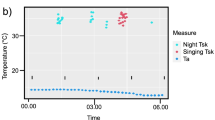
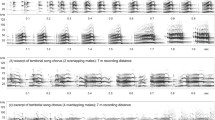
Abstract
Singing to create and defend territory boundaries is common among birds but rare in mammals. The African heart-nosed bat, Cardioderma cor, is hypothesized to use loud, low-frequency songs to reestablish foraging territories nightly. Territoriality can be defined ecologically, whereby an individual exclusively uses an area repeatedly, and behaviorally, through defense of an area. C. cor males sing on tightly abutting, exclusive areas nightly, which they return to throughout the season and sometimes across seasons. C. cor meets the ecological determinants of territoriality, but assessments of the use of song to maintain territories are lacking. We explore the singing behavior in this species by conducting song playback experiments within the borders of singing areas of 10 target individuals, with echolocation playbacks conducted as a control. In addition, we further explore the influence of song metrics on the behavioral response. Song playbacks prompted investigative and aggressive behavior, including passing by, approaching, and in one case, attacking the speaker, whereas echolocation did not. Additional post hoc analyses suggested that major song parameters, including song length, syllable frequency, intersyllable interval, and the number of double syllables comprising song stimuli influenced the level of response. For five bats we assessed whether their songs changed in response to the playback, and found that they sang faster, lower-frequency songs. These results are consistent with observations in other territorial animals including birds and gibbons, and provide a basis for further exploration of the territory defense hypothesis in the heart-nosed bat. We conclude that C. cor song features likely play an important role in mediating behavioral interactions within signaling networks of foraging bats.
Significance statement
It has been hypothesized that birdsong first evolved in support of territorial defense because it offered a cost-effective alternative to patrolling large spaces by flight. Singing-like behaviors have also been documented in several species of bats but never as a tool for maintaining foraging territories. However, evidence of foraging territoriality is scarce for bats, likely due to technical challenges associated with documenting such behaviors for a small, flying animal that may travel large distances at night. Here, we show for the first time that a bat responds to conspecific songs in a manner strikingly similar to many songbirds, providing support from outside songbirds for the hypothesis that territorial defense is a key selective pressure for singing in small, flying animals. This work provides the important basis for continuing to explore the role of singing, including song variability, in natural bat behavior outside of the roost.






Similar content being viewed by others
References
Abdi H (2003) Partial least squares (PLS) regression. In: Lewis-Beck M, Bryman A, Futing T (eds) Encylopedia of social sciences research methods. SAGE Publications, Thousand Oaks, CA, pp 792–795
Akçay C, Tom ME, Holmes D, Campbell SE, Beecher MD (2011) Sing softly and carry a big stick: signals of aggressive intent in the song sparrow. Anim Behav 82:377–382
Akçay C, Tom ME, Campbell SE, Beecher MD (2013) Song type matching is an honest early threat signal in a hierarchical animal communication system. Proc R Soc B 280:20122517
Bastian A, Schmidt S (2008) Affect cues in vocalizations of the bat, Megaderma lyra, during agonistic interactions. J Acoust Soc Am 124:598–608
Bastian A, Schmidt S (2009) Individual communication affects ritualized courtship: a case study in the bat (Megaderma lyra). In: Abstracts 31th International Ethological Conference, Rennes, France, p 140
Beecher MD, Campbell ES, Nordby JC (1998) The cognitive ecology of song communication and song learning in the song sparrow. In: Dukas R (ed) Cognitive ecology: the evolutionary ecology of information processing and decision making. University of Chicago Press, Chicago, pp 175–196
Behr O, von Helversen O (2004) Bat serenades–complex courtship songs of the sac-winged bat (Saccopteryx bilineata). Behav Ecol Sociobiol 56:106–115
Behr O, von Helversen O, Heckel G, Nagy M, Voigt CC, Mayer F (2006) Territorial songs indicate male quality in the sac-winged bat Saccopteryx bilineata (Chiroptera, Emballonuridae). Behav Ecol 17:810–817
