Abstract
To ensure effective acoustic communication, signals should reach receivers in the least distorted form possible. Animals use various short- and long-term strategies to avoid signal degradation and masking. However, we still have an insufficient understanding of how animals’ vocal behaviour is impacted by the vocalisations of other animals in their acoustic communities. We experimentally examined how two tropical, sedentary, territorial songbirds in Western Uganda—the scaly-breasted illadopsis (Illadopsis albipectus) and the green-backed camaroptera (Camaroptera brachyura)—modify their singing behaviour after the simulated appearance of new, unfamiliar acoustic competitors, whose songs vary in similarity to those of the species studied. We found that scaly-breasted illadopsis sang significantly less during the playback of songs of acoustically similar species than of acoustically different species or silence and avoided song overlapping with acoustically similar species but not with acoustically different species. Green-backed camaroptera sang significantly more during the playback of both acoustically similar and different simulated intruders than during the control containing silence, and patterns of overlap with the songs of both the acoustically similar and different species were random. Our results show that even a single-point noise source present within a territory can modify a bird’s singing behaviour. The new sound may affect species differently, depending in part on the level of acoustic similarity with the species’ song. To mitigate the effect of song masking, different species may use different strategies, such as temporal avoidance or signal redundancy. Studies examining the adaptive abilities of species in natural and modified habitats are needed to predict the consequences of changes in acoustic community structure.
Significance statement
To ensure effective communication, birds may use different strategies to avoid signal masking in common acoustic space, particularly in the complex acoustic environment of a tropical forest. While multiple studies have focused on responses to interference caused by anthropogenic noise, the effect of new individual species on the acoustic community structure has received little attention. We simulated intrusions by unfamiliar species with different levels of song similarity into the territories of two tropical songbird species. The appearance of new simulated acoustic intruders modified the birds’ singing behaviour, but the two study species responded differently. These results suggest that the level of acoustic similarity, as well as the species ecology, may affect the species response, which may be particularly important when predicting the effects of new species appearance as a result of changes in habitat and climate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
At any given location and time, multiple animal species are often heard vocalising. To ensure effective communication, acoustic signals should be adapted to the physical characteristics of the environment in which they are broadcast, so as to minimise attenuation and degradation (acoustic adaptation hypothesis; Morton 1975). Additionally, they should be produced in a frequency range and at a time when overlapping with other sounds present in the environment is minimal in order to avoid signal masking (acoustic partitioning hypothesis; Hödl 1977). That is, the evolution of acoustic signals is shaped in part by the combination of environmental conditions supporting the transmission of some signals and limiting others (Marten and Marler 1977), and by the sounds already present in the environment, resulting in variation across species in the frequency bands or time periods used for effective communication (Luther 2008). These factors can be regarded as opposite forces in signal evolution—convergence of signal parameters caused by acoustic adaptation and divergence of signal parameters caused by acoustic competition among vocalising animals.
Analyses of the acoustic structure of animal species assemblies have revealed several patterns of acoustic space partitioning, such as divergence of signal parameters (Chitnis et al. 2020) and vocalising in distinct frequency bands (Hödl 1977; Schmidt et al. 2013), at different times of day (Hart et al. 2015) or from different locations (Diwakar and Balakrishnan 2007; Luther 2009). However, past studies from different geographical regions and on different animal taxa have yielded inconsistent results, suggesting that acoustic signal partitioning takes place along multiple axes and can differ among species assemblies (Chek et al. 2003; Planqué and Slabbekoorn 2008; Luther 2009). Alternatively, convergent patterns of acoustic adaptation to local environments (Morton 1975) and the use of between-species social information may lead to the opposite pattern, in which ecologically and acoustically similar species become aggregated in space and time (Tobias et al. 2014). Irrespective of the similarity or divergence pattern, acoustic community structures can change dynamically and therefore may be an indicator of disturbance and as such be used as a tool in monitoring and conservation (Chhaya et al. 2021).
Animals can use several behavioural strategies to reduce the masking of their acoustic signals (Brumm and Slabbekoorn 2005). The first response to an acoustic competitor may be to increase one’s sound amplitude, so as to improve the sound’s signal-to-noise ratio (Brumm 2004; Hage et al. 2013). Other options include avoiding vocalising near the sound source (Goodwin and Shriver 2011), vocalising at different times of the day (Greenfield 1988), increasing one’s calling rate (Kaiser and Hammers 2009), singing longer songs (Ríos-Chelén et al. 2013), modifying intervals between vocalisations to avoid overlapping in time (Popp et al. 1985), adjusting signal redundancy (Brumm and Slater 2006), or modifying the frequencies of vocalisation to avoid overlapping in the frequency domain (Goodwin and Podos 2013; Hage et al. 2013; Zhao et al. 2018). The effectiveness of these strategies may depend on the acoustic characteristics of the competing sound, the individual species preferences, and prevailing environmental conditions.
