Abstract
In many taxa, melanin-based coloration is a signal of dominance or fighting ability and is associated with concentrations of hormones that may mediate aggressive behavior. Previous studies found that experimental manipulation of melanin-based signals can result in manipulated individuals receiving more social challenges in some but not all species. These differences could arise from mismatches between the signal, behavior, and hormone concentrations. In the present study, we experimentally manipulated the chest spotting of urban and rural male song sparrows (Melospiza melodia) following an assessment of their territorial aggression and initial concentrations of corticosterone and testosterone and then assessed their behavior and hormone concentrations 2 weeks later. We found that males generally displayed less territorial aggression in the second trial, consistent with our previous findings. Males in the enlarged treatment decreased aggression to a greater degree than those in the reduced treatment. The effect of the plumage manipulation was similar across the rural and urban habitats. Despite the changes in behavior we detected, we found no effects of the manipulation on concentrations of testosterone or corticosterone. Our results show that melanin-based spotting in male song sparrows is a signal of territorial aggression but the physiological mechanisms that mediate the relationships between chest spotting and behavior remain to be identified.
Significance statement
Many bird species use their plumage to signal their dominance status, fighting ability, or motivation during interactions with other individuals to resolve conflicts without a fight. Here, we asked whether chest spotting is a signal in territorial interactions among male song sparrows. We experimentally increased or reduced the extent of spotting in males and measured the change in their aggression. We found that reduced-spotting males showed a more moderate seasonal decrease of aggression compared to males with enlarged spotting reduced aggression, possibly because the former experienced more intrusions later on in the breeding season while the latter experienced fewer intrusions. These results are consistent with chest spotting size in song sparrows functioning as a signal of territory holding potential of the bearer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Signals convey information about an individual’s phenotype to receivers, and in order to be maintained in a population, signals should be honest and benefit both the signaler and receiver (Johnstone and Grafen 1993; Searcy and Nowicki 2005). For instance, intrasexual competitive interactions can be costly, and ornaments that reliably indicate dominance or fighting ability could benefit both signaler and receiver by allowing them to avoid a physical confrontation (Maynard Smith and Price 1973; Maynard Smith and Parker 1976). Melanin-based coloration is one trait that has frequently been examined in the context of intrasexual interactions and darker and larger ornaments are often associated with winning contests, fighting ability, or intensity of territorial aggression (Rohwer 1977; Chaine and Lyon 2008; Santos et al. 2011). Expression of melanin-based traits is often related to aspects of physiology that facilitate aggressive behavior including concentrations of hormones, such as androgens (Safran et al. 2008; Hasegawa et al. 2017) or glucocorticoids (Kittilsen et al. 2009; Saino et al. 2013) that both play important roles in mediating aggressive behavior in vertebrates (Wingfield et al. 2001; Summers et al. 2005; Haller 2014). Thus, individuals with larger or darker melanin-based traits are generally more aggressive and have the physiological mechanisms that support this behavior. However, the degree to which these relationships are fixed by genetic (Ducrest et al. 2008) or cellular (Galván and Alonso-Alvarez 2008) mechanisms associated with melanin production, and the degree to which the relationships are plastic and can be influenced by the current environment and individual condition, requires additional investigation.
Experimental manipulation of signals can reveal the nature of the relationship between signals and other aspects of phenotype. For instance, enhancement of signals that mediate aggressive interactions often results in manipulated individuals or models receiving more challenges from conspecifics (Rohwer 1977; Dale and Slagsvold 1996; van Dongen and Mulder 2007; Rick and Bakker 2008; Tannure-Nascimento et al. 2008; Laubach et al. 2013). In other cases however, individuals with enhanced signals are challenged less (Pryke et al. 2002) or those with reduced signals are challenged more than other individuals (Evans and Hatchwell 1992; Rémy et al. 2010; Theis et al. 2012; Dey et al. 2014).
Changes in aggressive or territorial behaviors may come about if there is a mismatch between the experimentally altered ornament and other behaviors of the individual. For example, if a signal of competitive dominance is accompanied by submissive behaviors by the individual bearing the signal, the signaler may be socially punished (Rohwer and Rohwer 1978; Tibbetts and Izzo 2010; Ligon and McGraw 2016; Webster et al. 2018). In contrast, an individual’s behavior may shift and become matched to the modified ornament through social feedback from conspecifics. Such social feedback can potentially resolve mismatches through changes in hormone concentrations that could facilitate the expression of behaviors that match the manipulated ornament. For instance, experimental darkening of the melanin chest patch of male barn swallows (Hirundo rustica erythrogaster) is associated with elevated testosterone compared to control males (Safran et al. 2008). Similarly, a later study found males that experienced a greater change in the color of their chest patch, from a lighter color to a much darker color, showed a greater increase in testosterone concentrations than males that experienced a more subtle change in coloration (Levin et al. 2018). Experimentally increased area of white in the crown of male white-crowned sparrows (Zonotrichia leucophrys gambelii) is associated with greater baseline concentrations of corticosterone and an attenuated release of stress-induced corticosterone (Laubach et al. 2013). These changes in physiology are likely the result of changes in social interactions with conspecifics that respond differently to the manipulated individual, which ultimately leads to changes in individual physiology and behavior (Vitousek et al. 2014).
The effect of experimental manipulation of signals may also vary because of the social context in which the signal is displayed, which may influence relationships between signals and other aspects of phenotype. For instance, the same level or intensity of ornament could be a relatively strong signal in a population of weak signalers, but a relatively weak signal in a population of strong signalers and this could alter the relationship between a signal and behavior or physiology in these two social contexts (Vitousek et al. 2014). Rapid environmental change, such as due to urbanization, is one potential factor that may lead to differences in social context between populations.
