and evolutionary work in Heliconius in particular (Merrill et al. 2015; Jiggins 2017), providing the basis for creating and testing adaptive hypothesis concerning the benefit of enlarged learning and memory circuits. Together, phylogenetic and ecological proximity, experimental tractability, and behavioural novelty make Heliconius a particularly attractive system for investigating cognitive evolution.
Behavioural ecology in the Heliconiini. a Heliconius hecale feeding on a diamond-shaped feeder during a shape learning assay with a star-shaped feeder in the background. b Heliconius numata feeding on Palicourea elata with pollen clump visible on its proboscis. c Heliconius erato laying on a fresh Passiflora biflora shoot
Traplining butterflies: evolution of a new cognitive ecology
Uniquely amongst butterflies, adult Heliconius actively feed on pollen (Fig. 2b) and exhibit associated derived foraging behaviours (Gilbert 1972; Ehrlich and Gilbert 1973). Heliconius predominantly collect pollen from a limited number of relatively rare, but pollen rich, plants that may flower continuously for up to a year (Gilbert 1972, 1975; Estrada and Jiggins 2002). In exploiting these uncommon, but highly and consistently rewarding resources, Heliconius establish “traplines,” stable foraging routes along which specific plants are repeatedly returned to for up to several months (Ehrlich and Gilbert 1973; Gilbert 1975; Mallet 1986; Finkbeiner 2014). This occurs within an individual home range of between 100 m2 and 1 km2, including a small number of roosting sites which Heliconius return to at night with high fidelity (Jones 1930; Mallet 1986; Murawski and Gilbert 1986). Evidence for the traplining abilities of Heliconius predominantly comes from mark-release-recapture studies of wild Heliconius that show individuals repeatedly returning to the same pollen sources over successive days, with a high degree of temporal consistency (Ehrlich and Gilbert 1973; Gilbert 1975; Mallet 1986; Finkbeiner 2014). Traplining routes are highly individualistic and differ between butterflies sharing the same roosting site, further suggesting that memory is guiding their movements, rather than individuals simply responding to environmental stimuli (Mallet 1986). A recent study demonstrated that wild Heliconius translocated hundreds of metres will quickly orientate towards, and return to, their site of origin after release, demonstrating a high degree of active site fidelity, again emphasising individual agency in shaping home ranges (Moura et al. 2021).
Combined, these behaviours suggest Heliconius possess an enhanced capacity for allocentric spatial learning similar to bumblebees and orchid bees (Janzen 1971; Heinrich 1979; Lihoreau et al. 2013). Such foraging behaviours are unknown in Lepidoptera, with the exception of unsubstantiated reports of putative traplining in wild Jalmenus evagoras and Speyeria cybele (Pierce and Elgar 1985; Horn 2021). The cognitive demands of traplining were noted soon after the description of Heliconius foraging behaviour, and are hypothesised to have played a major role in driving mushroom body expansion in Heliconius (Sivinski 1989; Gilbert 1991; Montgomery et al. 2016). In other insects, ablation experiments implicate the mushroom bodies in visual navigation in ants (Kamhi et al. 2020; Buehlmann et al. 2020) and a cockroach (Mizunami et al. 1998), giving credence to this hypothesis (Webb and Wystrach 2016).
Regrettably, there is little data on the foraging behaviours of other Heliconiini genera, or the extent to which they establish home ranges. Territorial behaviour, however, has been observed in Eueides tales and Eueides aliphera (Benson et al. 1989), and the Heliconiini Agraulis vanillae are reported to establish temporary home ranges (Mallet et al. 1987). Both empirical data and modelling indicate that maintaining a home range requires a degree of investment in spatial memory (Spencer 2012; Fagan et al. 2013; Heathcote et al. 2023). A stable home range is likely a pre-requisite for the emergence of trapline foraging, given memories of resource distribution would lose their value if individuals do not regularly forage in that location. Interestingly, Eueides, the sister genus to Heliconius, along with the Heliconiini Dryadula phaetusa (for which there is very little movement data), exhibit mushroom bodies of a size intermediate between Heliconius and other Heliconiini (Fig. 1b; Couto et al. 2023). One possible evolutionary scenario, therefore, is that an emergence of increased territorial behaviour and mushroom body size along the Heliconius + Eueides stem provided a foundation for the later, dramatic mushroom body expansion and traplining in Heliconius, with similar shifts occurring in Dryadula. However, this hypothesis cannot be formally tested without further comparative studies on the movement ecology of wild Heliconiini.
evertheless, alternative hypotheses concerning the adaptive value of mushroom body expansion in Heliconius have little support. For example, enlarged mushroom bodies have speculatively been linked to host plant use (de Castro et al. 2017). In common with other Heliconiini, Heliconius lay eggs exclusively on Passiflora plants (Fig. 2c) and the identification of these host plants appears to rely, in part, on the recognition and learning of the leaf shapes to identify suitable hosts (Dell’Aglio et al. 2016). Passiflora in turn have diversified their leave shape, potentially due to selection to escape Heliconiini search images (de Castro et al. 2017). Larger mushroom bodies could conceivably support a greater array of search images and shape-learning ability, allowing species to identify a greater range of Passiflora host plants. However, neither comparative data on host plant use (number of species or the morphological diversity of species used) nor behavioural data on shape learning ability support this hypothesis (Couto et al. 2023; Young et al. 2023c). Similarly, links to social ecology and information transfer have little support (Moura et al. 2023a; Couto et al. 2023). As such, the innovation of pollen feeding and trapline foraging remains the most plausible driver of mushroom body expansion in Heliconius.
Information use by foraging Heliconius
Despite sustained interest in the unique dietary adaptations of Heliconius, the mechanisms by which Heliconius navigate remain poorly described, and only recently have assumptions about their spatial learning abilities been directly tested. In an experimental setting, Heliconius can learn the location of food rewards across a range of spatial scales, including a T-maze approximately 60 m wide, with some evidence of improved performance at larger scales (Moura et al. 2023b). In addition, Heliconius reared in a 4-m × 4-m maze can locate food in that environment more quickly than individuals reared in a matched, but undivided cage, suggesting they improve in the ability to navigate the maze through experience (Hebberecht et al. 2023). These results support previous field studies in demonstrating that Heliconius can learn spatial information, and constitute the first experimental evidence of spatial learning in Lepidoptera. However, experimentally testing for interspecific variation in spatial learning ability at the large scales over which Heliconius naturally forage (hundreds of metres) presents several logistical challenges, particularly regarding suitable testing arenas and individual tracking. To date, these practical constrains have also limited our understanding of the sensory cues used to guide foraging behaviour.