Birds are one of the best models for studying communication strategies employed to avoid signal interference, because many species use songs to attract mates and defend territories (Catchpole and Slater 2008). However, most of the studies examining the effects of competing sounds on singing behaviour have focused on anthropogenic noise, which is less complex than biological noise, occupies lower frequencies, can have higher amplitudes, and may differently affect species singing low- or high-frequency songs (Goodwin and Shriver 2011). The effect of biological sounds has been examined in white-throated sparrows (Zonotrichia albicollis), with males decreasing their song durations and increasing their minimum song frequencies during spring peeper (Pseudacris crucifer) chorus and singing less intensively when other bird species were vocalising (Lenske and La 2014). Four common temperate forest bird species showed a strong tendency to stay quiet while other species were singing and to modify the intervals between their songs to avoid overlapping (Popp et al. 1985). Neotropical songbirds which shared their sound frequency range with nocturnal insects delayed their dawn chorus to avoid song masking (Stanley et al. 2016). Moreover, species with songs with similar structure partitioned signal space by singing from different places or at different times to minimise acoustic interference (Luther 2009). Thus, the strategies undertaken to avoid sound overlapping in birds seem to be species specific and environmentally variable. Generally speaking, more acoustically plastic species should have the edge over those with less song flexibility, both in the context of settling in new environments and in outcompeting acoustic intruders in natal environments. However, studies experimentally testing the effect of new acoustic competitors on the singing behaviour of natal species are still rare. To fully understand the complex mechanisms shaping acoustic community structure, we need to first know how various species modify their singing behaviour during interactions with other vocalising animals. Such information will be helpful in predicting the effect of new species appearance on acoustic community structure.
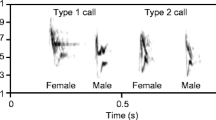
In this study, we experimentally examined how two tropical songbirds, the scaly-breasted illadopsis (Illadopsis albipectus) and the green-backed camaroptera (Camaroptera brachyura), respond to the appearance of songs of unfamiliar acoustic competitors representing different levels of acoustic similarity to their songs. Both species are monogamous, without sexual dimorphism. They live a sedentary life and occupy small territories, making them appropriate for our type of study, as it is more likely that the appearance of a new acoustic competitor would affect their behaviour. Depending on the region, they breed year round or in specific periods limited by rainfall, but in both species, the males defend territories and sing throughout the year (Collar and Robson 2020; Ryan 2020). The scaly-breasted illadopsis is a forest specialist of Central Africa (distribution range ca 2 mln km2), inhabiting primary forest, seasonal swamp forest, and transitional forest with dense ground cover and treefall gaps. The species song is narrow in frequency band and consists of an introductory note and 2–3 ascending whistling notes repeated every 5–35 s (Collar and Robson 2020) (Fig. 1, Supplementary material S1SongSimilarity). The green-backed camaroptera is a habitat generalist of sub-Saharan Africa (distribution range ca 25 mln km2), found in a wide spectrum of forest understory, forest clearings, forest edge, thickets, bush clumps, and well-wooded gardens (Ryan 2020). The male song is loud with a wide frequency range, containing the same type of syllable repeated multiple times (Fig. 1, S1SongSimilarity).
The experimental procedure: a examples of songs of green-backed camaroptera with the acoustically similar (marsh tit) and acoustically different species (Eurasian treecreeper), b examples of songs of scaly-breasted illadopsis with the acoustically similar (Papuan treecreeper) and acoustically different species (Eurasian treecreeper), c time sequence for an experimental trial. Each trial lasted 20 min and contained 258 s of playback (repeated 3 s song plus 3 s\ silence) and 42 s of additional silence, all repeated four times, alternately from two speakers. Each bird was exposed—in random order—to three 20-min trials, with each representing a distinct treatment: (1) silence, (2) sound samples of acoustically similar species, and (3) sound samples of acoustically different species
To assess these species’ potential strategies for song masking avoidance, we here broadcast sounds of acoustically similar and different bird species and observed how study subjects modified their singing behaviour accordingly. We expected that the appearance of acoustically similar intruders would compel the tested individuals to change their singing rates or song interval durations to avoid overlapping. We also expected birds’ responses to be species specific, due to species differences in song structure and frequency as well as different histories of acoustic ecological adaptation.