A number of studies have documented differences in territorial aggression (Fokidis et al. 2011; Atwell et al. 2014; Foltz et al. 2015; Davies and Sewall 2016), vocal signals (Mockford and Marshall 2009; Kight and Swaddle 2015; Rios-Chelen et al. 2015), and visual signals (Atwell et al. 2012; Beck et al. 2018; Giraudeau et al. 2018) between urban and rural habitats, suggesting that the social environment differs between these areas. Urban organisms often also differ from their rural counterparts in aspects of physiology, including concentrations of glucocorticoids (Partecke et al. 2006; Bonier et al. 2007; Atwell et al. 2012) and androgens (Atwell et al. 2014; Davies et al. 2018). These differences in hormone concentrations could be the result of differences in the social environment and mediate some of the behavioral differences between urban and rural individuals. Alternatively, hormone concentrations could differ between habitats due to other factors and disrupt expected relationships between signals, physiology, and behavior. Urban habitats, therefore, offer a unique opportunity to determine how relationships between signaler ornaments, behavior, and physiology are influenced by ecological conditions.
Song sparrows (Melospiza melodia) provide a good model system for experimentally addressing the relationships among melanin-based ornaments, hormones, and territorial aggression. This species commonly occurs in urban and rural habitats and males in urban habitats display greater territorial aggression than rural males during simulated territorial intrusions, which suggests the social environment differs between sites (Evans et al. 2010; Foltz et al. 2015; Davies and Sewall 2016). Both sexes display brown, melanin-based spotting on the breast. While we focus on the male territoriality and ornaments here, it is worth noting that females also show aggressive territory defense against intruding females (Elekonich 1999; Elekonich and Wingfield 2000; Lane and Sewall 2022). Previous work on this population indicated that urban males have significantly greater spotting area and tend to have darker spotting than rural males (Beck et al. 2018). Though the overall pattern of spotting and aggression is for urban males to have more spotting and be more aggressive, natural variation in chest spotting within habitat types is related to territorial aggression in a complex manner: urban birds have greater spotting area than rural males and are also more aggressive than rural males (Beck et al. 2018; Davies and Sewall 2016). Within rural populations, however, males with less chest spotting are more aggressive than those with greater spotting. Perhaps because of their greater spotting area overall, in urban habitats chest spotting area was not correlated with aggression (Beck et al. 2018). These different effects across and within habitats may be due to various confounds such as different resource abundance (Foltz et al. 2015). Given the complexity of behavior, physiology, and ornamentation in this system, understanding the possible role of ornaments requires manipulations that create mismatches among these traits.
To resolve the relationship between chest spotting and territorial aggression, we experimentally manipulated the chest spotting area in urban and rural song sparrows and asked if this manipulation would lead to correlated changes in territorial aggression and hormone concentrations. Based on the general literature on badge signals (Rohwer 1977; Chaine and Lyon 2008; Santos et al. 2011), we assume that the chest spotting area is positively correlated with the likelihood that the territory holder that bears it will be able to repel an intruder. Therefore, experimentally reducing a male’s spotting area is predicted to result in that male getting more frequently challenged in territorial interactions (Evans and Hatchwell 1992; Dey et al. 2014), which in turn would necessitate higher levels of investment in territory defense (i.e., higher territorial aggression) relative to before the manipulation. This change may, in turn, lead to greater circulating testosterone and corticosterone levels relative to before the manipulation. In contrast, males with enlarged spotting areas are expected to be challenged less because they are viewed as higher quality males (Chaine et al. 2013), and consequently these males would decrease their investment in territory defense (i.e., display lower aggression), and consequently lower initial concentrations of corticosterone and testosterone relative to before the manipulation. Once the chest spotting of a male has been altered, these effects should be cumulative and relatively persistent.
Our second goal was to determine if the habitat type influenced the association between experimentally manipulated spotting area and male behavior or physiology. As noted above, urban males have larger chest spotting area and are more aggressive than rural males but that natural variation in chest spotting area was only associated with aggression in rural males (Beck et al. 2018). Two alternative predictions are therefore possible from these findings: first, chest spotting may be a more salient signal of territorial aggression in the more competitive urban habitats (where males are more aggressive and have larger chest spotting areas) and therefore experimental manipulations of the spotting area may have a larger effect as described above in urban habitats. Alternatively, chest spotting may be a less informative signal in urban habitats compared to rural habitats. If that is the case, then we expect the manipulation to have a larger effect in rural habitats than in urban habitats.
Methods
Study site and species
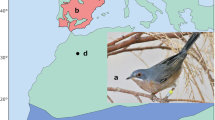
We conducted this research with male song sparrows living in Montgomery County, VA, USA, at six locations, three rural and three urban sites. The rural sites were located around the city of Blacksburg, VA, and the Kentland Farms of Virginia Tech. The three urban sites were located on the campuses of Radford University and Virginia Tech. A full description of sites is in Davies and Sewall (2016). Urbanization scores were calculated from aerial images by scoring the land covered by vegetation and built structures using the method developed by Seress and colleagues (Seress et al. 2014) that can be found in Davies and Sewall (2016). We assessed aggressive behavior of 40 male song sparrows from 09 April to 10 May 2017, which corresponds to early breeding season. Each male was tested twice, once before the chest spotting manipulation and once 2 to 3 weeks (mean ± SD: 17.7 ± 3.39 days) after the chest spotting manipulation. Three subjects in each treatment group could not be tested a second time. It was not possible to record data blind because our study involved focal animals in the field.
