Abstract
Marine environments are subject to increasing disturbance events, and coral reef ecosystems are particularly vulnerable. During periods of environmental change, organisms respond initially through rapid behavioural modifications. Whilst mean population level modifications to behaviour are well documented, how these shifts vary between individuals, and the relative trade-offs that are induced, are unknown. We test whether the frequency and time invested in different behaviours varies both between and within individuals with varying resource availability. To do this, we quantify differences in four key behavioural categories (aggression, exploration, feeding and sociability) at two sites of different resource availability, using an obligate corallivore butterflyfish species (Chaetodon lunulatus). Individuals on a low resource site held larger territories, investing more time in exploration, which was traded off with less time invested on aggression, feeding and sociability. Repeatability measures indicated that behavioural differences between sites could plausibly be driven by both plasticity of behaviour within individuals and habitat patchiness within feeding territories. By combining population-level means, co-correlation of different behaviours and individual-level analyses, we reveal potential mechanisms behind behavioural variation in C. lunulatus due to differences in resource availability.
Significance statement
Using observational methods, we identify differences in the behaviour of an obligate corallivorous butterflyfish (Chaetodon lunulatus) between a high and a low resource site. We use a combination of density surveys, territory mapping and behavioural observation methods in a comparative analysis to relate behaviour directly to the environment in which it occurs. Bringing together insights from game theory and optimal foraging, we also use our results to highlight how understanding the correlations of different behaviours can inform our understanding of the extent to which behaviours are plastic or fixed. Furthermore, by considering how multiple behaviours are correlated, we move away from exploring individual behaviours in isolation and provide an in-depth insight into how differences in behaviour both between individuals and at the population level can affect responses to declining resource availability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Behaviour mediates an organism’s complex interactions with both its biotic and abiotic environment. Spatial and temporal variation in the environment can affect various aspects of behaviour, such as predation and competition, which in turn can alter species interactions (Anholt 1997). Changes in behaviour therefore have the potential to shape ecological communities (Delarue et al. 2015). As a result, behaviour plays a pivotal role in how individuals can adjust to rapid environmental change (Wong and Candolin 2015; Nagelkerken and Munday 2016). For example, in marine systems, reef degradation and coral loss can influence reef fish recruitment and settlement (Dixson et al 2014), predator avoidance and boldness (Boström-Einarsson et al. 2018), aggression (Keith et al. 2018) and foraging (Nash et al. 2012). The capacity for a population to acclimate and/or adapt behaviourally to severe environmental change can be determined by factors such as if and how behaviour is transmitted between individuals (Keith and Bull 2017), geographic variation in biotic interactions (Early and Keith 2018) and the likelihood that initial acclimation contributes to evolutionary or ecological traps (Schlaepfer et al. 2002). Traps can occur when cues become unreliable under new environmental conditions, leading to maladaptive behaviour and reduced fitness (Schlaepfer et al. 2002). Individuals within a population do not always respond in the same way and can feedback to generate frequencies of alternative strategies within a population (Dall et al. 2004). Individual behaviour can, therefore, scale up to influence population dynamics, with broader community and ecosystem-level consequences (Wong and Candolin 2015). To understand any potential mechanisms or limitations on adaptation, there is a need to understand the role of individual variation and associated trade-offs in responses to environmental change.
Behavioural responses to environmental change can be measured at three levels: between population, between individuals within populations and within individuals. Population-level behavioural responses to environmental change have been well documented and reviewed (Tuomainen and Candolin 2011; Wong and Candolin 2015). Recent research has focused on how individual differences in behaviour affect how organisms respond to change (Mathot et al. 2012; Niemelä and Dingemanse 2018). Behavioural plasticity involves immediate changes in behaviour by individuals in response to the environment (Nussey et al. 2007). Plastic behaviours could allow individuals to buffer the effects of environmental change. For example, butterflyfishes can show flexibility in foraging behaviour following a decline in resource availability (Zambre and Arthur 2018). Personality refers to repeated correlated suites of behaviours that result in consistent responses by an individual across different behavioural contexts, such as mating or foraging, and across environmental gradients (Sih et al. 2004; Bell et al. 2009). When payoffs are based on the behavioural history of individuals, and the frequency with which different traits and strategies are expressed, these can be shaped over time through natural selection (Dall et al. 2004). Consistent behaviours across multiple contexts are used to indicate the presence of a personality (Hayes and Jenkins 1997; Bell et al. 2009). This consistency can be measured as behavioural repeatability: the proportion of variation across the population that can be attributed to differences between individuals (Lessells and Boag 1987). However, because repeatability is a statistic of the population, this measure cannot reveal the extent to which individuals vary in their consistency (Sih et al. 2004). It is therefore also important to account for the magnitude of individual variation around the mean population level (Dochtermann and Royauté 2019). Considering behaviour on an individual level can, therefore, reveal variation that is not apparent in population mean-level responses (Barbasch and Buston 2018; Gunn et al. 2021).