Behr O, Knörnschild M, von Helversen O (2009) Territorial counter-singing in male sac-winged bats (Saccoperyx bilineata): low-frequency songs trigger a stronger response. Behav Ecol Sociobiol 63:433–442
Bohn KM, Schmidt-French B, Ma ST, Pollak GD (2008) Syllable acoustics, temporal patterns, and call composition vary with behavioral context in Mexican free-tailed bats. J Acoust Soc Am 124:1838–1848
Bohn KM, Smarsh GC, Smotherman M (2013) Social context evokes rapid changes in bat song syntax. Anim Behav 85:1485–1491
Bradbury JW, Vehrencamp SL (2011) Principles of animal communication, 2nd edn. Sinauer Associates Inc., Massachusetts, pp 397–462
Brockelman WY (2009) Ecology and the social systems of gibbons. In: Lappan S, Whittaker D (eds) The gibbons: new perspectives on small ape socioecology and population biology. Springer, New York, pp 217–223
Burt JM, Bard SC, Campbell ES, Beecher MD (2002) Alternative forms of song matching in song sparrows. Anim Behav 63:1143–1151
Byers BE, Akresh ME, King DI (2016) Song and male quality in prairie warblers. Ethology 122:1–11
Cardoso GC (2014) Studying the silent side of birdsong. BMC Biol 12:62
Carter GG, Wilkinson GS (2016) Common vampire bat contact calls attract past food-sharing partners. Anim Behav 116:45–51
Catchpole CK, Slater PJB (2008) Bird song: biological themes and variations, 2nd edn. Cambridge University Press, Cambridge
Catchpole C, Leisler B, Dittami J (1986) Sexual differences in the responses of captive great reed warblers (Acrocephalus arundinaceus) to variation in song structure and repertoire size. Ethology 73:69–77
Chaverri G, Gillam EH, Vonhof MJ (2010) Social calls used by a leaf-roosting bat to signal location. Biol Lett 64:441–444
Denzinger A, Schnitzler H-U (2013) Bat guilds, a concept to classify the highly diverse foraging and echolocation behaviors of Microchiropteran bats. Front Physiol 4:164
Dhondt A (1966) A method to establish boundaries of bird territories. Gerfaut-Giervalk 56:404–408
DuBois AL, Nowicki S, Searcy WA (2009) Swamp sparrows modulate vocal performance in an aggressive context. Biol Lett 5:163–165
Falls JB (1978) Bird song and territorial behavior. In: Kramer L, Pliner P, Alloway T (eds) Aggression, dominance, and individual spacing. Academic Press, New York, pp 61–89
Fan PF, Xiao W, Huo S, Jiang XL (2009) Singing behavior and singing functions of black-crested gibbons (Nomascus concolor jingdongensis) at Mt. Wuliang, Central Yunnan, China. Am J Primatol 71:539–547
Fenton MB, Belwood JJ, Fullard JH, Kunz TH (1976) Responses of Myotis lucifigus (Chiroptera: Vespertilionidae) to calls of conspecifics and to other sounds. Can J Zool 54:1443–1448
Fichtel C, Hilgartner R (2013) Noises in the dark: vocal communication in Lepilemur ruficaudatus and other nocturnal pair-living primates. In: Masters J, Gamba M, Genin F (eds) Leaping ahead: advances in prosimian biology. Springer-Verlag, New York, pp 297–304
Fitch WT, Hauser MD (2002) Unpacking ‘honest’: vertebrate vocal production and the evolution of acoustic signals. In: Simmons AM, Fay RR, Popper AN (eds) Acoustic communication. Springer, New York, pp 65–137
Funghi C, Cardoso GC, Mota PG (2015) Increased syllable rate during aggressive singing in a bird with complex and fast song. J Avian Biol 46:283–288
Geberzahn N, Aubin T (2014) Assessing vocal performance in complex birdsong: a novel approach. BMC Biol 12:58
Guppy A, Coles RB, Pettigrew JD (1985) Echolocation and acoustic communication in the Australian ghost bat, Macroderma gigas (Microchiroptera: Megadermatidae). Aust Mammal 8:299–308
Hall ML, Kingma SA, Peters A (2013) Male songbird indicates body size with low-pitched advertising songs. PLoS One 8:e56717
Ham S, Hedwig D, Lappan S, Choe JC (2016) Song functions in nonduetting gibbons: evidence from playback experiments on Javan gibbons (Hylobates moloch). Int J Primatol 37:225–240
Hinde RA (1956) The biological significance of the territories of birds. Ibis 98:340–369
Janßen S, Schmidt S (2009) Evidence for a perception of prosodic cues in bat communication: contact call classification by Megaderma lyra. J Comp Physiol A 195:663–672
Jones DL (2015) Fathom toolbox for Matlab: software for multivariate ecological and oceanographic data analysis. College of Marine Science, University of Florida, St. Petersburg, FL, USA