Playback stimuli
The playback stimuli for the simulated territorial intrusions were recorded with Marantz PMD 660 or PMD 661 Solid State recorder and a Sennheiser ME66/K6 directional microphone. The stimulus files were made using the Syrinx software (John Burt, Portland, OR) by selecting a song with a good signal to noise ratio and manually filtering low-frequency (< 1000 Hz) noise. We selected 38 song types from 24 different males located near our study site in the same year. Each subject received a randomly selected playback stimulus from a male located at least 1 km from the focal male, so that males always heard unfamiliar songs. Subjects were presented with the same song type in both trials. Song sparrows respond to randomly selected song types of strangers (unfamiliar birds from far away) with equal intensity, suggesting a given song type is equivalent in function to any other song type (Searcy et al. 1981; Akçay and Beecher 2020).
Simulated territorial intrusion procedure
We identified the territories of the subjects by observing singing posts of already singing males, although we did not attempt to map the entire territory of the male. In some cases, we used short playbacks (1–2 songs) to elicit flights from the singing male to determine the extent of the territory. We then placed a speaker at a central location in the territory (in the approximate center of the observed singing posts of the male). The playback stimuli were broadcast at a volume of about 80 dB at 1 m and rate of one song every 10 s which approximates natural singing amplitude and rate of broadcast song in song sparrows (Anderson et al. 2008).
The simulated intrusion trials lasted about 3 min (the actual playback durations varied slightly between trials with a mean ± SD of 191 ± 21.1 s). During the trial, we recorded the distance between the male and the speaker, the number of broadcast (loud) songs the male sang, the number of soft songs (low amplitude songs that are given at close range), the number of wing waves, and the number of flights using the same recording equipment as above, throughout the trial. Soft vs. loud song distinction was made in the field by an experienced observer (CA or MB), which was shown to yield a reliable classification (Anderson et al. 2008).
Blood sampling
Following the simulated territory intrusions, males were captured in a mist net using conspecific song playback (average total playback duration including the behavioral trial which we scored 22 min 23 s ± 1 min 25 s standard error). Within 3 min of capture, we obtained a blood sample to quantify initial hormone concentrations, took morphological measurements, and banded males. Males were photographed and feathers collected from their chest for spectrophotometry, their chest spotting was manipulated (see detailed descriptions below), and they were then released at the point of capture. Blood samples were kept on ice until return to the lab where they were spun at 6708 g for 5 min and the plasma fraction frozen at − 80 °C in separate aliquots for the hormone assays.
Scoring behaviors during the simulated intrusions
All the simulated intrusion trials were scored by one observer (CA) by annotating the recordings in Syrinx. The number of loud songs, soft songs, wing waves, and flights was counted as was the proportion of the trial a male spent within 5 m of the speaker and the closest approach to the speaker. Soft songs and wing waves are both predictive of attack on a taxidermic mount in this species (Searcy et al. 2006; Akçay et al. 2013). Because the trials varied some in duration, we calculated rates for soft songs, wing waves, loud songs, and flights. Previous studies found that rates of these behaviors do not significantly change over even longer trial periods (Searcy et al. 2008; Akçay et al. 2014), thus taking the rates of these behaviors over 3-min trials is a valid approach.
Quantification of badge size and plumage color
To quantify badge size, we took a photograph of each bird on a white background with a metric ruler as a size standard. The camera was held perpendicular to the bird and we took 3–5 images of the chest of each individual. Between pictures, we smoothed the feathers which produced a slightly different view of the spotting. We used Image J (Rueden et al. 2017) to quantify the area covered in brown on each bird in the three pictures. We calculated the brown area from the neck to a line drawn horizontally across the chest, just below the bottom of the central portion of the spotting. These three measurements have high repeatability (Beck et al. 2018) and we used the average area from the three pictures as our estimate of spotting area. We did collect brown feathers for spectrophotometry but do not include that data here because our focus was on spotting area.
Manipulation of spotting area and effects on physiology and behavior
After collecting feathers from males, we manipulated the extent of brown spotting, alternating between enlarging and reducing the area. We increased spotting of 8 and 11 males in urban and rural habitat respectively and reduced spotting in 9 urban and 12 rural males. To reduce spotting, we used scissors to snip brown feathers from males. As song sparrows do not molt until the end of the breeding season, this reduced treatment would be persistent throughout the breeding season. To increase spotting, we applied a combination of black Sharpie and ShinHanart chestnut brown (BR998) marker to the breast of males (Fig. 1). Which the combination of these two markers did not precisely match the natural variation in spotting reflectance (Supplementary Fig. 1), this was the closest marker combination. To control for the application of marker, we colored the belly feathers of males that had their badge reduced in size with white Sharpie marker. To control for cutting in reduced badge males, we cut a similar number of white belly feathers from males that had their badge size increased. We then took a second picture of the male to confirm that we successfully increased or decreased the amount of chest spotting (Supplementary Fig. 2).
Example pre- and post-manipulation photos for male songs sparrows. Prior to manipulation, males were photographed on a white standard with a ruler for a size standard (a and c). Males had their chest spotting experimentally enlarged (b) using a combination of black Sharpie and chestnut art marker or experimentally reduced by having the brown feathers clipped (d)
To determine if the manipulation affected male behavior or hormone concentrations, we assessed male behavior using the previously described procedure approximately 2 weeks after the manipulation (mean ± SD = 17.7 ± 3.39 days, range: 13–26, see Fig. S3 in supplementary materials for the distribution of the inter-trial intervals). We attempted to recapture the male on the same day, but males were more difficult to recapture (compared to the initial capture) and we sometimes captured them 1–3 days following the assessment of their behavior. Once recaptured, we obtained a blood sample within 3 min and used these samples to quantify post-manipulation corticosterone and testosterone. We did not quantify whether the painted feathers in the enlarged treatment retained their colors during this second capture so as to not interfere with the breeding activities.