Although chemoreception is important at close proximities (Papaj 1986; Honda et al. 1998; Andersson and Dobson 2003a, b), visual cues are known to be important in Heliconius and other Lepidoptera in both hostplant and floral inspection (Swihart and Swihart 1970; Rausher 1978; Blackiston et al. 2011; Cepero et al. 2015; Dell’Aglio et al. 2016). Visual cues are also thought to be the predominant sensory modality used in instigating attraction behaviours from larger distances (Jiggins et al. 2001; Jiggins 2008; Merrill et al. 2011), with Heliconius able to orientate towards habitat patches at distances up to 60 m, suggesting this marks the upper limit of their visual range (Cardoso et al., in review). Trapline foraging in Heliconius is therefore similarly hypothesised to depend on learned visual cues, such as the visual scene provided by the forest canopy and structure (Gilbert 1975). In support of this, an early study in Heliconius charithonia reported that when branches that were used as a nocturnal roost were moved by a few meters during the day, individual butterflies returned to the original roost site rather than seeking out the displaced branches, suggesting they are not guided to roosts by scent marking (Jones 1930). A predominant role for visual cues is consistent with the visual specialisation of the mushroom body calyx in Heliconius (Couto et al. 2023), and the absence of any variation in antennal lobe morphology which would suggest limited reliance on detection of long distance odours (Montgomery et al. 2016; Couto et al. 2023). Theoretical modelling of mushroom body circuitry also suggests that increased numbers of Kenyon cells, as seen in Heliconius, can lead to an enhanced storage capacity for visual patterns (Ardin et al. 2016), such as scene memories, providing a substrate for visual navigation.
The use of visual cues in traplining Hymenoptera, including bumblebees (Heinrich 1979) and honeybees (Buatois and Lihoreau 2016), is also well established. Hymenoptera that display allocentric foraging are capable of using both individual landmarks and panoramic views when learning or recalling foraging routes (Cartwright and Collett 1983; Collett et al. 2002; De Ibarra et al. 2009; Mertes et al. 2014; Boeddeker et al. 2015). Whether similar cues are used by Heliconius is only beginning to be explored experimentally. However, a recent study exploring the use of landmark cues in spatial learning in Heliconius erato suggests that although the butterflies are able to learn the location of a rewarded feeder in conjunction with a fixed, adjacent landmark, when this landmark is moved, the trained spatial preference remains fixed, suggesting local landmark cues are not essential for spatial learning (Moura 2022). In support of this inference, butterflies were unable to learn an association between a food reward and an adjacent landmark when both were moved in parallel between trials (Moura 2022). In contrast, when the panoramic views seen through the transparent cage walls were blocked, spatial learning was diminished (but not prevented entirety), suggesting the use of broader visual scenes (Moura 2022). This suggests that Heliconius may be predominantly relying on scene memories for navigation, similar to natural navigational processes in ants and bees (Graham and Cheng 2009; Wystrach et al. 2011). The incomplete abolition of spatial learning when background scene views are blocked, however, also suggests other processes may be important, such as a solar or geomagnetic cues.
Comparative cognition in the Heliconiini: ecologically relevant shifts in learning and memory
Brain tissue is energetically expensive (Niven and Laughlin 2008), and neural elaboration of the kind detected in Heliconius can be expected to underpin adaptive, beneficial shifts in cognition. However, adaptations in neural systems must support ecologically-relevant behaviours (Logan et al. 2018; Poirier et al. 2020). Given the broad ecological similarities between Heliconius and other Heliconiini, with overlapping habitats and use of Passiflora hostplants, their behavioural motivation and performance in many behavioural tasks can be expected to be similar. Indeed, several studies have failed to show cognitive enhancements in Heliconius over other Heliconiini. For example, both Heliconius hecale and Dryas iulia can learn to use time-cues to modulate food-colour associations in an experimental setting, with Dryas iulia actually showing greater time-dependent shifts (Toure et al. 2020). This suggests that time learning, which is important for the temporal faithfulness of Heliconius trap lines (Gilbert 1975), predates the emergence of traplining and does not functionally rely on expanded mushroom bodies, most likely as it is also beneficial for nectar feeding (Barp et al. 2011). Similarly, experiments involving three Heliconius and three outgroup Heliconiini, showed that all species were equally able to reverse a learned colour-food association after 4 days of training (Young et al. 2023b). Although reversal learning is often used as a proxy for behavioural or cognitive flexibility (Izquierdo et al. 2017), this lack of difference may reflect the broad importance of reversal learning for animals experiencing changing environments. Finally, as noted above, shape learning experiments across six Heliconiini species did not show an overall difference in performance between Heliconius and non-Heliconius species, despite some interspecific variation, suggesting host-plant leaf shape recognition and learning was not a major driver of mushroom body expansion in the tribe (Young et al. 2023c).
Heliconius have, however, shown improved performance in two visual cognitive tasks potentially relevant to navigation during trapline foraging: visual non-elemental learning and long-term memory (Couto et al. 2023; Young et al. 2023a). Broadly, spatial learning has been considered a form of non-elemental learning as it involves the integration of several different, potentially multimodal, cues (Sutherland and Rudy 1989; Buatois and Gerlai 2020). For example, in the whip spider Phrynus marginemaculatus, which also possess greatly expanded mushroom bodies, shelters can be located through the configural learning of combined tactile and olfactory cues (Flanigan et al. 2021). Traplining in Heliconius may therefore reflect, in part, an ability to learn complex cues composed of multiple elements. Consistent with this premise, Heliconius erato outperform Dryas iulia in visual, non-elemental learning tasks, showing greater accuracy in a positive patterning assay (A−, B−, AB+), and successfully solve a biconditional discrimination problem (AB+, CD+, A−, BD−), while Dryas iulia do not (Couto et al. 2023). Within insects, visual non-elemental learning has only previously been shown in bumblebees (Zhou et al. 2020) and honeybees (Schubert et al. 2002), in which the mushroom bodies are essential for non-elemental olfactory learning tasks, but not necessarily simple elemental learning (Devaud et al. 2015). The convergent abilities of Heliconius and bees to solve these more complex tasks may therefore represent a response to similar selective pressures relating to central place foraging and, at least indirectly, to mushroom body expansion.
While individual pollen sources exhibit day-to-day variability, they are a generally reliable resource over the long-term and Heliconius traplines can therefore remain constant for several months (Mallet 1986). Efficient traplining for pollen, therefore, appears to rely on the formation of stable, long-term visual memories. The value of long-term memory may also be increased in Heliconius which live much longer than related genera, as a result of pollen feeding providing an adult source of amino acids (Dunlap-Pianka et al. 1977). A study of three Heliconius and three outgroup Heliconiini species shows Heliconius indeed consistently have more stable long-term memories of visual cues (Young et al. 2023a). Heliconius melpomene retained learnt food-colour associations after 13 days without reinforcement, to our knowledge the longest memory recall demonstrated in any insect. In contrast, by 8 days, all three non-pollen feeding outgroups show random foraging preferences. Mushroom bodies are known to be central to long-term olfactory memories in insects (Pascual and Préat 2001; Hourcade et al. 2010b) and this result is suggestive of a similarly important role in visual long-term memory for Heliconius.
The emerging picture from these behavioural experiments is that distinct cognitive shifts are evident in Heliconius, relative to other Heliconiini, but these are limited to specific abilities which are putatively necessary for traplining (Table 1). These behavioural changes are likely shared across Heliconius species, and coincide with the most substantial episode of mushroom body expansion in the Heliconiini, and an increased proportion of calyx innervated by visual projection neurons. This is consistent with the apparent cognitive demands of traplining being a major selective pressure shaping brain evolution in the Heliconiini. Collectively, these results also provide a reminder of the importance of designing ecologically informed tests when conducting comparative cognitive experiments, and the risk of using any single task as a measure of cognitive ability (Logan et al. 2018). Notably, however, the experimental tractability of Heliconius has already provided the first experimental evidence of the ability to learn spatial, visual non-elemental, and time cues in any Lepidoptera.