Materials and methods
Study site
We conducted our study in Kibale National Park, located in Western Uganda. The 766-km2 park protects a mixture of moist evergreen rainforest, dry tropical forest, woodland, and savanna crossed by swamps and streams. Mean annual temperature oscillates around 20 °C. Depending on the region, rainfall ranges from 1500 to 1700 mm and falls in two wet seasons: from March to May and from September to November (Struhsaker 1997; Chapman et al. 2010). Kibale National Park is home to 13 primate species (the highest diversity and abundance of primates in Africa), more than 375 bird species, and 350 tree species.
Our study site was located in the central-west area of the park, near the Makerere University Biological Field Station (N 0.56136°, E 30.35778°; altitude ranges from 1500 to 1600 m a.s.l.). This part of the park is covered by both primary and secondary evergreen rainforest, with many trees exceeding 50 m in height.
Playback preparation
Experimental sound samples were prepared using the Avisoft SASLab Pro 5.2.12 software (https://www.avisoft.com). We matched each of the study species with one acoustically similar and one acoustically different “intruder” species. As a control, we used silence. Acoustic similarity was based on the frequency range and structure of the species’ songs (see S1SongSimilarity for more details). The geographical distribution range of chosen intruder species did not overlap with the distribution range of the study species; thus, the songs of acoustically similar and different species were unfamiliar to tested birds. As acoustically similar species, we used the Papuan treecreeper (Cormobates placens) (sedentary species restricted to New Guinea) for the scaly-breasted illadopsis and the marsh tit (Poecile palustris) (short-distance migrant found in Europe and Asia) for the green-backed camaroptera. For both tested species, we used the Eurasian treecreeper (Certhia familiaris) (short-distance migrant found in Europe and Asia) as an acoustically different species. For each simulated intruder species, we selected three high-quality recordings of songs belonging to three different individuals, available at a public database (www.xeno-canto.org). We shortened the song duration to 3 s, removed all background noise, and saved each file in 16 bit/44.1 kHz wav format. These modified songs were used to prepare the final playback sound sample, which contained the tested song (3 s in duration) plus 3 s of silence, repeated 43 times (258 s of playback, followed by an additional 42 s of silence; hereafter referred to as the “5-min phase” of our experiment). A single playback trial lasted 20 min and contained the 5-min phase broadcast four times alternately from two speakers, imitating an intruder’s movement. In our tests of different individuals, we randomly assigned sound samples of acoustically similar and different species (three individuals of each species; we used nine unique combinations of acoustically similar and acoustically different species). Each combination was used to test two birds, and in the case of green-backed camaroptera, one combination was also used to test three birds. The sound samples of tested bird species and corresponding acoustically similar and different species can be found in Fig. 1 and S1SongSimilarity.
Experiment design
We conducted experiments in the dry season, from 8th to 25th June 2022, corresponding to the breeding period for both species (Collar and Robson 2020; Ryan 2020). Following our observations of the daily patterns of singing activity of the study species, we started the experiments involving scaly-breasted illadopsis earlier in the morning (from 06:12 to 09:42) than those involving green-backed camaroptera (from 07:49 to 11:19; sunrise between 06:52 and 06:56, local time). On the day before each experiment, we spent ca 30 min within the bird’s territory to determine the locations from which the tested individual sang most often. During the experiment, we placed two speakers (Ultimate Ears Boom 2) spaced ca 25 m from each other on tree branches (ca 5 m above ground level). We tried to place the speakers in the middle of the territory, near the points from which the tested individual sang the most. The speakers were connected via Bluetooth to an iPhone 7. We standardised the sound pressure level (90 ± 1 dB measured at 1 m using UNI-T UT351 sound level meter) of each playback song sample. Each tested individual was exposed to its three 20-min experimental trials such that the treatments were arranged in random order. We started each trial only when a tested individual had started to sing spontaneously. We started the next trial more than 5 min after the end of the previous one (the bird must sing before each treatment), without moving the speakers. All trials on the tested individual were conducted during a given day, except for three scaly-breasted illadopsis individuals; for two of these birds, we conducted two trials on one day and the third trial on the following day, and for the third bird, one trial was performed on the first day and two on the following day.
During the experiment, the acoustic responses of tested individuals were recorded by a field observer (MB) using a Marantz PMD661 recorder connected to a Sennheiser ME67 shotgun microphone with a K6 powering module (wav file, 48 kHz/16-bit sampling, mono recording). The observer also noted the position of the bird in relation to the speaker and any changes in behaviour. Overall, we tested 18 males of scaly-breasted illadopsis and 19 males of green-backed camaroptera. It was not possible to record data blind because our study involved focal animals in the field.