Quantification of testosterone and corticosterone
Details regarding the quantification of testosterone and corticosterone can be found elsewhere (Davies and Sewall 2016; Davies et al. 2018). Briefly, we quantified plasma testosterone and corticosterone using validated enzyme-linked immunoassays (Enzo Life Sciences, Farmingdale, NY) following the manufacturer’s instructions. For both hormones, we assayed samples in duplicate and assigned all the samples from a given bird to the same assay plate. The average intra- and inter-assay coefficients of variation for the testosterone assay were 6.6% and 9.5%, respectively, and for the corticosterone assay they were 6.8% and 7.8%, respectively. The average assay sensitivities for the testosterone and corticosterone assays were 2.7 pg/mL and 19.8 pg/mL, respectively.
Analysis
All analyses were carried out in R 4.2.3 (R Core Team 2021). Because many of the behaviors measured during simulated intrusions were correlated with each other, we used a principal components analysis (PCA) to combine these behaviors into a single measure of territorial aggression. We entered flight rates, closest approach, proportion of time spent within 5 m, and rates of soft songs and rates of wing waves into the model. The first principal component (PC1) explained 54.39% of the variance and received positive loadings for the number of flights, number of wing waves, number of soft songs, and the proportion of the trial within 5 m and a negative loading for the closest approach (Table 1). Thus, higher PC1 scores (henceforth, aggression scores) are indicative of a bird showing greater territorial aggression. Aggression scores calculated from these behavioral variables are highly predictive of attack on a taxidermic mount in this species (Akçay et al. 2014).
To verify that subjects in the two treatments did not differ in their initial extent of chest spotting, we carried out a linear regression analysis in which manipulation (reduced or enlarged), habitat type (urban vs. rural), and their interaction were the predictor variables and chest spotting area as the dependent variable. We then analyzed the behavioral and physiological data using linear mixed models (LMMs) using the lme function in package nlme (Pinheiro et al. 2017). In all the models, we included male as a random factor to account for repeated sampling of the same individual. We included the following fixed factors and all their interactions (three two-way and one three-way interactions) in the full model: manipulation (reduced or enlarged), trial number (1st or 2nd), habitat (urban or rural). The three-way interaction was included to assess whether the manipulation led to a change in the response variable between the first and second trial depending on the habitat. We used a model averaging approach using AICc because several candidate models had relatively close ΔAICc (< 6) to the model with the lowest AICc (see Supplementary Tables S1 and S2 for the full model and the model selection table). To determine the relative fit of models and average the models, we used the dredge and model.avg functions in the package MuMIn (Bartoń 2013). Following Harrison and colleagues (Harrison et al. 2018), we averaged models within 6 ΔAICc of the best fit model. We carried out separate LMMs as described above for the aggression scores, testosterone and corticosterone concentrations as response variables, with the latter two variables log-transformed.
Results
Spotting area manipulation and effects on behavior
Prior to the manipulation, male chest spotting initially ranged from 129.48 to 413.48 mm2. Male song sparrows did not differ in the initial extent of their chest spotting between the two treatment groups, or between habitat types (Table 2, Fig. S2). There was also no significant interaction of habitat and manipulation in the pre-manipulation extent of chest spotting. There was a significant effect of manipulation on the extent of chest spotting (i.e., we effectively altered chest spotting area; Table 2). Results of paired t-tests indicated that removing feathers significantly reduced chest spotting (t = 6.14, df = 20, p < 0.0001) while using markers to increase chest spotting resulted in significantly greater spotting area (t = 8.27, df = 18, p < 0.0001).
Males showed significantly lower aggression in the second trials compared to first trials and this effect was dependent on manipulation: birds in the enlarged treatment reduced aggression to a greater extent than males in the reduced treatment (Table 3, Fig. 2). We also found a significant interaction between trial number and habitat: males in both habitats showed a decrease in territorial aggression in the second trial, but this decrease was more pronounced for males in rural habitats. Indeed, there was no difference in aggression between urban and rural birds in the first trial (unpaired t-test: t(38) = 1.44, p = 0.16), but urban birds were more aggressive in the second trial (unpaired t-test t(32) = 3.23, p = 0.003; Fig. 2). The three-way interaction of habitat type, trial number, and manipulation was not significant in the averaged model (Table 3).
Spotting area manipulation and hormone concentrations
Despite the effect of the spotting manipulation on the change in aggressive behavior, we found no effect of the manipulation on hormone concentrations: the averaged models (Table 4) for both corticosterone and testosterone showed no significant effects of any of the predictor variables or their interactions (see Supplementary Tables S3 and S4 in the Electronic Supplementary Materials for the full models).
Discussion
Melanin-based coloration is a signal that is often associated with dominance or fighting ability (reviewed in Santos et al. 2011) and with concentrations of hormones such as testosterone or corticosterone (Saino et al. 2013; Hasegawa et al. 2017) that mediate aggressive behavior in vertebrates (Wingfield et al. 2001; Haller 2014). In the present study, we experimentally addressed how manipulating a melanin-based plumage signal is related to these changes in circulating hormones and aggressive behavior.
We predicted that experimentally reducing chest spotting area would cause subjects to increase investment into territory defense via physical aggression because they experience increased intrusion pressure from other males as a result of reduced chest spotting area. In contrast, we expected experimental enlargement of the chest spotting to result in a reduction in aggression, presumably as the result of fewer social challenges to the bearer. We found that aggression levels generally decreased from the first to second trial, consistent with a previous study in this population that showed seasonal decline of aggression in both urban and rural birds (Davies and Sewall 2016). The plumage manipulation, however, had a significant effect on this decline. Specifically, males with experimentally reduced chest spotting showed less of a decline in aggression between trials than males with experimentally enlarged spotting areas. This suggests that our enlarged spotting manipulation was not effective but supports our prediction that reduced spotting should lead to higher aggression, as has been found in some previous studies (Chaine et al. 2013; Dey et al. 2014). The effect was similar in both rural and urban habitats. Habitat type did influence reduction in aggression: rural birds showed stronger reductions in the second trials than urban birds.