Evolutionary shifts in mushroom body plasticity
The cognitive shifts observed in Heliconius are associated not only with volumetric expansion of the mushroom but also involve changes in the environmental sensitivity of mushroom body maturation. Heliconius mushroom bodies are indeed highly plastic and show considerable post-eclosion growth, nearly doubling in size after the adults eclose (Montgomery et al. 2016; Young et al. 2023a; Alcalde et al. 2023), similar to the extent of plasticity observed in Hymenoptera (Durst et al. 1994; Molina and O’Donnell 2008; Li et al. 2017; Kraft et al. 2019). Post-eclosion expansion of the mushroom bodies in Heliconius appears sensitive to environmental experience, being significantly larger in wild-caught butterflies than captive-reared individuals (Montgomery et al. 2016), and in butterflies reared in larger cages compared to those in smaller cages which limit the expression of natural flight behaviours (Hebberecht et al. 2023). The Heliconiini Agraulis vanillae also shows evidence of post-eclosion growth of the mushroom bodies (Kroutov et al. 2002), but it appears less extensive than in Heliconius. This post-eclosion growth seems to be a product of synaptic pruning and likely dendritic branching, as adult neurogenesis appears to occur at extremely low levels, if at all, in the Heliconiini mushroom bodies (Alcalde et al. 2023), another trait shared with honeybees (Fahrbach et al. 1995). This contrasts with some regions of the vertebrate brain, where adult neurogenesis in the hippocampus appears integral to certain learning processes (Augusto-Oliveira et al. 2019), and some groups of insects (Cayre et al. 2007; Trebels et al. 2020) where adult neurogenesis can have an important role in supporting long term memory (Kulkarni et al. 2023).
The mushroom body calyx is comprised synaptic complexes called microglomeruli which are formed by a projection neuron bouton synapsing with dendritic spines from multiple Kenyon cells. Interestingly, the number of synaptic boutons (as defined by anti-SYNORF staining; Fahrbach and Van Nest 2016) per Kenyon cell appears to be lower in the Heliconius mushroom body calyx than in Dryas (Young et al. 2023a). This potentially suggests Heliconius mushroom body circuits may receive sparser innervation from projection neurons, although data on the number of projection neurons is currently lacking. The interspecific difference in synaptic bouton density is further exacerbated by synaptic pruning during maturation, which is significantly more pronounced in Heliconius erato than Dryas iulia, and likely occurs in the absence of changes in projection or Kenyon cell number (Alcalde et al. 2023). Hence, learning and memory in Heliconius seem predominantly a function of the re-organisation, or differential pruning and maintenance of synaptic boutons. This is consistent with findings in honeybees (Groh et al. 2012) and desert ants where age and visual experience are associated with synaptic pruning in the mushroom body calyx (Stieb et al. 2010, 2012). The apparent increased sparsity of connectivity in mushroom body circuits in Heliconius would likely have functional ramifications (Olshausen and Field 2004).
Further support for synaptic re-organisation in the calyx being central to visual learning in Heliconius comes from experiments linking synaptic density to learning performance. Heliconius erato exposed to associative food-colour training show a greater number of calyx synapses than individuals raised in a “non-learning” environment where colour cues are equally rewarded and punished (Young et al. 2023a). A higher number of mushroom body synapses has similarly been linked to visual and olfactory learning and memory in Hymenoptera (Hourcade et al. 2010a; Falibene et al. 2015; Li et al. 2017). However, no such effect is detected in the Heliconiini Dryas iulia, despite also learning the colour-food associations (Young et al. 2023a). In addition, recall accuracy of learned food-colour associations is positively correlated with calyx synapse number in Heliconius erato, but not Dryas iulia (Young et al. 2023a). These results suggest a difference between these species in how visual associative memories are consolidated in the mushroom body, with Heliconius erato exhibiting a greater plastic response. Such findings illustrate the importance of investigating not only volumetric changes in the brain, but also its connectivity on a cellular level, and how detailed investigations of select species can inform broader comparative studies.
Towards an integrated cognitive ecology of Heliconius butterflies
Current work on Heliconiini butterflies has synthesised phylogenetic data, large- and fine-scale neuroanatomy and behavioural experiments to provide clear evidence of rapid mushroom body expansion in the Heliconius genus coinciding with distinct cognitive shifts and a novel foraging behaviour. This has established Heliconius as an important case study in cognitive evolution and opens several clear paths for investigating key questions in cognitive ecology and evolution.
Do shared ecological pressures lead to cognitive convergences?
Heliconius and traplining Hymenoptera both need to remember resource locations over large spatial scales, but to what extent have they evolved a common navigational toolset? Although both field observations and experiments shows that Heliconius are capable of learning and using spatial information (Ehrlich and Gilbert 1973; Mallet 1986; Moura et al. 2021; Moura et al. 2023b), the mechanisms supporting this ability are unknown. Insects variously utilise a range of visual information for navigation including panoramic views, individual landmarks, and celestial cues in addition to non-visual methods such as the use of olfactory cues, path integration, and magnetic compasses (Webb and Wystrach 2016; Freas and Spetch 2023). Given the visual specialisation of the Heliconius mushroom body, visual information is expected to be important, similar to Hymenoptera (Freas and Spetch 2023). Current evidence favours the use of panoramic views over individual landmark cues, supplemented by other, as yet unidentified, processes (Moura 2022). However, there is a need to test these mechanisms over larger, more naturalistic scales, and study how Heliconius may integrate visual information with other navigational tools.
How does ecology lead to selection for specific, adaptive behaviours?
Behavioural experiments show that mushroom body expansion in Heliconius coincides with enhanced ability for select cognitive tasks (Table 1), but further assays will better clarify the nature of this shift. Current evidence points to the demands of traplining as a key selective pressure, and experiments should be expanded to include outgroup Heliconiini and test spatial learning at larger scales and in more complex environments. The increased visual specialisation of the Heliconius mushroom body invites speculation that the enhancements in visual long-term memory and non-elemental learning may not be replicated in the olfactory modality, but this is yet to be tested. There is also a dearth of behavioural and field data for Eueides, the sister genus of Heliconius, and Dryadula, which both exhibit mushroom bodies intermediate in size between Heliconius and other Heliconiini (Couto et al. 2023). Investigating the ecological correlates of these independent, but more modest periods of mushroom body expansion could further illuminate the evolutionary transition along the Heliconius stem. Interestingly, the four Heliconius species from the “Neruda” clade do not feed on pollen, but nevertheless exhibit mushroom bodies within the size range of other Heliconius. Little is known about Neruda foraging ecology, and their phylogenetic position remains subject to debate, but such data could provide an example of how neural and behavioural adaptations can be co-opted for new purposes.
How are behavioural adaptations supported by shifts in both brain composition and connectivity?