Acoustic analysis
Acoustic analyses of subjects’ vocal behaviour were conducted using the Raven Pro 1.6.4 software (window type = Hamming, frame size = 1024, overlap = 75%). Each song of a tested bird was marked and assessed within two criteria: (1) whether it was produced during the 258 s of playback or during the 42 s of silent intervals that followed and (2) whether the song overlapped with the experimental sound sample, defined as when > 50% of the song duration of the tested bird coincided with a song from the playback file.
Statistical analysis
To examine whether birds change their singing behaviour in response to the simulated appearance of different acoustic competitors, we ran three separate generalised linear mixed models (GLMMs) for each species.
To test the effect of different acoustic competitors on singing rate, we specified territory ID as a subject on which we conducted repeated measurements. The repeated measurement contained three experimental treatments in which we nested four 5-min phases of treatment. As a dependent variable, we used the number of songs produced in each 5-min phase of each trial. We included three fixed effects: treatment type (acoustically similar species, acoustically different species, silence), order of trials and order of phases within a trial, and one random effect—territory ID. Data were fitted by a negative binomial distribution with a log link function.
To test whether the first reaction of a bird towards the playback depends on the type of simulated acoustic competitor, we compared the bird’s singing rate in the 30 s before versus 30 s after the first song in the first 5-min phase of each trial. In the GLMM, we nested treatment order and period (30 s before or 30 s after the first sound sample song) within the territory ID. We specified the number of songs produced in each 30-s period as a dependent variable; the treatment, period, and crossed effect of treatment and period as fixed effects; and territory ID as a random effect. Data were fitted by a negative binomial distribution with a log link function.
We also examined whether the proportion of songs which overlapped playback songs depends on the type of acoustic competitor. In our experiment, the duration of a playback song and silence between playback songs was identical (3 s); thus, the probability of overlapping versus not overlapping the playback song by the tested individual was the same. Because the tested birds did not sing in every 5-min phase of the experiment, we summed the number of songs produced during the whole trial and compared the proportion of songs which overlapped and did not overlap the playback (songs produced in the 42 silence periods between playback phases were excluded from this analysis). In the model, we specified territory ID as a subject and treatment as a repeated measure. As a dependent variable, we used the proportion of overlapped songs to all songs sung by the tested bird. We specified two fixed effects, treatment and treatment order, and one random effect, territory ID. Data were fitted by a normal distribution with an identity link function.
All statistical analyses were conducted using the IBM SPSS Statistics 28.0.1.0 software. All p-values are two tailed. Raw data can be found in Supplementary material S2Dataset.
Results
Singing rate
In each scaly-breasted illadopsis territory, we recorded on average 315 (SD = 101.6; from 121 to 475) songs. Tested birds sang during each treatment (on average 105 ± 47.6; from 17 to 210 songs) but not in every 5-min phase of the experiment. We found significant effects of treatment (F2,208 = 9.844, p < 0.001), order of treatments (F2,208 = 3.300, p = 0.039) and order of 5-min phase within trials (F3,208 = 7.478, p < 0.001) on the number of songs produced. Scaly-breasted illadopsis sang significantly fewer songs during the playback of acoustically similar species than during the playback of acoustically different species or silence and decreased singing rate both in succeeding treatments and repeated phases within trials (Fig. 2, Table 1).
Differences in the number of songs sung by a scaly-breasted illadopsis and b green-backed camaroptera during the three experimental treatments. Each trial lasted 20 min and contained four 5-min phases. Each phase contained 258 s of playback (3-s song broadcasted every 3 s) and 42 s of silence at the end. Mean number (± SE) of songs per 5-min phase is given
For the green-backed camaroptera, we recorded on average 378 (SD = 146.1; from 140 to 645) songs. Tested birds sang 126 ± 69.3 (from 0 to 281) songs on average per treatment. We found a significant effect of treatment (F2,220 = 7.918, p < 0.001) but no effects of treatment order (F2,220 = 0.390, p = 0.678) or order of 5-min phases within trials (F3,220 = 2.183, p = 0.091) on the number of songs produced. Tested birds sang significantly less intensively during silence than during playbacks of both acoustically similar and different species. However, the difference in singing rate between treatments of acoustically similar and different species was not statistically significant (Fig. 2, Table 1).