Evidence suggests that differences in behaviour within and between individuals can constrain the available strategies for coping with environmental change (Koolhaas et al. 1999). For example, less aggressive, but more exploratory individuals could be better able to cope with low resource availability by investing less in territory defence, which is costly where the resource reward is low (Righton et al. 1998). Instead, individuals invest more in exploratory foraging to locate increasingly sparse food resources (Chandler et al. 2016). Exploratory behaviours might be beneficial when individuals experience nutritional deficit, whereas aggressive behaviours are beneficial where resource availability is intermediate (Wyman and Hotaling 1988). Empirical evidence supports this idea, such that resource availability has been identified as a determinant of territoriality across various taxa. Specifically, territoriality is lower, and territory sizes larger, where resource availability is lower (Justino et al. 2012; Mazzamuto et al. 2020). Individuals that persist in changed environments may do so because they show a high degree of behavioural plasticity, i.e. have shifted their levels of aggression and exploration, and/or because the population of persisting individuals show specific, consistent variation in behaviour, i.e. specific personality types (Kralj-Fišer and Schneider 2012). To reveal how differences in behaviour within and between individuals can affect responses to environmental change and how that alters the frequency of different behaviours within a population through plasticity or selection, or a combination of both (Dingemanse et al. 2010), we must move beyond exploring behaviours in isolation and consider explicitly how multiple behaviours are correlated, and thus potentially trade-off against one another, in different contexts (Dall et al. 2004; Sih et al. 2004).
Butterflyfishes are an excellent model system to understand the impact of resource levels on behaviour at both the population and individual level. Global declines in live coral cover directly translate to a decline in resource availability for butterflyfish species. Their diet and, as a result, their abundance, body condition and behaviour, are subsequently influenced directly by the availability and quality of live coral (Graham et al. 2011). Butterflyfish territories have been shown to expand or break down when coral cover is low (Tricas 1989), especially in specialist obligate corallivores (Pratchett et al. 2014). The underlying mechanism appears to be linked to foraging strategies; reduced food resources lead to a nutritional deficit, so individuals must travel further to locate food and territorial boundaries break down (Zambre and Arthur 2018). The same mechanisms are also linked to reduced aggression (Berumen and Pratchett 2006; Keith et al. 2018) and changes in social behaviour (Thompson et al. 2019). As behaviour can influence the survival of individuals (Moiron et al. 2020), understanding variation in behavioural responses between butterflyfish individuals and identifying the drivers of these responses could have important implications for their persistence.
We test the extent to which the frequency of butterflyfish behaviours, and the variation in these behaviours across individuals, is influenced by resource levels (i.e. coral cover), using existing variation in coral cover and an obligate corallivore (Chaetodon lunulatus). Specifically, using population and individual level approaches, we (1) test whether territory size is influenced by resource availability such that territories with higher resource availability are smaller; (2) test whether individuals at reefs with high resource availability are more likely to exhibit aggressive behaviours, whilst those at reefs with low resource availability are more likely to exhibit exploratory behaviours; and (3) calculate both behavioural repeatability estimates and estimates of individual variability around the population mean to determine variation in behaviour between individuals within sites. If behaviours are highly repeatable, then any differences in behaviour between sites are likely a consequence of selection on fixed behavioural types.