Data from the Heliconiini show that comparative cognition benefits from consideration of both the overall composition of the brain and its connectivity at a cellular level. Although no other neuropil in the Heliconius brain exhibits expansion on the scale of the mushroom bodies (Couto et al. 2023), investigating the size, structure, and function of the central complex, a brain region also implicated in insect navigation (Pfeiffer and Homberg 2014; Varga et al. 2017; Le Moël et al. 2019), is of clear interest, given the possibility that the mushroom body and central complex have co-evolved across the tribe. Likewise, the structure of the mushroom body lobes and extent and direction of output neurons remains unknown in the Heliconiini. At the same time, the role of the environment in shaping these circuits will also be an important layer of information to combine with anatomical descriptions. The mushroom bodies of Heliconius erato and Dryas iulia also vary in their sensitivity to visual experience (Young et al. 2023a), but the nature of this difference is only beginning to be unravelled. Future studies are necessary to determine whether this reflects a broad shift between Heliconius and other Heliconiini and to more precisely characterise these changes in how information is processed and stored at a cellular level.
How do molecular mechanisms constrain and facilitate cognitive evolution?
Recently sequenced genomes covering all the major Heliconiini lineages offer the possibility of uncovering the genetic underpinnings of mushroom body elaboration, and associated derived behaviours, in the tribe (Edelman et al. 2019; Cicconardi et al. 2023). Combined with work identifying the key stages of mushroom body development and associated changes in gene expression, this genomic toolkit could be used for manipulative developmental experiments (Livraghi et al. 2018). Investigating differential gene expression specifically associated with learning and memory formation (Wang et al. 2013; Li et al. 2018) could further reveal the genetic bases of the cognitive shifts in Heliconius. A complimentary approach would be to assess the role of DNA methylation in learning and memory across the Heliconiini, given its importance for honeybees (Lockett et al. 2010; Biergans et al. 2012). These investigations will help to uncover the molecular processes controlling brain development and neural plasticity, and how this shapes the evolution of brains and behaviour.
In summary, although in its infancy as a neuro-cognitive study system, the wealth of ecological and evolutionary data, combined with close phylogenetic relatedness and extreme neuro-phenotypic variation, means the Heliconiini tribe is well-positioned as an important model for investigating cognitive evolution. The foundations are currently being laid for future studies synthesising genomic, neuroanatomical, and behavioural data in a highly tractable system. Further characterising the links in Heliconius between neural adaptations and cognitive shifts will help to unravel how the structure and function of the brain is shaped by ecological pressures and supports novel behaviours.
References
Alcalde AA, Young FJ, Melo-Flórez L, Couto A, Cross S, McMillan WO, Montgomery SH (2023) Adult neurogenesis does not explain the extensive post-eclosion growth ofHeliconiusmushroom bodiesRoyal Society Open. Science 10(10). https://doi.org/10.1098/rsos.230755
Andersson S, Dobson HEM (2003a) Antennal responses to floral scents in the butterfly Heliconius melpomene. J Chem Ecol 29:2319–2330. https://doi.org/10.1023/A:1026278531806
Andersson S, Dobson HEM (2003b) Behavioral foraging responses by the butterfly Heliconius melpomene to Lantana camara floral scent. J Chem Ecol 29:2303–2318. https://doi.org/10.1023/A:1026226514968
Ardin P, Peng F, Mangan M et al (2016) Using an insect mushroom body circuit to encode route memory in complex natural environments. PLoS Comput Biol 12:1–22. https://doi.org/10.1371/journal.pcbi.1004683
Augusto-Oliveira M, Arrifano G, Malva J, Crespo-Lopez M (2019) Adult hippocampal neurogenesis in different taxonomic groups: possible functional similarities and striking controversies. Cells 8:125. https://doi.org/10.3390/cells8020125
Barp EA, Soares GLG, Giani EJM et al (2011) Variation in nectar and pollen availability, sucrose preference, and daily response in the use of flowers by Heliconius erato phyllis. J Insect Behavior 24:200–219. https://doi.org/10.1007/s10905-010-9248-2
Barton RA, Harvey PH (2000) Mosaic evolution of brain structure in mammals. Nature 405:4. https://doi.org/10.1038/35016580
Barton RA, Venditti C (2014) Rapid evolution of the cerebellum in humans and other great apes. Curr Biol 24:2440–2444. https://doi.org/10.1016/j.cub.2014.08.056
Benson WW, Haddad CFB, Zikan M (1989) Territorial behavior and dominance in some Heliconiine butterflies (Nymphalidae). J Lepidopterists’ Soc 43:33–49
Benson-Amram S, Dantzer B, Stricker G et al (2016) Brain size predicts problem-solving ability in mammalian carnivores. Proc Natl Acad Sci USA 113:2532–2537. https://doi.org/10.1073/pnas.1505913113
Biergans SD, Jones JC, Treiber N et al (2012) DNA methylation mediates the discriminatory power of associative long-term memory in honeybees. PLoS ONE 7:39349. https://doi.org/10.1371/journal.pone.0039349
Blackiston D, Briscoe AD, Weiss MR (2011) Color vision and learning in the monarch butterfly, Danaus plexippus (Nymphalidae). J Exp Biol 214:509–520. https://doi.org/10.1242/jeb.048728
Boeddeker N, Mertes M, Dittmar L, Egelhaaf M (2015) Bumblebee homing: the fine structure of head turning movements. PLoS ONE 10:e0135020. https://doi.org/10.1371/journal.pone.0135020
Buatois A, Gerlai R (2020) Elemental and configural associative learning in spatial tasks: could zebrafish be used to advance our knowledge? Front Behav Neurosci 14:570704. https://doi.org/10.3389/fnbeh.2020.570704
Buatois A, Lihoreau M (2016) Evidence of trapline foraging in honeybees. J Exp Biol 219:2426–2429. https://doi.org/10.1242/jeb.143214
Buehlmann C, Wozniak B, Goulard R et al (2020) Mushroom bodies are required for learned visual navigation, but not for innate visual behavior, in ants. Curr Bio 30:3438–3443.e2. https://doi.org/10.1016/j.cub.2020.07.013
Cartwright BA, Collett TS (1983) Landmark learning in bees: experiments and models. J Comp Physiol 151:521–543. https://doi.org/10.1007/BF00605469
Cayre M, Scotto-lomassese S, Malaterre J, Strambi C (2007) Understanding the regulation and function of adult neurogenesis : contribution from an insect model, the House Cricket. 385–395. https://doi.org/10.1093/chemse/bjm010