First reaction
The number of songs produced by scaly-breasted illadopsis was significantly higher in the 30 s before than in the 30 s after the first song of the playback (F1,102 = 10.091, p = 0.002) but did not differ among treatments (F2,102 = 2.537, p = 0.084). However, we found a significant interaction between treatment and time period (F2,102 = 5.566, p = 0.005), suggesting that the proportion of songs produced in the 30 s before and 30 s after the first song varied depending on the treatment. Scaly-breasted illadopsis significantly decreased its singing rate in the 30 s after the first song of acoustically similar species playback, whereas during the playback of acoustically different species or during the silence, this difference was not statistically significant (Fig. 3, Table 2).
The number of songs produced by green-backed camaroptera varied significantly between treatments (F2,108 = 3.884, p = 0.023) and periods of time (F1,108 = 3.884, p < 0.001). We also detected an interaction between treatment and period of time (F2,108 = 9.188, p < 0.001). Green-backed camaroptera produced significantly fewer songs after the first song of a playback than before the playback. Fewer songs were also produced during silence than during the playback of similar species. Green-backed camaroptera decreased their singing rate after the first song significantly more during the playback of similar species than either different species or silence (Fig. 3, Table 2).
Song overlapping
In scaly-breasted illadopsis, we found significant differences across treatment in the tendency to overlap playback songs (F1,33 = 188.935, p < 0.001) and no effect of treatment order (F1,33 = 1.897, p = 0.178). The playback of acoustically similar species overlapped with an average of 29% (± 8.4%) of the time, while the playback of acoustically different species overlapped with an average of 61% (± 7.6%) of the time (Fig. 4, Table 3).
Proportion of songs (mean value ± SE) of a scaly-breasted illadopsis and b green-backed camaroptera which overlapped playback song relative to the total number of songs the bird sang during the treatment. Graph is based on four 258-s phases in which playback song was broadcasted in each trial. Duration of playback song and interval between songs was equal (3 s); therefore, the probability of singing during silence and playback song was the same
In green-backed camaroptera, we found no significant effects of treatment on the tendency to overlap playback songs (F1,35 = 0.667, p = 0.420) or of treatment order (F1,35 = 0.738, p = 0.738). The playback of acoustically similar species overlapped with an average of 49% (± 7.5%) of the time, while the playback of acoustically different species overlapped with an average of 50% (± 4.7%) of the time (Fig. 4, Table 3).
Discussion
Our study design aimed to simulate the appearance of new and unfamiliar acoustic competitors in our tested species’ natal environment. The results experimentally confirmed different species-specific strategies for song masking avoidance by tropical songbirds in their natural habitats, free of anthropogenic noise. The scaly-breasted illadopsis was found to sing fewer songs during the playback of acoustically similar species than during the playback of an acoustically different one or the silent control treatment. Such behaviour is consistent with the temporal acoustic partitioning hypothesis, which predicts that animals will modify their vocal activity and sing more often when their song frequency band is free of other sounds (Hödl 1977). Such mechanisms have been reported for tropical birds avoiding the noise of cicadas (Hart et al. 2015), temperate forest birds avoiding song overlapping with other bird species (Popp et al. 1985), city birds avoiding morning peaks of anthropogenic noise (Shannon et al. 2016), or noise generated by planes (Gallardo Cruz et al. 2021). Moreover, singing rate decreased in successive trials and over the course of individual trials. Such results are not surprising, since many bird species sing more intensively in the early morning rather than later (Gil and Llusia 2020), and the duration of song bouts is limited, interchanging with phases of silence (Catchpole and Slater 2008).
Over short time frames, scaly-breasted illadopsis were seen to adjust their singing rate to the repeated pattern of playback containing 3 s of song and 3 s of silence. During the playback of acoustically different species, tested birds overlapped playback songs over 60% of the time. Yet, when the playback of acoustically similar species was broadcast, the test birds overlapped playback songs just 29% of the time. Thus, avoiding song overlapping with acoustically similar species and singing in the silent intervals between songs, while ignoring acoustically different species, can be considered another strategy for minimising acoustic signal masking (Brumm and Slater 2006; Yang et al. 2014). Moreover, modifications of singing behaviour only in response to acoustically similar species suggest that the effect of the appearance of new sounds in the environment—regardless of their source—on the species will depend on the level of acoustic similarity between the new sound and the species vocalisations. Therefore, the same introduced noise should have different effects on different species. We note that while our study species were unfamiliar with both the acoustically similar and acoustically different species, due to geographic isolation, we did not control for varying phylogenetic distances among simulated competitors and the species tested. Beyond the potential effect of song similarity or phylogenetic affinity on signal masking, birds’ responses to the songs of newly arrived species might also be influenced by the perception of more acoustically similar signals as belonging to a potential competitor sharing a more similar ecological niche (similar size, type of environment; Morton 1975; Mikula et al. 2021) as compared to differently singing species.