Materials and methods
Study species and field sites
We collected field data using SCUBA between the 15th June and the 7th August 2019. Pilot data from abundance surveys were used to identify a species with strong pair bonding and territory defence behaviour. The most abundant obligate corallivore fitting this criterion was the oval butterflyfish, Chaetodon lunulatus. Yabuta and Berumen (2014) characterise C. lunulatus as monogamous and territorial with a strong pair bond, allowing us to observe the same breeding pair repeatedly in situ, by returning to the same territory on multiple occasions. It was not possible to record data blind because our study involved focal animals in the field.
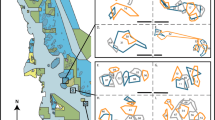
The Wakatobi Marine National Park (WMNP) is located within the province of Southeast Sulawesi, Indonesia (5°41′S 124°0′E), within the coral triangle. Based on hard coral cover (resource availability), we selected two permanent monitoring sites around Hoga Island: Pak Kasims (PK) and Sampela (SAM) (Fig. S1). The high resource site, PK, was characterised by hard coral cover of 40–50%, with abundant soft coral (Clifton et al. 2010). In contrast, the low resource site, SAM, consisted of a gently sloping reef with coral cover of 10–20% and various rubble slips (Powell et al. 2015). The sites are similar in terms of wave exposure, reef aspect and depth, with all work conducted between 3 and 10 m across both sites.
Survey methods
Coral cover
We used a long-term reef monitoring programme to obtain coral cover data for 2019. Using permanent transect locations, three replicate 50 m transects were conducted per reef zone (flat, crest and slope) at each of the two sites and benthic composition recorded every 25 cm along each transects. We extracted the percentage of hard coral cover from each transect for analysis.
Territory mapping
We collected intensive behavioural data for ten C. lunulatus pairs at each of the two study sites. We mapped the territories for five of these pairs at each of the two sites following the methods in Berumen and Pratchett (2006). We observed a 2-min acclimation period to ensure the behaviour of the focal pair was not influenced by the presence of observers. We then followed the focal pair from a minimum distance of 2 m for 15 min. Visual markers (flagging tape attached to fishing weights) were placed at the pair’s location every minute. This observation time was based on pilot studies by Berumen and Pratchett (2006) that determined 15-min observation periods were optimal to accurately estimate C. lunulatus territory size. When pairs separated, we followed a single individual for the remainder of the observation period. Following the observation, two 30 × 30 m transects were laid perpendicular to one another outside of the points to provide X, Y axes. We recorded the marker locations as Cartesian coordinates from the transect origin.
Density
We conducted C. lunulatus density surveys through an underwater visual census (UVC) using a 50 × 5 m belt transect, which was laid out during counts. We used 10 replicate transects at each of three reef zones (flat, crest and slope) across the two sites, equating to 30 transects and an area of 7,500 m2 per site. As previous research indicated that territoriality in C. lunulatus could be influenced by a dominant competitor, we also recorded the density of C. baronessa along the transects (Berumen and Pratchett 2006).
Behavioural observations
We recorded 10-min behavioural observations for 10 pairs at each of the two sites, repeating our observations of each pair five times, resulting in a total of 100 behavioural observations. To keep the time between observations as consistent as possible, we observed individuals at both sites on each survey day, and all individuals were surveyed approximately every 5–7 days. The location of each territory was marked with a weight and flagging tape. Before placing the flagging tape, we observed the chosen breeding pair for approximately 5 min. The flagging tape was then placed within the approximate centre of the territory. Observations were recorded on a waterproof Nikon Coolpix camera from a minimum distance of 2 m. As with the territory mapping, we observed a 2-min acclimation period before we began recording behaviour. We also used this acclimation period to observe the pair and ensure that we were following the correct breeding pair, and not a pair from a neighbouring territory. Behaviours were then recorded from video analyses. We selected one individual from each breeding pair as a focal individual and used a continuous sampling method to record the length of time spent exhibiting each behavioural trait. We also identified any species the focal pair interacted with during video analyses. Butterflyfish show minimal sexual dimorphism (Yabuta 2008), so we were unable to discern between the two individuals within pairs when selecting the focal individual for each behavioural observation. Nonetheless, for C. lunulatus, monogamous bonds are long term (Reese 1975), and pairs have been shown to move throughout the feeding territory in coordination, with both individuals displaying equal levels of aggression (Nowicki et al. 2018). We therefore assumed behaviour to be synonymous between individuals making up a breeding pair.