Cepero LC, Rosenwald LC, Weiss MR (2015) The relative importance of flower color and shape for the foraging Monarch butterfly (Lepidoptera: Nymphalidae). J Insect Behav 28:499–511. https://doi.org/10.1007/s10905-015-9519-z
Chittka L, Niven J (2009) Are bigger brains better? Curr Biol 19:R995–R1008. https://doi.org/10.1016/j.cub.2009.08.023
Cicconardi F, Milanetti E, Pinheiro De Castro EC et al (2023) Evolutionary dynamics of genome size and content during the adaptive radiation of Heliconiini butterflies. Nat Commun 14:5620. https://doi.org/10.1038/s41467-023-41412-5
Clutton-Brock TH, Harvey PH (1980) Primates, brains and ecology. J Zool 190:309–323
Collett M, Harland D, Collett TS (2002) The use of landmarks and panoramic context in the performance of local vectors by navigating honeybees. J Exp Biol 205:807–814. https://doi.org/10.1242/jeb.205.6.807
Couto A, Wainwright JB, Morris BJ, Montgomery SH (2020) Linking ecological specialisation to adaptations in butterfly brains and sensory systems. Curr Opin Insect Sci 42:55–60. https://doi.org/10.1016/j.cois.2020.09.002
Couto A, Young FJ, Atzeni D et al (2023) Rapid expansion and visual specialisation of learning and memory centres in the brains of Heliconiini butterflies. Nat Commun 14:4024. https://doi.org/10.1038/s41467-023-39618-8
de Belle JS, Heisenberg M (1994) Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science 263:692–695. https://doi.org/10.1126/science.830328
de Castro ÉCP, Zagrobelny M, Cardoso MZ, Bak S (2017) The arms race between heliconiine butterflies and Passiflora plants - new insights on an ancient subject. Biol Rev 555–573. https://doi.org/10.1111/brv.12357
De Ibarra NH, Philippides A, Riabinina O, Collett TS (2009) Preferred viewing directions of bumblebees (Bombus terrestris L.) when learning and approaching their nest site. J Exp Biol 212:3193–3204. https://doi.org/10.1242/jeb.029751
DeCasien AR, Williams SA, Higham JP (2017) Primate brain size is predicted by diet but not sociality. Nat Ecol Evol 1:1–7. https://doi.org/10.1038/s41559-017-0112
Dell’Aglio DD, Losada ME, Jiggins CD (2016) Butterfly learning and the diversification of plant leaf shape. Front Ecol Evol 4:81. https://doi.org/10.3389/FEVO.2016.00081
Dell’Aglio D, McMillan W, Montgomery S (2022) Shifting balances in the weighting of sensory modalities are predicted by divergence in brain morphology in incipient species of Heliconius butterflies. Animal Behaviour 185:83–90. https://doi.org/10.1016/j.anbehav.2022.01.003
Devaud JM, Blunk A, Podufall J et al (2007) Using local anaesthetics to block neuronal activity and map specific learning tasks to the mushroom bodies of an insect brain. Eur J Neurosci 26:3193–3206. https://doi.org/10.1111/j.1460-9568.2007.05904.x
Devaud J-M, Papouin T, Carcaud J et al (2015) Neural substrate for higher-order learning in an insect: mushroom bodies are necessary for configural discriminations. Proc Natl Acad Sci USA 112:E5854–E5862. https://doi.org/10.1073/pnas.1508422112
Dunbar RIM (1992) Neocortex size as a constraint on group size in primates. J Human Evol 22:469–493. https://doi.org/10.1016/0047-2484(92)90081-J
Dunbar RIM, Shultz S (2007) Evolution in the social brain. Sci (New York, NY) 317:1344–1347. https://doi.org/10.1126/science.1145463
Dunbar RIM, Shultz S (2017) Why are there so many explanations for primate brain evolution? Phil Trans R Soc B 372:20160244. https://doi.org/10.1098/rstb.2016.0244
Dunlap-Pianka H, Boggs CL, Gilbert LE (1977) Ovarian dynamics in Heliconiine butterflies: programmed senescence versus eternal youth. Science 197:487–490. https://doi.org/10.1126/science.197.4302.487
Durst C, Eichmüller S, Menzel R (1994) Development and experience lead to increased volume of subcompartments of the honeybee mushroom body. Behavior Neural Biol 62:259–263. https://doi.org/10.1016/S0163-1047(05)80025-1
Edelman NB, Frandsen PB, Miyagi M et al (2019) Genomic architecture and introgression shape a butterfly radiation. Science 366:594–599. https://doi.org/10.1126/science.aaw2090
Ehrlich PR, Gilbert LE (1973) Population structure and dynamics of the tropical butterfly, Heliconius ethilla. Biotropica 5:69–83. https://doi.org/10.2307/2989656
Estrada C, Jiggins CD (2002) Patterns of pollen feeding and habitat preference among Heliconius species. Ecol Entomol 27:448–456. https://doi.org/10.1046/j.1365-2311.2002.00434.x
Fagan WF, Lewis MA, Auger-Méthé M et al (2013) Spatial memory and animal movement. Ecol Lett 16:1316–1329. https://doi.org/10.1111/ele.12165
Fahrbach SE, Van Nest BN (2016) Synapsin-based approaches to brain plasticity in adult social insects. Curr Opin Insect Sci 18:27–34. https://doi.org/10.1016/j.cois.2016.08.009
Fahrbach SE, Strande JL, Robinson GE (1995) Neurogenesis is absent in the brains of adult honey bees and does not explain behavioral neuroplasticity. Neurosci Lett 197:145–148. https://doi.org/10.1016/0304-3940(95)11913-H
Falibene A, Roces F, Rössler W (2015) Long-term avoidance memory formation is associated with a transient increase in mushroom body synaptic complexes in leaf-cutting ants. Front Behavior Neurosci 9:211–212. https://doi.org/10.3389/fnbeh.2015.00084
Farris SM (2013) Evolution of complex higher brain centers and behaviors: behavioral correlates of mushroom body elaboration in insects. Brain Behav Evol 82:9–18. https://doi.org/10.1159/000352057
Farris SM (2015) Evolution of brain elaboration. Phil Trans R Soc B 370:20150054. https://doi.org/10.1098/rstb.2015.0054
Farris SM, Roberts NS (2005) Coevolution of generalist feeding ecologies and gyrencephalic mushroom bodies in insects. Proc Natl Acad Sci 102:17394–17399. https://doi.org/10.1073/pnas.0508430102
Farris SM, Schulmeister S (2011) Parasitoidism, not sociality, is associated with the evolution of elaborate mushroom bodies in the brains of hymenopteran insects. Proc R Soc B 278:940–951. https://doi.org/10.1098/rspb.2010.2161
Farris SM, Van Dyke JW (2015) Evolution and function of the insect mushroom bodies: contributions from comparative and model systems studies. Curr Opin Insect Sci 12:19–25. https://doi.org/10.1016/j.cois.2015.08.006
Finkbeiner SD (2014) Communal roosting in Heliconius butterflies (Nymphalidae): roost recruitment, establishment, fidelity, and resource use trends based on age and sex. J Lepidopterists’ Soc 68:10–16. https://doi.org/10.18473/lepi.v68i1.a2
Flanigan KAS, Wiegmann DD, Hebets EA, Bingman VP (2021) Multisensory integration supports configural learning of a home refuge in the whip spider Phrynus marginemaculatus. J Exp Biol 224:jeb238444. https://doi.org/10.1242/jeb.238444