As we saw with scaly-breasted illadopsis, green-backed camaroptera decreased their singing rates in the first 30 s of playback significantly more when broadcasting an acoustically similar species than when broadcasting an acoustically different one or silence, suggesting that both species differentiated between these two types of experimental sounds. However, when we looked across experimental treatments, we found species-distinct patterns of response to various kinds of simulated acoustic intruders. Green-backed camaroptera sang significantly more intensively during the playbacks of both acoustically similar and different intruders than during the silence. In addition, we did not observe a significant decrease in singing rate in successive treatments or over the course of treatment phases, which could be explained by the species having a more regular singing activity pattern throughout the day and producing more but shorter song bouts. When we compared the proportion of songs of tested birds which avoided and which overlapped the playback songs, in both types of intruders, we observed almost perfect random distributions. Such behaviour suggests that, regardless of the type of noise, green-backed camaroptera tried to mask the sounds of intruders by increasing the singing rate. This is consistent with the signal redundancy hypothesis, which predicts that reprising the same information will increase the chance of it reaching the receiver (Shannon and Weaver 1949). In our study, we did not attempt to test another possible strategy for mitigating the effect of noise—improving the signal-to-noise ratio by increasing the song amplitude (Brumm 2004).
The observed species-specific response towards acoustically similar and different species might be explained in part by species differences in song structure. The song of scaly-breasted illadopsis contains 2–3 ascending whistling notes produced in a very narrow frequency band (Fig. 1). This type of acoustic signal can be easily masked by sounds covering the same frequency band, as in the song of Papuan treecreepers that we used here (Fig. 1). It thus makes sense that scaly-breasted illadopsis would avoid singing when an acoustically similar species also vocalised, adjusting their singing rate to fit the gaps between the songs of similar species while effectively ignoring the vocalisations of acoustically different species which do not cover the frequency band. In contrast, green-backed camaropteras produce short, broad-band frequency notes repeated multiple times in a single song (Fig. 1). This type of redundant signal is resistant to masking by biological sounds, and the chances that all notes sung by green-backed camaroptera would be masked by a song similar in structure, such as that of a marsh tit (Fig. 1), are marginal. It thus makes sense that green-backed camaroptera would sing as often during the playback of acoustically similar and acoustically different intruders and also did not avoid song overlapping by the playback song. What was surprising was that green-backed camaroptera sang significantly more during the playback of both types of intruders than during the silent control playback, thus singing more in potentially the most unfavourable conditions for signal transmission. Similar patterns have been observed in canaries (Serinus canaria) examined in aviaries, where males were seen to start singing during bursts of noise rather than during silent gaps (Goto et al. 2023), or in the wild population of serins (Serinus serinus) in which males sang more in areas with noise pollution (Díaz et al. 2011). Such behaviour supports the redundancy of signal hypothesis, which assumes that in a noisy environment, the same information should be sent more times in order to reach the receiver (Shannon and Weaver 1949). Alternatively, singing by other species may be a cue of optimal conditions for vocalising, because of low predation risk (Møller 1992).
Scaly-breasted illadopsis and green-backed camaroptera differ not just in their songs but also in their habitat specialisation (primary forests vs. forests, woodlands, and thickets respectively) and in their distribution range (2 mln vs. 25 mln km2; respectively; Collar and Robson 2020; Ryan 2020). According to the acoustic niche hypothesis (Krause 1993), in mature ecosystems, we are more likely to observe highly specialised species that should strongly avoid acoustic competition by singing in unique and narrow frequency bandwidths or at different times. In contrast, more open or disturbed ecosystems should contain acoustic generalist species, with a less organised acoustic community structure producing songs with wide frequency bands without restrictions on the time of day. Our results suggest that acoustic specialists and acoustic generalists may also respond differently to new sources of noise in their natal environments and may be variously affected by new acoustic competitors. However, more studies are needed to reach more general conclusions.
In conclusion, our study showed that a single-point noise source, in the form of simulated competing species, modified the singing behaviour of two tropical, sedentary songbird species. The responses towards acoustically similar and different intruders were species specific, suggesting that novel sounds in an environment can affect different species in different ways. Scaly-breasted illadopsis on the one hand avoided singing during the playback of acoustically similar species and modified singing rate to minimise song overlapping by acoustically similar species, indicating the use of temporal avoidance strategy to minimise signal masking. Green-backed camaroptera, on the other hand, sang significantly more during the playback of both acoustically similar and different intruders and did not avoid song overlapping by both playback types. Such outcomes are consistent with the signal redundancy hypothesis. The observed species-specific responses towards various kinds of intruders might be explained by differences in song structure and acoustic specialisation. Further studies examining the adaptation abilities of various species to new acoustic conditions—both in natural as well as modified habitats—are needed, to predict the effects of ongoing changes in acoustic community structure on the effectiveness of acoustic communication. This is especially important as habitat destruction and climate change lead to new, previously allopatric species coming into contact with each other more than in previous years and given that it is unknown how natal species will react to novel community members.