We assigned observed behaviours to four behavioural categories (aggression, sociability, exploration and feeding), each consisting of one or more discrete behaviours (Table 1). Aggression was defined as an intra- or interspecific agonistic interaction initiated by the focal individual. Sociability was defined as the response to the presence of a conspecific (Réale et al. 2007), specifically the amount of time the focal individual spent within 2 m of the breeding partner (Table 1). Exploration captured two swimming traits, previously defined by Zambre and Arthur (2018) for butterflyfish: searching for food, defined as when individuals swim slowly, angled towards the benthos between patches, and all other swimming movements, here labelled as travelling. Travelling consisted of faster, streamlined swimming (Zambre and Arthur 2018). Feeding was defined as when individuals were taking bites out of live coral (Réale et al. 2007). Aggression, sociability and exploration are three commonly used behavioural categories, all of which have been shown to be repeatable to various extents (Bell et al. 2009; Table 1). We assigned the two discrete foraging behaviours (searching and feeding) into separate categories (exploration and feeding respectively), so that the time spent looking for food, and the time spent feeding, could be quantified separately. Additional behaviours, specifically the use of cleaning stations and resting, were also recorded. However, as these behaviours were observed rarely (< 0.05% of time across all observations) and were not the focal behaviours for this study, they have been excluded from analyses.
Statistical analyses
All our analyses were conducted using R v3.5.1 (R Core Team 2019). To analyse territory size, we plotted the Cartesian coordinates and calculated the minimum convex polygon to establish the area of each territory. We compared differences in territory size across the two sites using a one-way ANOVA.
Population level
For the population level analyses, we calculated the mean time spent on each behavioural category from the five observations for each breeding pair. We ran a multivariate analysis of variance (MANOVA), with time spent on each behavioural category (aggression, sociability, exploration and feeding) as the response variables, with site as the predictor variable. We then ran one-way ANOVAs with a Bonferroni correction for multiple comparisons, comparing time spent on each behavioural category separately, to identify which categories were different between the two sites. Based on the output from these tests, we also analysed the amount of time spent on searching, travelling and feeding using ANOVAs to compare differences between behaviours both within and across the two sites. Due to the small sample size in our study, we also bootstrapped the raw behavioural data and plotted the distributions of the 1000 bootstrap estimates and used non-overlapping confidence intervals to identify significant differences in behaviour between the two sites.
We compared C. lunulatus and C. baronessa densities across the two sites using basic Gaussian generalised linear models, with abundance as the response variable and site as a fixed effect. We also ran two additional models with reef zone as an additional factor, and then with an interaction between reef zone and species. We used a likelihood ratio test to compare these three models. Furthermore, for C. lunulatus, we determined whether aggression was a consequence of abundance by recording both aggressive and non-aggressive interactions between C. lunulatus pairs and calculating the probability that any given encounter would result in aggression. The probability was calculated by dividing the number of aggressive interactions by the number of total interactions. We then compared the probability of aggression for each focal individual between the two sites using a generalised linear model with probability of aggression as the response variable and site as the predictor variable.
Individual level
To visualise correlations of behaviours of individual pairs, we used a principal component analysis (PCA) with the Kaiser–Meyer–Olkin (KMO) criterion as a measure of sampling accuracy (Kaiser and Rice 1974) and Bartlett’s test of sphericity (Bartlett 1951) to ensure our data fit the assumptions of PCA. Kaiser and Rice (1974) suggest that a sampling adequacy of less than 0.5 indicates that the data are not suitable for further PCA analysis. To evaluate differences in traits between pairs within each site, we ran additional MANOVA tests comparing the time spent on each of the four behaviours (aggression, feeding, exploration and sociability) between the ten pairs within each of the two sites. For these tests, the behavioural traits (aggression, searching, travelling, feeding and sociability) were the response variables, with pair ID as the predictor variable. We used two separate MANOVAs, one for each site, rather than a single model with study site as a covariate, because the subsequent outputs from each test were used to calculate behavioural repeatability using an ANOVA approach (see below).