Kaňuch P, Aghová T, Meheret Y, Šumbera R, Bryja J (2015) New discoveries on the ecology and echolocation of the heart-nosed bat Cardioderma cor with a contribution to the phylogeny of Megadermatidae. Afr Zool 50:53–57
Kinzey WG, Robinson JG (1983) Intergroup loud calls, range size, and spacing in Callicebus torquatus. Am J Phys Anthropol 60:539–544
Knӧrnschild M, Jung K, Nagy M, Metz M, Kalko E (2012) Bat echolocation calls facilitate social communication. Proc R Soc Lond B 279:4827–4835
Koren L, Mokady O, Geffen E (2008) Social status and cortisol levels in singing rock hyraxes. Horm Behav 54:212–216
Kroodsma DE (1989) Suggested experimental designs for song playbacks. Auk 37:600–609
Kulzer E, Nelson JE, McKean JL, Mohres FP (1984) Prey-catching behaviour and echolocation in the Australian ghost bat, Macroderma gigas (Microchiroptera: Megadermatidae). Austr Mammal 7:37–50
Langmore NE (2000) Why female birds sing. In: Espmark Y, Amundsen T, Rosenqvist G (eds) Animal signals: signalling and signal design in animal communication. Tapir Academic Press, Trondheim, pp 317–327
Lawrence BD, Simmons JA (1982) Measurements of atmospheric attenuation at ultrasonic frequencies and the significance for echolocation by bats. J Acoust Soc Am 71:585–590
Leippert D, Frank E, Gabriel P, Kutter S, Scheiderman KD, von Stillfried N, Weller F (2002) Prey-correlated spectral changes in echolocation sounds of the Indian false vampire Megaderma lyra. Ethology 108:139–156
Linhart P, Jaska P, Petruskova T, Petrusek A, Fuchs R (2013) Being angry, singing fast? Signalling of aggressive motivation by syllable rate in a songbird with slow song. Behav Process 100:139–145
Maher CR, Lott DF (1995) Definitions of territoriality in the study of variation in vertebrate spacing systems. Anim Behav 49:1581–1597
Marler P (1969) Colobus guereza: territoriality and group composition. Science 163:93–95
McWilliam A (1987) Territoriality and pair behavior of the African false vampire bat, Cardioderma cor (Chiroptera: Megadermatidae), in coastal Kenya. J Zool 213:243–252
Mitani JC (1984) The behavioral regulation of monogamy in gibbons (Hylobates muelleri). Behav Ecol Sociobiol 15:225–229
Mitani JC (1985a) Gibbon song duets and inter-group spacing. Behaviour 92:59–95
Mitani JC (1985b) Location-specific responses of gibbons (Hylobates muelleri) to male songs. Z Tierpsychol 70:219–224
Mitani JC (1987) Territoriality and monogamy among agile gibbons (Hylobates agilis). Behav Ecol Sociobiol 20:265–269
Neuweiler G (1990) Auditory adaptations for prey capture in echolocating bats. Physiol Rev 70:615–641
Pereira HM, Bergman A, Roughgarden J (2003) Socially stable territories: the negotiation of space by interacting foragers. Am Nat 161:143–152
Podos J (1997) A performance constraint on the evolution of trilled vocalizations in a songbird family (Passeriformes: Emberizidae). Evolution 51:537–551
Puechmaille SJ, Borissov IM, Zsebok S, Allegrini B, Hizem M, Kuenzel S, Schuchmann M, Teeling EC, Siemers BM (2014) Female mate choice can drive the evolution of high frequency echolocation in bats: a case study with Rhinolophus mehelyi. PLoS One 9:e103452
Raemaekers JJ, Raemaekers PM (1985) Field playback of loud calls to gibbons (Hylobates lar): territorial, sex-specifi, and species-specific responses. Anim Behav 33:481–493
Ratcliffe J (2015) Ultrasonic and superfast: design constraints on echolocation in bats. J Acoust Soc Am 138:1931
Rendall D, Owren MJ, Ryan MJ (2009) What do animal signals mean? Anim Behav 78:233–240
Richards DG (1981) Estimation of distance of singing conspecifics by the Carolina wren. Auk 98:127–133
Ryan MJ, Tuttle MD (1987) The role of prey-generated sound, vision, and echolocation in prey localization by the African bat Cardioderma cor (Megadermatidae). J Comp Physiol A 161:59–66
Schmidt S (2000) The role of echolocation in the hunting of terrestrial prey—new evidence for an underestimated strategy in the gleaning bat, Megaderma lyra. J Comp Physiol A 186:975–988
Schmidt S (2013) Beyond echolocation: emotional acoustic communication in bats. In: Altenmüller E, Schmidt S, Zimmerman E (eds) Evolution of emotional communication: from sounds in nonhuman mammals to speech and music and man. Oxford University Press, Oxford, pp 92–104
Searcy WA, Beecher MD (2009) Song as an aggressive signal in songbirds. Anim Behav 78:1281–1292