Despite the change in behavior, we found no changes in concentrations of either corticosterone or testosterone at capture. This finding suggests that there is plasticity in the relationship between melanin coloration and hormone concentrations and/or that another physiological mechanism links variation in color with variation in territorial aggression. The hormone results however come with large caveats as further discussed below.
The effect of the experimental manipulation of chest spotting on aggression during the second trial may have come about through social feedback from male competitors in two non-mutually exclusive ways. First, males that received reduced chest spotting may have been challenged more and therefore may have maintained a higher level of aggression later in the season (Rohwer and Rohwer 1978). This is consistent with the earlier finding that rural song sparrows show a negative correlation between spotting size and aggression (Beck et al. 2018). A similar relationship between ornamentation and aggression was reported in male red-collared widowbirds (Euplectes ardens). In this species, males with naturally large collars spend less time in territory defense suggesting they are challenged less than males with smaller collars (Pryke et al. 2001). Alternatively, it is possible that males that received enlarged chest spotting may have been challenged less and therefore invested less in territorial defense (Chaine et al. 2013). This would mean that song sparrows may not perceive a mismatch between the enlarged spotting and the behavior of the subject (cf. Rohwer and Rohwer 1978; Tibbetts and Izzo 2010). However, the enlarged spotting manipulation may not have been effective due to the imperfect match in color spectra and males in this treatment may simply show seasonal decreases in territoriality (Davies and Sewall 2016). Future studies can test these possibilities in song sparrows by measuring the frequency of territorial challenges by conspecifics and time spent in territory defense by males with experimentally enlarged or reduced chest spotting.
Another potential explanation for our finding that male behavior changed after experimental manipulation of chest spotting is that dynamics between males and females, not male-male interactions, provided social feedback that influenced subjects’ behavior. After the spotting enlargement, males may have been perceived as more attractive by social and extra-pair mates, which may have decreased their need to invest in territorial defense for mate guarding, as the male is under less threat of losing paternity (Moser-Purdy et al. 2017). A recent experimental manipulation in barn swallows showed interactions between males and their social mate, and to a lesser extent other females in the population increased following experimental darkening of the ventral plumage. This manipulation had the greatest effect on males that experienced the greatest shifts in color (Levin et al. 2018). In other species, females paired to more ornamented males often produce fewer extra-pair offspring (Safran et al. 2005; Lehtonen et al. 2009; Reudink et al. 2009; Eikenaar et al. 2011). Whether there is a directional female preference for the extent of chest spotting in song sparrows is not yet known.
One caveat for the enlarged treatment in our experiment is warranted. Although we tried to match the reflectance values of the painted feathers to the reflectance of the naturally pigmented feathers, they were not an exact match (Fig. S1). We also did not measure the chest spotting of the subjects during the second capture. Although the reduced chest spotting treatment would have persisted (due to the fact that molting does not happen until the end of the breeding season; Arcese et al. 2020), the painted feathers may have lost of their color in the intervening period. Some variation is to be expected in a field experiment like the present study, but it also means that we cannot be sure if the males in this treatment indeed were perceived as having a greater chest spotting. Nonetheless, given that these males had their normal chest feathers intact, these males at least have their regular chest spotting. Indeed, the seasonal decrease in aggression in this group is consistent with a previous study in population with unmanipulated males (Davies and Sewall 2016). In other words, males in this treatment group were at the very least perceived with their natural chest spotting intact, providing a valid comparison to the reduced treatment group.
We expected to detect an interaction between habitat type and the manipulation because male song sparrows in urban areas have significantly greater spotting area (Beck et al. 2018) and show greater territorial aggression than those in rural areas (Foltz et al. 2015; Davies and Sewall 2016; Akçay et al. 2020). As a result, we predicted a reduction in spotting area would have a greater effect on urban birds compared to rural birds because in the former a reduced chest spotting male would be perceived as competitively inferior among more aggressive neighbors with intact chest spotting. Contrary to our hypothesis, the manipulation had similar effects in urban and rural habitats. It is possible that our sample size was not large enough to detect a three-way interaction between habitat, manipulation, and trial number. It is also worth noting that in our sample, urban and rural birds did not show a significant difference in the initial extent of the chest spotting (Fig. S2). This finding is in contrast to an earlier study in our population which found urban males to have higher extent of chest spotting than rural males (Beck et al. 2018). It is possible that in our initial sampling we may have sampled disproportionately males with large chest spotting in rural areas compared to the average rural population, which may have masked any interaction of habitat with the experimental manipulation. This possibility warrants further research.
Habitat type did influence aggression irrespective of the manipulation: urban birds reduced their aggression from the first to second trials to a lesser extent than the rural birds. Indeed, when analyzed separately, aggression scores in the first trials were not significantly different between urban and rural birds, but in second trials urban birds showed higher aggression compared to rural birds. One possibility is that urban habitats are more fragmented by unsuitable landcover such as parking lots and buildings which might mean that birds in these habitats experience more sustained competition for the existing territories (Marzluff 2001; Juárez et al. 2020). This may lead to higher levels of investment in territorial defense throughout the breeding season. Another possibility is that urban habitats may have higher levels of food resources over the breeding season, allowing urban birds to maintain a relatively higher level of aggression (Foltz et al. 2015). Finally, urban and rural birds may differ in behavioral plasticity such that urban birds are more consistent in their behavior over time than rural birds. Few previous studies examined this possibility. In one such study, urban male great tits (Parus major) showed lower repeatabilities of territorial behavior over a few days than rural males (Hardman and Dalesman 2018). Whether the same holds for song sparrows is currently not known. The relative influence of plasticity and environmental conditions on the persistence of higher aggression in urban habitats warrants further research.