Repeatability estimates
We calculated the repeatability of each behaviour between individuals at each site based on the proportion data using the intraclass correlation coefficient (ICC, Nakagawa and Schielzeth 2010; Wolak et al. 2012). We took an ANOVA-based approach and calculated 1000 bootstrapped ICC estimates using ANOVA variance components in the ICC package (Wolak et al. 2012), using 2.5 and 97.5% confidence intervals from bootstrapped ICC estimates to identify any significant differences in repeatability between the two sites. To understand the variability of individuals around the population mean, as well as from each other, we used the mean standardisation (as defined in Dochtermann and Royauté 2019) of both the among and within (residual) individual variance (mean-scaled individual and mean-scaled residual variation) from bootstrapped variance components from the within site MANOVA tests, using equations in Dochtermann and Royauté (2019). Mean-scaled individual variation (Ia) calculates the proportion of variation, relative to the mean that can be explained by differences in the expression of behaviours between individuals. In contrast, mean-scaled residual variation (Iw) calculates the proportion of variation, relative to the mean that can be explained by differences in behaviour that are a consequence of unmeasured sources of variation (Dochtermann and Royauté 2019).
Results
Coral cover
For 2019, the mean coral cover across the reef flat, crest and slope was 35.69% at PK, the high resource site, compared to 17.67% at SAM, the low resource site. Coral cover was significantly higher at PK than SAM across both 2018 and 2019 (Fig. S2, ANOVA: F = 71.01, df = 1, P ≤ 0.001).
Population level
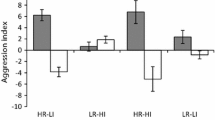
Territory sizes at the low resource site were significantly larger than those at the high resource site with mean (± standard error) territory sizes of 179.7 ± 35.6 m2 and 65.0 ± 13.8 m2 respectively (Fig. 1, Table S1, ANOVA, F = 9.04, df = 1.8, P = 0.02). Overall, behaviour also differed significantly between the two sites (Fig. 2, MANOVA: F = 6.87, df = 3.4, P < 0.01). Specifically, individuals at the higher resource site invested more time in aggression (ANOVA: F = 16.67, df = 1.18, P < 0.001), sociability (ANOVA: F = 7.87, df = 1.18, P = 0.01) and feeding (ANOVA: F = 20.17, df = 1.18, P < 0.001) and less time was invested in exploration (ANOVA: F = 24.96, df = 1.18, P < 0.001). These differences were also apparent from the 1000 bootstrap estimates, such that confidence intervals for the two sites did not overlap for any of the behaviours (Fig. S3).
C. lunulatus behaviour at a high (blue) and low (red) resource site. Behaviours are measured as the time spent on each of the four behavioural categories (left-handed Y axis). n = 10 individuals per site, with five replicate values per individual. Black points represent the mean time spent in each behaviour by an individual such that one point refers to a single individual. Boxplot lines are median values, box lengths represent interquartile ranges and whiskers are 25th and 75th percentiles. A second Y axis is presented for aggression, due to the rarity with which this behaviour occurs
For comparing C. lunulatus density across the two study sites, the likelihood ratio test identified the model with an interaction term for reef zone and site to be the best fit for the data (χ2 = 258.53, df = 4, P = < 0.001). The density of C. lunulatus differed significantly between the two sites (Fig. S4, GLM: t = 7.11, df = 1.58, P < 0.001). Despite this difference, conspecific aggression was not influenced by density—the number of encounters between conspecific pairs was the same between the two sites (GLM: t = − 1.45, df = 1.58, P = 0.17); yet, the probability that an encounter would be aggressive was significantly higher at the higher resource site (GLM: t = − 2.30, df = 1.58, P = 0.03). Likelihood ratio tests also identified the model with an interaction term for reef zone and study site to be optimal for comparing the density of C. lunulatus and C. baronessa at both the high (χ2 = 22.16, df = 4, P = < 0.001) and the low (χ2 = 62.49, df = 4, P = < 0.001) resource site. C. lunulatus density was greater than C. baronessa at the high resource site (Fig. S5, GLM: t = 4.578, df = 1.58, P = < 0.001) but was equivalent at the low resource site (Fig. S5, GLM: t = 1.633, df = 1.58, P = 0.108).