Data availability
All data generated and analysed during this study are included in this published article as a supplementary file.
References
Brumm H (2004) The impact of environmental noise on song amplitude in a territorial bird. J Anim Ecol 73:434–440
Brumm H, Slabbekoorn H (2005) Acoustic communication in noise. Adv Stud Behav 35:151–209
Brumm H, Slater PJB (2006) Ambient noise, motor fatigue, and serial redundancy in chaffinch song. Behav Ecol Sociobiol 60:475–481
Catchpole CK, Slater PJB (2008) Bird song. Biological themes and variation, 2nd edn. Cambridge University Press, Cambridge
Chapman CA, Chapman LJ, Jacob AL, Rothman JM, Omeja P, Reyna-Hurtado R, Hartter J, Lawes MJ (2010) Tropical tree community shifts: implications for wildlife conservation. Biol Conserv 143:366–374
Chek AA, Bogart JP, Lougheed SC (2003) Mating signal partitioning in multi-species assemblages: a null model test using frogs. Ecol Lett 6:235–247
Chhaya V, Lahiri S, Jagan MA, Mohan R, Pathaw NA, Krishnan A (2021) Community bioacoustics: studying acoustic community structure for ecological and conservation insights. Front Ecol Evol 9:706445
Chitnis SS, Rajan S, Krishnan A (2020) Sympatric wren-warblers partition acoustic signal space and song perch height. Behav Ecol 31:559–567
Collar N, Robson C (2020) Scaly-breasted Illadopsis (Illadopsisalbipectus). In: del Hoyo J, Elliott A, Sargatal J, Christie D, de Juana E (eds) Birds of the world. Cornell Lab of Ornithology, Ithaca, NY. https://doi.org/10.2173/bow.scbill1.01
Díaz M, Parra A, Gallardo C (2011) Serins respond to anthropogenic noise by increasing vocal activity. Behav Ecol 22:332–336
Diwakar S, Balakrishnan R (2007) Vertical stratification in an acoustically communicating ensiferan assemblage of a tropical evergreen forest in southern India. J Trop Ecol 23:479–486
Gallardo Cruz KV, Paxton KL, Hart PJ (2021) Temporal changes in songbird vocalizations associated with helicopter noise in Hawai’i’s protected natural areas. Landsc Ecol 36:829–843
Gil D, Llusia D (2020) The bird dawn chorus revisited. In: Aubin T, Mathevon N (eds) Coding strategies in vertebrate acoustic communication. Springer, Cham, pp 45–90
Goodwin SE, Podos J (2013) Shift of song frequencies in response to masking tones. Anim Behav 85:435–440
Goodwin SE, Shriver WG (2011) Effects of traffic noise on occupancy patterns of forest birds. Conserv Biol 25:406–411
Goto H, de Framond L, Leitner S, Brumm H (2023) Bursts of white noise trigger song in domestic Canaries. J Ornithol. https://doi.org/10.1007/s10336-023-02070-y
Greenfield MD (1988) Interspecific acoustic interactions among katydids Neoconocephalus: inhibition-induced shifts in diel periodicity. Anim Behav 36:684–695
Hage SR, Jiang T, Berquist SW, Feng J, Metzner W (2013) Ambient noise induces independent shifts in call frequency and amplitude within the Lombard effect in echolocating bats. P Natl Acad Sci USA 110:4063–4068
Hart PJ, Hall R, Ray W, Beck A, Zook J (2015) Cicadas impact bird communication in a noisy tropical rainforest. Behav Ecol 26:839–842
Hödl W (1977) Call differences and calling site segregation in anuran species from central amazonian floating meadows. Oecologia 28:351–363
Kaiser K, Hammers JL (2009) The effect of anthropogenic noise on male advertisement call rate in the neotropical treefrog, Dendropsophus triangulum. Behaviour 146:1053–1069
Krause BL (1993) The niche hypothesis: a virtual symphony of animal sounds, the origins of musical expression and the health of habitats. Soundscape Newslett 6:6–10
Lenske AK, La VT (2014) White-throated sparrows alter songs differentially in response to chorusing anurans and other background noise. Behav Process 105:28–35
Luther DA (2008) Signaller: receiver coordination and the timing of communication in Amazonian birds. Biol Lett 4:651–654
Luther D (2009) The influence of the acoustic community on songs of birds in a neotropical rain forest. Behav Ecol 20:864–871
Marten K, Marler P (1977) Sound transmission and its significance for animal vocalization. Behav Ecol Sociobiol 2:271–290