Effects of manipulation of hormone concentrations
We found no effect of the spotting area manipulation on concentrations of testosterone or corticosterone in blood samples collected immediately after capture, despite the change in territorial aggression. Circulating levels of both testosterone and corticosterone can be positively associated with territorial aggression (Nelson and Trainor 2007) although the relationship is often complex (George and Rosvall 2022). Previous work in this population indicated that initial corticosterone is positively associated with territorial aggression but that initial and GnRH-induced testosterone are unrelated to an individual’s territorial aggression in response to conspecific playback (Davies et al. 2018). We predicted that initial concentrations of corticosterone and testosterone would increase in males with reduced-spotting areas because we expected that they would experience more social challenges and thus need to engage in more territorial defense (Wingfield 1994; Deviche et al. 2014). One explanation for our failure to detect an effect of the manipulation on hormone concentrations is that corticosterone and testosterone vary seasonally in this population (Davies and Sewall 2016; Davies et al. 2018), and it is possible that this seasonal variation masked any effect of the manipulation on hormone concentrations. Crucially, we did not control for breeding stage of the males which may have masked any effects of social challenges on testosterone and corticosterone. It is also possible that the variation in the capture times from the start of the simulated intrusion may have masked corticosterone and testosterone responses in the short term. Nevertheless, the observed differences in aggressive behaviors depending on habitat and manipulation in the absence of differences in circulating hormone levels underscores the need for future studies to understand the physiological mechanism of plumage signals and territorial aggression.
Conclusion
We found that aggression decreased over the breeding season but that an experimental reduction of chest spotting in male song sparrows was associated with dampened reductions in aggression in both urban and rural song sparrows compared to experimentally enlarged chest spotting. These findings add weight to the hypothesis that chest spotting is an important signal of resource-holding potential or mate quality in both habitats (Davies and Sewall 2016; Beck et al. 2018). While our plumage manipulation had similar effects in urban and rural habitats, aggressive behavior nonetheless differed between these habitats, with urban birds showing sustained aggression later in the season while rural birds decreased aggression to a greater extent. Questions remain about the mechanisms regulating this behavioral response to manipulations of chest spotting. Nonetheless, our finding that urban and rural birds differ in response to the manipulation highlights the benefit of using experimental manipulations across urban and rural habitats to understand the behavioral changes that widespread urbanization causes in wildlife.
Data availability
The data and R-code to reproduce the analyses reported in the manuscript are provided as electronic supplementary materials with the manuscript.
References
Akçay Ç, Beecher MD (2020) Song sparrows do not discriminate between their own song and stranger song. Behav Process 178:104184
Akçay Ç, Tom ME, Campbell SE, Beecher MD (2013) Song type matching is an honest early threat signal in a hierarchical animal communication system. Proc R Soc B 280:20122517
Akçay Ç, Campbell SE, Beecher MD (2014) Individual differences affect honest signaling in a songbird. Proc R Soc B 281:20132496
Akçay Ç, Beck ML, Sewall KB (2020) Are signals of aggressive intent less honest in urban habitats? Behav Ecol 31:213–221
Anderson RC, Searcy WA, Peters S, Nowicki S (2008) Soft song in song sparrows: acoustic structure and implications for signal function. Ethology 114:662–676
Arcese P, Sogge MK, Marr AB, Patten MA (2020) Song sparrow (Melospiza melodia), version 1.0. In: Poole AF, Gill FB (eds) Birds of the world. Cornell Lab of Ornithology, Ithaca, NY, USA. https://doi.org/10.2173/bow.sonspa.01
ASAB, ABS (2020) Guidelines for the treatment of animals in behavioural research and teaching. Anim Behav 159:I–XI
Atwell JW, Cardoso GC, Whittaker DJ, Campbell-Nelson S, Robertson KW, Ketterson ED (2012) Boldness behavior and stress physiology in a novel urban environment suggest rapid correlated evolutionary adaptation. Behav Ecol 23:960–969
Atwell JW, Cardoso GC, Whittaker DJ, Price TD, Ketterson ED (2014) Hormonal, behavioral, and life-history traits exhibit correlated shifts in relation to population establishment in a novel environment. Am Nat 184:E147–E160
Bartoń K (2013) MuMIn: multi-model inference. R package version 1. https://cran.r-project.org/web/packages/MuMIn/index.html
Beck ML, Davies S, Sewall KB (2018) Urbanization alters the relationship between coloration and territorial aggression, but not hormones, in song sparrows. Anim Behav 142:119–128
Bonier F, Martin PR, Sheldon KS, Jensen JP, Foltz SL, Wingfield JC (2007) Sex-specific consequences of life in the city. Behav Ecol 18:121–129
Chaine AS, Lyon BE (2008) Intrasexual selection on multiple plumage ornaments in the lark bunting. Anim Behav 76:657–667
Chaine AS, Roth AM, Shizuka D, Lyon BE (2013) Experimental confirmation that avian plumage traits function as multiple status signals in winter contests. Anim Behav 86:409–415
Dale S, Slagsvold T (1996) Plumage coloration and conspicuousness in birds: experiments with the pied flycatcher. Auk 113:849–857