When behaviours within the ‘exploration’ category were analysed separately with feeding behaviour, time spent searching and feeding differed at the two sites (Fig. S5). Individuals at the higher resource site invested less time in searching (ANOVA: F = 11.3, df = 1.18, P = 0.003) and more time in feeding (ANOVA: F = 20.17, df = 1.18, P < 0.001) compared to individuals at the lower resource site. Within the higher resource site, there was no significant difference identified between the time spent on searching and feeding (ANOVA: F = 0.25, df = 1.18, P = 0.63). In contrast, at the lower resource site, a greater proportion of time was spent searching than feeding (ANOVA: F = 49.39, df = 1.18, P < 0.001).
Individual level
At the high resource site, there was no significant variation between individuals in the time spent on any of the four behaviours (MANOVA: F = 0.592, df = 9.40 P = 0.552). There was significant variation between individuals at the low resource site in the proportion of time spent feeding (ANOVA: F = 2.23, df = 9, 40 P = 0.04). The proportion of variance from the ANOVAs attributed to both within and between individual variations was similar between the two sites, with the exception of feeding, where between individual variation accounted for 70% of total variance at the low resource site. In other words, within the low resource site, individuals varied in the amount of time they invested in feeding (Fig. 3).
From left to right: breakdown of ANOVA variance components from raw data (n = 10 per site, with five replicates per individual), bootstrapped distributions of repeatability estimates and summary statistics from bootstrapped data for each of the five measured behaviours at the high and low resource site. Summary statistics (mean repeatability (R), mean-scaled between individual variation (Ia) and mean-scaled residual individual variation (Iw)) were calculated from 1000 bootstrapped estimates of the raw behavioural data and ANOVA variance components. Confidence intervals in the distribution plots (centre) are 2.5 and 97.5% limits of bootstrapped repeatability distributions. Solid and dashed lines are confidence intervals for the high and low resource sites respectively
Repeatability estimates
Raw repeatability estimates for each trait were small, and most were negative, suggesting a high level of statistical noise. Bootstrapping the raw estimates reduced this statistical noise, and bootstrapped repeatability estimates were all positive, but no estimate exceeded 0.3 (Fig. 3). Feeding at the low resource site was the most repeatable behavioural trait (R = 0.299). For all traits, confidence intervals for both bootstrapped repeatability (Fig. 3) and mean-scaled between individual variation estimates (Fig. S7) at each site overlapped, indicating no significant difference in repeatability between the two sites. Although for aggression and feeding, the mean standardisation estimate was higher at the low resource site, and by an order of magnitude for aggression, confidence intervals overlapped for all behaviours between the two sites (Fig. S7). However, mean-scaled residual variation was significantly different for feeding (high: mean = 9.091, 2.5% CI = 9.016, 97.5% CI = 9.165; low: mean = 17.272, 2.5%CI = 17.117, 97.5% CI = 17.427), such that confidence intervals did not overlap (Fig. S8). Residual mean standardisation estimates were higher at the low resource site.
The PCA is included to visualise the data, with site and individual pairs overlaid as groups (Fig. 4, S9). Although the KMO sampling adequacy criterion produced a low value of 0.48, which is slightly below the acceptance threshold, the Bartlett test of sphericity was significant (P < 0.05). We therefore deemed that the PCA would be an acceptable tool to visualise the data. From PCA loading values, PC1 had positive associations with searching and travelling, and a negative association with aggression, feeding and sociability. PC2 had a strong positive association with aggression. The PCA suggests that sociability and aggression are positively correlated with one another, as are travelling and searching, whilst feeding is negatively correlated with the exploratory behaviours (travelling and searching).
The position of each of the 20 (n = 10 per site) breeding pair in relation of both the principal components, and each behaviour. The two sample sites, high (blue circles) and low (red triangles) resource availability, have been overlaid as an additional group. Ellipses around each site mean (larger symbols) are based on 95% confidence limits. PCA loadings for PC1 and PC2 are presented in the inset
Discussion
Using an observational approach, we have identified both broad (population level) and fine (individual level) scale differences in the behaviour of C. lunulatus between two sites of different resource availabilities. At the population mean level, we have shown that C. lunulatus in an area of high resource availability maintain small territories and invest time in aggressive territory defence and feeding. In contrast, C. lunulatus in an area of low resource availability held larger territories and spent less time on territory defence and more time invested in searching for food. At the individual level, we identified significant differences in the time spent feeding among individuals at the low resource availability site, and feeding was also the most repeatable behaviour at this site. By considering both population and individual-level analyses, combined with a consideration of the co-correlation of different behaviours, we can provide a deeper insight into the behavioural responses of C. lunulatus, than would be possible by considering the population means of individual behaviours in isolation.