Smarsh GC, Smotherman M (2015a) Singing away from home: songs are used on foraging territories in the heart-nosed bat, Cardioderma cor. In: Proceedings of Meetings on Acoustics, vol. 25 010002. Acoustical Society of America, Jacksonville
Smarsh GC, Smotherman M (2015b) Intra- and interspecific variability of echolocation pulse acoustics in the African megadermatid bats. Acta Chiropterol 17:429–443
Smotherman M, Knörnschild M, Smarsh G, Bohn K (2016) The origins and diversity of bat songs. J Comp Physiol A 202:535–554
Taylor AM, Reby D (2010) The contribution of source-filter theory to mammal vocal communication research. J Zool 280:221–236
Temeles EJ (1994) The role of neighbours in territorial systems: when are they ‘dear enemies’? Anim Behav 47:339–350
Terleph TA, Malaivijitnond S, Reichard UH (2016) Age related decline in female lar gibbon great call performance suggest that call features correlate with physical condition. BMC Biol 16:4
Tibbetts EA, Dale J (2007) Individual recognition: it is good to be different. Trends Ecol Evol 22:529–537
Tinbergen N (1957) The functions of territory. Bird Study 4:14–27
Vaughan T (1976) Nocturnal behavior of the African false vampire bat (Cardioderma cor). J Mammal 57:227–248
Vaughan TA, Vaughan RP (1986) Seasonality and the behavior of the African yellow-winged bat. J Mammal 67:91–102
Waters DA, Jones G (1995) Echolocation call structure and intensity in five species of insectivorous bats. J Exp Biol 198:475–489
Wiley RH (2013) Specificity and multiplicity in the recognition of individuals: implications for the evolution of social behaviour. Biol Rev 88:179–195
Wilkinson GS, Boughman JW (1998) Social calls coordinate foraging in greater spear-nosed bats. Anim Behav 55:337–350
Acknowledgements
We thank the reviewers for their critical assessments of this work. We feel that their suggestions have greatly improved this manuscript. We thank Felix Mpelembwa, Nuhu Bahaty Mhapa, Alfred Absolem Mollel, and Nickodemasy Obeid for their assistance in field work. We thank the officers of Kikavuchini, Mkalama, and Longoi Villages, the Machame Weru Weru Ward, and the Hai District for their cooperation. Dassa Nkini of the Tanzania Conservation Resource Centre assisted with permit acquisition. We thank Brian Pierce, Thomas DeWitt, David Jones, Mirjam Knörnschild, and Kirsten Bohn for advice and discussion on statistics and experimental design. This research was funded by the National Science Foundation Graduate Research Fellowship Program, Bat Conservation International, and Wildlife Acoustics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national, and institutional guidelines for the use of animals were followed. We acquired all necessary permits and permissions to work with this species and in these regions: Institutional Animal Care and Use Committee, AUP 2012-087; Tanzania Commission for Science and Technology, 2014-53-ER-2012-58, 2013-65-NA-2012-58, and NA-2012-58.
Conflict of interest
The authors declare that they have no conflict of interest.
Data availability
The datasets analyzed from the current study are available from the corresponding author on reasonable request.
Additional information
Communicated by M. Knoernschild
Electronic supplementary material
Online Resource 1
Video of a typical Cardioderma cor male singing in a perch. Individuals rotate while perched, broadcasting songs in bouts orally throughout the night (WMV 711 kb)
Online Resource 2
Spectrogram of an example contact call of C. cor, produced by both males and females while foraging. Contact calls consist of varying numbers of “upsweep” syllables (GIF 34 kb)
Online Resource 3
Song playlist for playback experiments. Each playlist consisted of a song repeated 20 times to create a set, which was repeated twice (Set 1 and Set 2) with 1 min of silence in between. Precontrol (PreC) and Postcontrol (PostC) time periods consisted of 5 min of silence at the beginning and the end of the playlist (GIF 8 kb)
Online Resource 4
Audio file of an example of a song playlist for playback. The silent PreC and PostC time periods on the ends of this playlist are five minutes in duration each (MP2 22126 kb)
Rights and permissions
About this article
Cite this article
Smarsh, G.C., Smotherman, M. Behavioral response to conspecific songs on foraging territories of the heart-nosed bat. Behav Ecol Sociobiol 71, 142 (2017). https://doi.org/10.1007/s00265-017-2370-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-017-2370-9