Davies S, Sewall KB (2016) Agonistic urban birds: elevated territorial aggression of urban song sparrows is individually consistent within a breeding period. Biol Lett 12:20160315
Davies S, Beck ML, Sewall KB (2018) Territorial aggression in urban and rural Song Sparrows is correlated with corticosterone but not testosterone. Horm Behav 98:8–15
Deviche P, Beouche-Helias B, Davies S, Gao S, Lane S, Valle S (2014) Regulation of plasma testosterone, corticosterone, and metabolites in response to stress, reproductive stage, and social challenges in a desert male songbird. Gen Comp Endocrinol 203:120–131
Dey CJ, Dale J, Quinn JS (2014) Manipulating the appearance of a badge of status causes changes in true badge expression. Proc R Soc B 281:20132680
Ducrest AL, Keller L, Roulin A (2008) Pleiotropy in the melanocortin system, coloration and behavioral syndromes. Trends Ecol Evol 23:502–510
Eikenaar C, Whitham M, Komdeur J, van der Velde M, Moore IT (2011) Testosterone, plumage colouration and extra-pair paternity in male North-American barn swallows. PLoS One 6:e23288
Elekonich MM (1999) Female song sparrow, Melospiza melodia, response to simulated conspecific and heterospecific intrusion across three seasons. Anim Behav 59:551–557
Elekonich MM, Wingfield JC (2000) Seasonality and hormonal control of territorial aggression in female song sparrows (Passeriformes: Emberizidae: Melospiza melodia). Ethology 106:493–510
Evans MR, Hatchwell BJ (1992) An experimental study of male adornment in the scarlet-tufted malachite sunbird: I. The role of pectoral tufts in territory defence. Behav Ecol Sociobiol 29:413–419
Evans J, Boudreau K, Hyman J (2010) Behavioural syndromes in urban and rural populations of song sparrows. Ethology 116:588–595
Fokidis HB, Orchinik M, Deviche P (2011) Context-specific territorial behavior in urban birds: no evidence for involvement of testosterone or corticosterone. Horm Behav 59:133–143
Foltz SL, Ross AE, Laing BT, Rock RP, Battle KE, Moore IT (2015) Get off my lawn: increased aggression in urban song sparrows is related to resource availability. Behav Ecol 26:1548–1557
Galván I, Alonso-Alvarez C (2008) An intracellular antioxidant determines the expression of a melanin-based signal in a bird. PLoS ONE 3:e3335
George EM, Rosvall KA (2022) Bidirectional relationships between testosterone and aggression: a critical analysis of four predictions. Integr Comp Biol 62:474–486
Giraudeau M, Toomey MB, Hutton P, McGraw KJ (2018) Expression of and choice for condition-dependent carotenoid-based color in an urbanizing context. Behav Ecol 29:1307–1315
Haller J (2014) The glucocorticoid/aggression relationship in animals and humans: an analysis sensitive to behavioral characteristics, glucocorticoid secretion patterns, and neural mechanisms. In: Miczek K, Meyer-Lindenberg A (eds) Neuroscience of aggression. Springer-Verlag, Berlin, pp 73–109
Hardman SI, Dalesman S (2018) Repeatability and degree of territorial aggression differs among urban and rural great tits (Parus major). Sci Rep 8:5042
Harrison XA, Donaldson L, Correa-Cano ME, Evans J, Fisher DN, Goodwin CED, Robinson BS, Hodgson DJ, Inger R (2018) A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 6:e4794
Hasegawa M, Arai E, Sato M, Sakai H (2017) Plasma testosterone levels increase with expression of male ornaments during mating, but not incubation, in Japanese barn swallows. Zool Sci 34:261–266
Johnstone RF, Grafen A (1993) Dishonesty and the handicap principle. Anim Behav 46:759–764
Juárez R, Chacón-Madrigal E, Sandoval L (2020) Urbanization has opposite effects on the territory size of two passerine birds. Avian Res 11:11
Kight CR, Swaddle JP (2015) Eastern bluebirds alter their song in response to anthropogenic changes in the acoustic environment. Integr Comp Biol 55:418–431
Kittilsen S, Schjolden J, Beitnes-Johansen I, Shaw JC, Pottinger TG, Sorensen C, Braastad BO, Bakken M, Overli O (2009) Melanin-based skin spots reflect stress responsiveness in salmonid fish. Horm Behav 56:292–298
Lane SJ, Sewall KB (2022) What about females? Urban female song sparrows elevate aggressive signaling compared to rural. Integr Comp Biol 62:487–495
Laubach ZM, Blumstein DT, Romero LM, Sampson G, Foufopoulos J (2013) Are white-crowned sparrow badges reliable signals? Behav Ecol Sociobiol 67:481–492
Lehtonen PK, Primmer CR, Laaksonen T (2009) Different traits affect gain of extrapair paternity and loss of paternity in the pied flycatcher, Ficedula hypoleuca. Anim Behav 77:1103–1110
Levin II, Fosdick BK, Tsunekage T, Aberle MA, Burns CMB, Hund AK, Safran RJ (2018) Experimental manipulation of a signal trait reveals complex phenotype-behaviour coordination. Sci Rep 8:15533
Ligon RA, McGraw KJ (2016) Social costs enforce honesty of a dynamic signal of motivation. Proc R Soc B 283:20161873
Marzluff JM (2001) Worldwide urbanization and its effects on birds. In: Marzluff JM, Bowman R, Donnelly R (eds) Avian ecology and conservation in an urbanizing world. Springer, US, pp 19–47
Maynard Smith J, Parker GA (1976) The logic of asymmetric contests. Anim Behav 24:159–175
Maynard Smith J, Price GR (1973) The logic of animal conflict. Nature 246:16–18
Mockford EJ, Marshall RC (2009) Effects of urban noise on song and response behaviour in great tits. Proc R Soc Lond B 276:2979–2985
Moser-Purdy C, MacDougall-Shackleton EA, Mennill DJ (2017) Enemies are not always dear: male song sparrows adjust dear enemy effect expression in response to female fertility. Anim Behav 126:17–22
Nelson RJ, Trainor BC (2007) Neural mechanisms of aggression. Nat Rev Neurosci 8:536–546
Partecke J, Schwabl I, Gwinner E (2006) Stress and the city: urbanization and its effects on the stress physiology in European Blackbirds. Ecology 87:1945–1952