Freas CA, Spetch ML (2023) Varieties of visual navigation in insects. Anim Cogn 26:319–342. https://doi.org/10.1007/s10071-022-01720-7
Gilbert LE (1972) Pollen feeding and reproductive biology of Heliconius butterflies. Proc Natl Acad Sci USA 69:1403–1407. https://doi.org/10.1073/pnas.69.6.1403
Gilbert LE (1975) Ecological consequences of a coevolved mutualism between butterflies and plants. In: Coevolution of animals and plants. University of Texas Press, Austin, pp 210–240
Gilbert LE (1991) Biodiversity of a Central American Heliconius community: pattern, process, and problems. In: Plant–animal interactions: evolutionary ecology in tropical and temperate regions. Wiley, New York, pp 403–427
Gordon DG, Zelaya A, Ronk K, Traniello JFA (2018) Interspecific comparison of mushroom body synaptic complexes of dimorphic workers in the ant genus Pheidole. Neurosci Lett 662:110–114. https://doi.org/10.1016/j.neulet.2017.10.009
Grabowski M, Kopperud BT, Tsuboi M, Hansen TF (2023) Both diet and sociality affect primate brain-size evolution. System Biol 72:404–418. https://doi.org/10.1093/sysbio/syac075
Graham P, Cheng K (2009) Ants use the panoramic skyline as a visual cue during navigation. Curr Biol 19:R935–R937. https://doi.org/10.1016/j.cub.2009.08.015
Groh C, Lu Z, Meinertzhagen IA, R??ssler W (2012) Age-related plasticity in the synaptic ultrastructure of neurons in the mushroom body calyx of the adult honeybee Apis mellifera. J Comp Neurol 520:3509–3527. https://doi.org/10.1002/cne.23102
Hampton RR, Shettleworth SJ (1996) Hippocampal lesions impair memory for location but not color in passerine birds. Behavior Neurosci 110:831–835. https://doi.org/10.1037/0735-7044.110.4.831
Hawkins J, Ahmad S (2016) Why neurons have thousands of synapses, a theory of sequence memory in neocortex. Front Neural Circuits 10. https://doi.org/10.3389/fncir.2016.00023
Healy SD, Krebs JR (1992) Food storing and the hippocampus in corvids: amount and volume are correlated. Proc R Soc Lond B 248:241–245. https://doi.org/10.1098/rspb.1992.0068
Healy SD, Rowe C (2007) A critique of comparative studies of brain size. Proc: Biol Sci 274:453–464
Heathcote RJP, Whiteside MA, Beardsworth CE et al (2023) Spatial memory predicts home range size and predation risk in pheasants. Nat Ecol Evol 7:461–471. https://doi.org/10.1038/s41559-022-01950-5
Hebberecht L, Wainwright JB, Thompson C et al (2023) Plasticity and genetic effects contribute to different axes of neural divergence in a community of mimetic Heliconius butterflies. J Evol Biol jeb.14188. https://doi.org/10.1111/jeb.14188
Heinrich B (1979) Resource heterogeneity and patterns of movement in foraging bumblebees. Oecologia 40:235–245. https://doi.org/10.1007/BF00345321
Heisenberg M, Borst A, Wagner S, Byers D (1985) Drosophila mushroom body mutants are deficient in olfactory learning. J Neurogen 2:1–30. https://doi.org/10.3109/01677068509100140
Herculano-Houzel S, Catania K, Manger PR, Kaas JH (2015) Mammalian brains are made of these: a dataset of the numbers and densities of neuronal and nonneuronal cells in the brain of Glires, Primates, Scandentia, Eulipotyphlans, Afrotherians and Artiodactyls, and their relationship with body mass. Brain Behav Evol 86:145–163. https://doi.org/10.1159/000437413
Honda K, Ômura H, Hayashi N (1998) Identification of floral volatiles from Ligustrum japonicum that stimulate flower-visiting by cabbage butterfly, Pieris rapae. J Chem Ecol 24:2167–2180. https://doi.org/10.1023/A:1020750029362
Horn HS (2021) Setting and running a trapline: the great spangled fritillary Speyeria cybele. In: Social butterflies. Princeton University Press
Hourcade B, Muenz TS, Sandoz JC et al (2010a) Long-term memory leads to synaptic reorganization in the mushroom bodies: a memory trace in the insect brain? J Neurosci 30:6461–6465. https://doi.org/10.1523/JNEUROSCI.0841-10.2010
Hourcade B, Muenz TS, Sandoz J-CC et al (2010b) Long-term memory leads to synaptic reorganization in the mushroom bodies: a memory trace in the insect brain? J Neurosci 30:6461–6465. https://doi.org/10.1523/JNEUROSCI.0841-10.2010
Izquierdo A, Brigman JL, Radke AK et al (2017) The neural basis of reversal learning: an updated perspective. Neuroscience 345:12–26. https://doi.org/10.1016/j.neuroscience.2016.03.021
Janzen DH (1971) Euglossine bees as long-distance pollinators of tropical plants. Science 171:203–205. https://doi.org/10.1126/science.171.3967.203
Jerison HJ (1973) Evolution of the brain and intelligence. Academic Press, New York
Jerison HJ (1985) Animal intelligence as encephalization. Phil Trans R Soc Lond B 308:21–35. https://doi.org/10.1098/rstb.1985.0007
Jiggins CD (2008) Ecological speciation in mimetic butterflies. BioScience 58:541–548. https://doi.org/10.1641/B580610
Jiggins CD (2017) The ecology and evolution of Heliconius butterflies, 1st edn. Oxford University Press, Oxford
Jiggins CD, Naisbit RE, Coe RL, Mallet J (2001) Reproductive isolation caused by colour pattern mimicry. Nature 411:302–305. https://doi.org/10.1038/35077075
Jones FM (1930) The sleeping Heliconias of Florida: a biological mystery in a fascinating setting. Am Museum Nat History 30:635–644
Kamhi JF, Barron AB, Narendra A (2020) Vertical lobes of the mushroom bodies are essential for view-based navigation in Australian Myrmecia Ants. Curr Biol 30:1–6. https://doi.org/10.1016/j.cub.2020.06.030
Kinoshita M, Shimohigasshi M, Tominaga Y et al (2015) Topographically distinct visual and olfactory inputs to the mushroom body in the Swallowtail butterfly, Papilio xuthus: Visual inputs in butterfly mushroom body. J Comp Neurol 523:162–182. https://doi.org/10.1002/cne.23674
Kozak KM, Wahlberg N, Neild AFE et al (2015) Multilocus species trees show the recent adaptive radiation of the mimetic Heliconius butterflies. Syst Biol 64:505–524. https://doi.org/10.1093/sysbio/syv007
Kraft N, Spaethe J, Rössler W, Groh C (2019) Neuronal plasticity in the mushroom-body calyx of bumble bee workers during early adult development. Dev Neurobiol 79:287–302. https://doi.org/10.1002/dneu.22678
Krebs JR, Sherry DF, Healy SD et al (1989) Hippocampal specialization of food-storing birds. Proc Nat Acad Sci USA 86:1388–1392. https://doi.org/10.1073/pnas.86.4.1388
Kroutov V, Reep RL, Fukuda T (2002) Experience-related changes in the brain of Agraulis vanillae (L.) (Nymphalidae). J Lepidopterists’ Soc 56:193–198