Mikula P, Valcu M, Brumm H, Bulla M, Forstmeier W, Petrusková T, Kempenaers B, Albrecht T (2021) A global analysis of song frequency in passerines provides no support for the acoustic adaptation hypothesis but suggests a role for sexual selection. Ecol Lett 24:477–486
Møller AP (1992) Interspecific response to playback of bird song. Ethology 90:315–320
Morton ES (1975) Ecological sources of selection on avian sounds. Am Nat 109:17–34
Planqué R, Slabbekoorn H (2008) Spectral overlap in songs and temporal avoidance in a peruvian bird assemblage. Ethology 114:262–271
Popp JW, Ficken RW, Reinartz JA (1985) Short-term temporal avoidance of interspecific acoustic interference among forest birds. Auk 102:744–748
Ríos-Chelén AA, Quirós-Guerrero E, Gil D, Macías Garcia C (2013) Dealing with urban noise: vermilion flycatchers sing longer songs in noisier territories. Behav Ecol Sociobiol 67:145–152
Ryan P (2020) Green-backed Camaroptera (Camaroptera brachyura), version 1.0. In: del Hoyo J, Elliott A, Sargatal J, Christie D, de Juana E (eds) Birds of the world. Cornell Lab of Ornithology, Ithaca
Schmidt AKD, Römer H, Riede K (2013) Spectral niche segregation and community organization in a tropical cricket assemblage. Behav Ecol 24:470–480
Shannon CE, Weaver W (1949) The mathematical theory of communication. The University of Illinois Press, Urbana
Shannon G, McKenna MF, Angeloni LM et al (2016) A synthesis of two decades of research documenting the effects of noise on wildlife. Biol Rev 91:982–1005
Stanley CQ, Walter MH, Venkatraman MX, Wilkinson GS (2016) Insect noise avoidance in the dawn chorus of Neotropical birds. Anim Behav 112:255–265
Struhsaker TT (1997) Ecology of an African Rain Forest. University Press of Florida, Gainesville
Tobias JA, Planqué R, Cram DL, Seddon AN (2014) Species interactions and the structure of complex communication networks. P Natl Acad Sci USA 111:1020–1025
Yang XJ, Ma XR, Slabbekoorn H (2014) Timing vocal behaviour: Experimental evidence for song overlap avoidance in Eurasian wrens. Behav Process 103:84–90
Zhao L, Sun X, Chen Q, Yang Y, Wang J, Ran J, Brauth SE, Tang Y, Cui J (2018) Males increase call frequency, not intensity, in response to noise, revealing no Lombard effect in the little torrent frog. Ecol Evol 8:11733–11741
Acknowledgements
The authors would like to thank the Uganda Wildlife Authority (COD/96/05), Uganda National Council for Science and Technology (NS302ES), and the personnel at the Makerere University Biological Field Station in Kanyawara for permission to work in Kibale National Park. Special thanks to Paul Mugisha for his invaluable assistance in the field and to Innocent Kato for help in logistics. We would like to thank editor and two anonymous reviewers for their helpful comments and suggestions on the initial version of the manuscript.
Funding
This work was financially supported by the National Science Centre, Poland (Grant No 2019/35/D/NZ8/04416).
Author information
Authors and Affiliations
Contributions
MB, AS, and ES conceived and developed the idea of the study. MB conducted playback experiment and bioacoustic and statistical analyses and wrote the first draft of the manuscript. All authors contributed to the interpretation of results. All authors contributed substantially to revision.
Corresponding author
Ethics declarations
Ethics approval
Our study was noninvasive and did not require ethics committee approval. All applicable national and institutional guidelines for the use of animals were followed. Our study was approved by the Uganda Wildlife Authority (COD/96/05) and Uganda National Council for Science and Technology (NS302ES).
Competing interests
The authors declare no competing interests.
Additional information
Communicated by J. Podos
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Budka, M., Staniewicz, A. & Sokołowska, E. Interspecific avoidance of song overlap in tropical songbirds: species-specific responses to acoustically similar and different intruders. Behav Ecol Sociobiol 77, 79 (2023). https://doi.org/10.1007/s00265-023-03356-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-023-03356-0