Pinheiro J, Bates D, DebRoy S, Sarkar D, Heisterkamp S, Van Willigen B, Maintainer R (2017) Package ‘nlme’. Linear and nonlinear mixed effects models, version 3. https://cran.r-project.org/web/packages/nlme/index.html
Pryke SR, Lawes MJ, Andersson S (2001) Agonistic carotenoid signalling in male red-collared widowbirds: aggression related to the colour signal of both the territory owner and model intruder. Anim Behav 62:695–704
Pryke SR, Andersson S, Lawes MJ, Piper SE (2002) Carotenoid status signaling in captive and wild Red-collared Widowbirds: independent effects of badge size and color. Behav Ecol 13:622–631
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Rémy A, Grégoire A, Perret P, Doutrelant C (2010) Mediating male-male interactions: the role of the UV blue crest coloration in blue tits. Behav Ecol Sociobiol 64:1839–1847
Reudink MW, Marra PP, Boag PT, Ratcliffe LM (2009) Plumage coloration predicts paternity and polygyny in the American redstart. Anim Behav 77:495–501
Rick IP, Bakker TCM (2008) Males do not see only red: UV wavelengths and male territorial aggression in the three-spined stickleback (Gasterosteus aculeatus). Naturwissenschaften 95:631–638
Rios-Chelen AA, Lee GC, Patricelli GL (2015) Anthropogenic noise is associated with changes in acoustic but not visual signals in red-winged blackbirds. Behav Ecol Sociobiol 69:1139–1151
Rohwer S (1977) Status signaling in Harris sparrows: some experiments in deception. Behaviour 61:107–129
Rohwer S, Rohwer FC (1978) Status signaling in Harris’ sparrows: experimental deceptions achieved. Anim Behav 39:1110–1115
Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW (2017) Image J2: ImageJ for the next generatin of scientific image data. BMC Bioinformatics 18:529
Safran RJ, Neuman CR, McGraw KJ, Lovette IJ (2005) Dynamic paternity allocation as a function of male plumage color in barn swallows. Science 309:2210–2212
Safran RJ, Adelman JS, McGraw KJ, Hau M (2008) Sexual signal exaggeration affects physiological state in male barn swallows. Curr Biol 18:R461–R462
Saino N, Canova L, Costanzo A, Rubolini D, Roulin A, Møller AP (2013) Immune and stress responses covary with melanin-based coloration in the barn swallow. Evol Biol 40:521–531
Santos ESA, Scheck D, Nakagawa S (2011) Dominance and plumage traits: meta-analysis and metaregression analysis. Anim Behav 82:3–19
Searcy WA, Nowicki S (2005) The evolution of animal communication. Princeton University Press, Princeton
Searcy WA, McArthur PD, Peters SS, Marler P (1981) Response of male song and swamp sparrows to neighbour, stranger, and self songs. Behaviour 77:152–163
Searcy WA, Anderson RC, Nowicki S (2006) Bird song as a signal of aggressive intent. Behav Ecol Sociobiol 60:234–241
Searcy WA, Anderson RC, Nowicki S (2008) Is bird song a reliable signal of aggressive intent? A reply. Behav Ecol Sociobiol 62:1213–1216
Seress G, Lipovits Á, Bókony V, Czúni L (2014) Quantifying the urban gradient: a practical method for broad measurements. Landsc Urban Plan 131:42–50
Summers CH, Watt MJ, Ling TL, Forster GL, Carpenter RE, Korzan WJ, Lukkes JL, Øverli Ø (2005) Glucocorticoid interaction with aggression in non-mammalian vertebrates: reciprocal action. Eur J Pharmacol 526:21–35
Tannure-Nascimento IC, Nascimento FS, Zucchi R (2008) The look of royalty: visual and odour signals of reproductive status in a paper wasp. Proc R Soc Lond B 275:2555–2561
Theis A, Salzburger W, Egger B (2012) The function of anal fin egg-spots in the cichlid fish Astatotilapia burtoni. PLoS ONE 7:e29878
Tibbetts EA, Izzo A (2010) Social punishment of dishonest signalers caused by mismatch between signal and behavior. Curr Biol 20:1637–1640
van Dongen WFD, Mulder RA (2007) Relative importance of multiple plumage ornaments as status signals in Golden Whistlers (Pachycephala pectoralis). Behav Ecol Sociobiol 62:77–86
Vitousek MN, Zonana DM, Safran RJ (2014) An integrative view of the signaling phenotype: dynamic links between signals, physiology, behavior and social context. Curr Zool 60:739–754
Webster MS, Ligon RA, Leighton GM (2018) Social costs are an underappreciated force for honest signalling in animal aggregations. Anim Behav 143:167–176
Wingfield JC (1994) Regulation of territorial behavior in the sedentary song sparrow, Melospiza melodia morphna. Horm Behav 28:1–15
Wingfield JC, Lynn SE, Soma KK (2001) Avoiding the ‘costs’ of testosterone: ecological bases of hormone-behavior interactions. Brain Behav Evol 57:239–251
Acknowledgements
We would like to thank Sam Lane for his help in the field and Ben Vernasco for logistical support and the reviewers for their comments which has greatly improved the manuscript.
Funding
This work was supported by funding from Virginia Tech Global Change Center and Fralin Life Sciences Institute as well as funding from National Science Foundation grant IOS 2114288 to KBS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The research conducted here complied with the guidelines for the use of animals in behavioral research and teaching (ASAB/ABS 2020). All procedures in the study reported here were conducted under the appropriate State (Virginia Department of Game and Inland Fisheries permit #058685) and U.S. Federal permits (US Fish and Wildlife Service Banding Permit # 23818) and approved by the Virginia Tech Institutional Animal Care and Use Committee (protocol # 16–176).
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by K. McGraw.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Beck, M.L., Sewall, K.B. & Akҫay, Ҫ. Experimental manipulation of chest spotting alters territorial aggression in urban and rural song sparrows. Behav Ecol Sociobiol 77, 136 (2023). https://doi.org/10.1007/s00265-023-03396-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-023-03396-6