Kulkarni A, Ewen-Campen B, Terao K et al (2023) oskar acts with the transcription factor Creb to regulate long-term memory in crickets. Proc Natl Acad Sci USA 120:e2218506120. https://doi.org/10.1073/pnas.2218506120
Le Moël F, Stone T, Lihoreau M et al (2019) The central complex as a potential substrate for vector based navigation. Front Psychol 10:690. https://doi.org/10.3389/fpsyg.2019.00690
Lefebvre L, Reader SM, Sol D (2004) Brains, innovations and evolution in birds and primates. Brain Behavior Evol 63:233–246. https://doi.org/10.1159/000076784
Li L, MaBouDi H, Egertova M et al (2017) A possible structural correlate of learning performance on a colour discrimination task in the brain of the bumblebee. Proc Royal Soc B 284:20171323. https://doi.org/10.1098/rspb.2017.1323
Li L, Su S, Perry CJ et al (2018) Large-scale transcriptome changes in the process of long-term visual memory formation in the bumblebee, Bombus terrestris. Scientific Reports 8:1–10. https://doi.org/10.1038/s41598-017-18836-3
Li F, Lindsey JW, Marin EC et al (2020) The connectome of the adult Drosophila mushroom body provides insights into function. eLife 9:e62576. https://doi.org/10.7554/eLife.62576
Lihoreau M, Raine NE, Reynolds AM et al (2013) Unravelling the mechanisms of trapline foraging in bees. Commun Integ Biol 6:e22701. https://doi.org/10.4161/cib.22701
Liu L, Wolf R, Ernst R, Heisenberg M (1999) Context generalization in Drosophila visual learning requires the mushroom bodies. Nature 400:753–756. https://doi.org/10.1038/23456
Livraghi L, Martin A, Gibbs M, et al (2018) CRISPR/Cas9 as the key to unlocking the secrets of butterfly wing pattern development and its evolution. In: Advances in insect physiology. Elsevier, pp 85–115
Lockett GA, Helliwell P, Maleszka R (2010) Involvement of DNA methylation in memory processing in the honey bee. NeuroReport 21:812–816. https://doi.org/10.1097/WNR.0b013e32833ce5be
Logan CJ, Avin S, Boogert N et al (2018) Beyond brain size: uncovering the neural correlates of behavioral and cognitive specialization. Comp Cognit Behavior Rev 13:55–89. https://doi.org/10.3819/CCBR.2018.130008
Mallet J (1986) Gregarious roosting and home range in Heliconius butterflies. Natl Geographic Res 2:198–215
Mallet J, Longino JT, Murawski D et al (1987) Handling effects in Heliconius: where do all the butterflies go? J Animal Ecol 56:377–386
Merrill RM, Gompert Z, Dembeck LM et al (2011) Mate preference across the speciation continuum in a clade of mimetic butterflies. Evolution 65:1489–1500. https://doi.org/10.1111/j.1558-5646.2010.01216.x
Merrill RM, Dasmahapatra KK, Davey JW et al (2015) The diversification of Heliconius butterflies: what have we learned in 150 years? J Evol Biol 28:1417–1438. https://doi.org/10.1111/jeb.12672
Mertes M, Dittmar L, Egelhaaf M, Boeddeker N (2014) Visual motion-sensitive neurons in the bumblebee brain convey information about landmarks during a navigational task. Front Behav Neurosci 8. https://doi.org/10.3389/fnbeh.2014.00335
Mizunami M, Weibrecht JM, Strausfeld NJ (1998) Mushroom bodies of the cockroach: their participation in place memory. J Comp Neurol 402:520–537. https://doi.org/10.1002/(SICI)1096-9861(19981228)402:4<520::AID-CNE6>3.0.CO;2-K
Mobbs PG (1982) The brain of the honeybee Apis mellifera. I. The connections and spatial organization of the mushroom bodies. Philos Trans Royal Soc London B, Biol Sci 298:309–354. https://doi.org/10.1098/rstb.1982.0086
Molina Y, O’Donnell S (2008) Age, sex, and dominance-related mushroom body plasticity in the paperwasp Mischocyttarus mastigophorus. Dev Neurobiol 68:950–959. https://doi.org/10.1002/dneu.20633
Montgomery SH, Merrill RM, Ott SR (2016) Brain composition in Heliconius butterflies, posteclosion growth and experience-dependent neuropil plasticity. J Comp Neurol 524:1747–1769. https://doi.org/10.1002/cne.23993
Moura PA (2022) Memória espacial e aprendizado em borboletas neotropicais. PhD thesis, Universidade Federal do Rio Grande do Norte
Moura PA, Corso G, Montgomery SH, Cardoso MZ (2021) True site fidelity in pollen-feeding butterflies. Funct Ecol 36:572–582. https://doi.org/10.1111/1365-2435.13976
Moura PA, Cardoso MZ, Montgomery SH (2023a) No evidence of social learning in a socially roosting butterfly in an associative learning task. Biol Lett 19:20220490. https://doi.org/10.1098/rsbl.2022.0490
Moura PA, Young FJ, Monllor M et al (2023b) Long-term spatial memory across large spatial scales in Heliconius butterflies. Curr Biol 33:R797–R798. https://doi.org/10.1016/j.cub.2023.06.009
Mumford D (1992) On the computational architecture of the neocortex: II The role of cortico-cortical loops. Biol Cybern 66:241–251. https://doi.org/10.1007/BF00198477
Murawski DA, Gilbert LE (1986) Pollen flow in Psiguria warscewiczii: a comparison of Heliconius butterflies and hummingbirds. Oecologia 68:161–167. https://doi.org/10.1007/BF00384782
Neder R (1959) Allometrisches Wachstum von Hirnteilen bei drei verschieden grossen Schabenarten. Zool Jahrb Anat 77:411–464
Niven JE, Laughlin SB (2008) Energy limitation as a selective pressure on the evolution of sensory systems. J Exp Biol 211:1792–1804. https://doi.org/10.1242/jeb.017574
Norman KA (2010) How hippocampus and cortex contribute to recognition memory: revisiting the complementary learning systems model. Hippocampus 20:1217–1227. https://doi.org/10.1002/hipo.20855
Olkowicz S, Kocourek M, Lučan RK et al (2016) Birds have primate-like numbers of neurons in the forebrain. Proc Natl Acad Sci USA 113:7255–7260. https://doi.org/10.1073/pnas.1517131113
Olshausen B, Field D (2004) Sparse coding of sensory inputs. Curr Opin Neurobiol 14:481–487. https://doi.org/10.1016/j.conb.2004.07.007
Papaj DR (1986) Conditioning of leaf-shape discrimination by chemical cues in the butterfly, Battus philenor. Animal Behaviour 34:1281–1288. https://doi.org/10.1016/S0003-3472(86)80199-3
Pascual A, Préat T (2001) Localization of long-term memory within the Drosophila mushroom body. Science 294:1115–1117. https://doi.org/10.1126/science.1064200
Peters RS, Krogmann L, Mayer C et al (2017) Evolutionary history of the hymenoptera. Curr Biol 27:1013–1018. https://doi.org/10.1016/j.cub.2017.01.027
Pfeiffer K, Homberg U (2014) Organization and functional roles of the central complex in the insect brain. Annu Rev Entomol 59:165–184. https://doi.org/10.1146/annurev-ento-011613-162031
Pierce NE, Elgar MA (1985) The influence of ants on host plant selection by Jalmenus evagoras, a myrmecophilous lycaenid butterfly. Behav Ecol Sociobiol 16:209–222. https://doi.org/10.1007/BF00310983
Poirier M-A, Kozlovsky DY, Morand-Ferron J, Careau V (2020) How general is cognitive ability in non-human animals? A meta-analytical and multi-level reanalysis approach. Proc R Soc B 287:20201853. https://doi.org/10.1098/rspb.2020.1853
Pravosudov VV, Clayton NS (2002) A test of the adaptive specialization hypothesis: population differences in caching, memory, and the hippocampus in black-capped chickadees (Poecile atricapilla). Behavior Neurosci 116:515–522. https://doi.org/10.1037/0735-7044.116.4.515
Pritchard DJ, Hurly TA, Tello-Ramos MC, Healy SD (2016) Why study cognition in the wild (and how to test it)?: cognition in the wild. Jrnl Exper Analysis Behavior 105:41–55. https://doi.org/10.1002/jeab.195
Rausher MD (1978) Search image for leaf shape in a butterfly. Science 200:1071–1073. https://doi.org/10.1126/science.200.4345.1071
Reader SM, Laland KN (2002) Social intelligence, innovation, and enhanced brain size in primates. Proc Nat Acad Sci 99:4436–4441. https://doi.org/10.1073/pnas.062041299
Rolls ET (1989) Functions of neuronal networks in the hippocampus and neocortex in memory. In: Neural Models of Plasticity. Elsevier, pp 240–265
Schubert M, Lachnit H, Francucci S, Giurfa M (2002) Nonelemental visual learning in honeybees. Animal Behaviour 64:175–184. https://doi.org/10.1006/anbe.2002.3055
Schumacher EL, Carlson BA (2022) Convergent mosaic brain evolution is associated with the evolution of novel electrosensory systems in teleost fishes. eLife 11:e74159. https://doi.org/10.7554/eLife.74159
Shettleworth SJ (2003) Memory and hippocampal specialization in food-storing birds: challenges for research on comparative cognition. Brain Behav Evol 62:108–116. https://doi.org/10.1159/000072441
Sivinski J (1989) Mushroom body development in Nymphalid butterflies: a correlate of learning? J Insect Behav 2:277–283. https://doi.org/10.1007/BF01053299
Sol D, Garcia N, Iwaniuk A et al (2010) Evolutionary divergence in brain size between migratory and resident birds. PLoS ONE 5:e9617. https://doi.org/10.1371/journal.pone.0009617
Spencer WD (2012) Home ranges and the value of spatial information. J Mammal 93:929–947. https://doi.org/10.1644/12-MAMM-S-061.1
Stieb SM, Muenz TS, Wehner R, Rössler W (2010) Visual experience and age affect synaptic organization in the mushroom bodies of the desert ant Cataglyphis fortis. Dev Neurobiol 70:408–423. https://doi.org/10.1002/dneu.20785
Stieb SM, Hellwig A, Wehner R, Rössler W (2012) Visual experience affects both behavioral and neuronal aspects in the individual life history of the desert ant Cataglyphis fortis. Devel Neurobio 72:729–742. https://doi.org/10.1002/dneu.20982
Sukhum KV, Shen J, Carlson BA (2018) Extreme enlargement of the cerebellum in a clade of teleost fishes that evolved a novel active sensory system. Curr Biol 28:3857–3863.e3. https://doi.org/10.1016/j.cub.2018.10.038
Sutherland RJ, Rudy JW (1989) Configural association theory: the role of the hippocampal formation in learning, memory, and amnesia. Psychobiology 17:129–144. https://doi.org/10.3758/BF03337828
Swihart CA, Swihart SL (1970) Colour selection and learned feeding preferences in the butterfly, Heliconius charitonius Linn. Anim Behav 18:60–64. https://doi.org/10.1016/0003-3472(70)90071-0
Tihelka E, Cai C, Giacomelli M et al (2021) The evolution of insect biodiversity. Curr Biol 31:R1299–R1311. https://doi.org/10.1016/j.cub.2021.08.057
Toure MW, Young FJ, McMillan WO, Montgomery SH (2020) Heliconiini butterflies can learn time-dependent reward associations. Biol Lett 16:1–5. https://doi.org/10.1098/rsbl.2020.0424
Trebels B, Dippel S, Schaaf M et al (2020) Adult neurogenesis in the mushroom bodies of red flour beetles (Tribolium castaneum, Herbst) is influenced by the olfactory environment. Sci Rep 10:1090. https://doi.org/10.1038/s41598-020-57639-x
van Dijk LJA, Janz N, Schapers A et al (2017) Experience-dependent mushroom body plasticity in butterflies: consequences of search complexity and host range. Proc R Soc B 284:20171594. https://doi.org/10.1098/rspb.2017.1594
Varga AG, Kathman ND, Martin JP et al (2017) Spatial navigation and the central complex: sensory acquisition, orientation, and motor control. Front Behav Neurosci 11. https://doi.org/10.3389/fnbeh.2017.00004
Vogt K, Schnaitmann C, Dylla KV et al (2014) Shared mushroom body circuits underlie visual and olfactory memories in Drosophila. eLife 3:e02395. https://doi.org/10.7554/eLife.02395
Wang Z-L, Wang H, Qin Q-H, Zeng Z-J (2013) Gene expression analysis following olfactory learning in Apis mellifera. Mol Biol Rep 40:1631–1639. https://doi.org/10.1007/s11033-012-2212-9
Webb B, Wystrach A (2016) Neural mechanisms of insect navigation. Curr Opin Insect Sci 15:27–39. https://doi.org/10.1016/j.cois.2016.02.011
Wystrach A, Beugnon G, Cheng K (2011) Landmarks or panoramas: what do navigating ants attend to for guidance? Front Zool 8:21. https://doi.org/10.1186/1742-9994-8-21
Young FJ, Montgomery SH (2020) Pollen feeding in Heliconius butterflies: the singular evolution of an adaptive suite. Proc R Soc B 287:20201304. https://doi.org/10.1098/rspb.2020.1304
Young FJ, Alcalde A, Melo-Florez L, et al (2023a) Enhanced long-term memory and increased mushroom body plasticity in Heliconius butterflies
Young FJ, Melo-Flórez L, McMillan WO, Montgomery SH (2023b) Reversal learning of visual cues in Heliconiini butterflies
Young FJ, Monllor M, McMillan WO, Montgomery SH (2023c) Patterns of host plant use do not explain mushroom body expansion in Heliconiini butterflies. Proc R Soc B 290:20231155. https://doi.org/10.1098/rspb.2023.1155
Zars T (2000) Behavioral functions of the insect mushroom bodies. Curr Opin Neurobiol 10:790–795. https://doi.org/10.1016/S0959-4388(00)00147-1
Zhou Y, Sun L, Peng X et al (2020) Chromatic, achromatic and bimodal negative patterning discrimination by free-flying bumble bees. Animal Behaviour 169:93–101. https://doi.org/10.1016/j.anbehav.2020.09.009
Funding
This work was funded by an ERC Starter Grant (758508) and a NERC IRF (NE/N014936/1) to SHM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
This article is a contribution to the Topical Collection “Toward a Cognitive Ecology of Invertebrates” - Guest Editors: Aurore Avarguès-Weber and Mathieu Lihoreau
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Young, F.J., Montgomery, S.H. Heliconiini butterflies as a case study in evolutionary cognitive ecology: behavioural innovation and mushroom body expansion. Behav Ecol Sociobiol 77, 131 (2023). https://doi.org/10.1007/s00265-023-03399-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-023-03399-3