Optimal foraging theory predicts that an increase in time spent foraging should yield an increase in food acquisition (MacArthur and Pianka 1966). One of the assumptions of optimal foraging theory is that all feeding patches are of similar quality, which is not the case across our two study sites, and it is also possible that within sites, there is variation in patch quality. Although territories were larger and the time invested in searching for food was greater at the low resource site, the time invested in actual bites of coral prey was lower. This suggests that individuals had to invest more time searching, i.e. moving between coral patches, because the available food was more sparsely distributed (Tricas 1989). The food maintenance hypothesis predicts that territory size is determined according to the food supply needed for short-term energetic needs (Stimson 1973). At the high resource site, food was readily available within a small space; thus, the time spent searching prior to being able to take a bite of prey was minimal. Individuals then had the time and energy to invest in territory defence (Davies and Hartley 1996). At the low resource site, food resources were sparse such that even when time was redistributed from aggression to searching, the nutritional gain was still lower than at the high resource site. A negative correlation between exploratory traits and feeding therefore emerged across both sites. This potential trade-off highlights the need to consider multiple traits together to fully understand the response of individuals to environmental change (Sih et al. 2004).
The differences in territory size that we identified for C. lunulatus in sites with different resources differ from previous work, which found territory size decreased with resource availability in the dominant specialist C. baronessa but not in generalist corallivores, including C. lunulatus (Berumen and Pratchett 2006). These data were collected in a different region, with different abundances, densities and diversity of obligate corallivorous butterflyfish, suggesting there is a degree of context specificity in butterflyfish territoriality at the species level. Heterospecific aggression is more prominent in C. baronessa than C. lunulatus (Berumen and Pratchett 2006; Blowes et al. 2013). As the density of C. lunulatus was greater than that of C. baronessa at the high resource site, i.e. where C. lunulatus aggression is greater, the presence of more aggressive competitors, such as C. baronessa, could therefore be a determinate of territoriality in C. lunulatus, along with resource availability.
Differences in aggression and exploration behaviour between our two study sites are consistent with the principles of game theory, specifically the Hawk-Dove game (Maynard Smith and Price 1973), and the principles of the model of economic defendability (Wyman and Hotaling 1988). Under normal (high coral) conditions, aggression within C. lunulatus individuals is high. Increasing disturbance to an intermediate level increases the value of the resource (Wyman and Hotaling 1988), in this case, coral within a C. lunulatus territory. In the Hawk-Dove game, if the value of a resource increases, or the ‘odds’ are higher, it pays for an individual to play ‘Hawk’ and act aggressively more often than under the original conditions (Maynard Smith 1982). This way, individuals can maximise the use of available resources by feeding on what high-quality resources remain (Cole et al. 2008). With additional disturbance, there will be a threshold beyond which aggression will decrease (Peiman and Robinson 2010; Keith et al. 2018) and exploration increase, as identified in our study, due to a nutritional deficit and resource availability trade-off (Berumen and Pratchett 2006). The cost of being aggressive is then outweighed by the benefit of playing ‘dove’, and remaining passive, thus a higher frequency of individuals adopting the low aggression strategy is expected in the population, as seen in our results.
The threshold of behavioural change is likely to be variable across butterflyfish species, locations and even individuals, such that individual state (e.g. physiological, environmental, morphological) influences the cost–benefit trade-off of aggressive interactions (Dingemanse and Wolf 2010). This threshold may also be determined by the extent to which behaviours are fixed or plastic within an individual. If behaviours are a result of fixed ‘personalities’, then a frequency change in behaviour can only occur via selection (Dall et al. 2004), whereas if behaviours are plastic, then changes can occur within generations. Identifying the threshold at which behavioural responses occur is important for management efforts, as behavioural modifications have implications to the future distribution and persistence of species (Delarue et al. 2015).
High repeatability between individuals within a population indicates high behavioural consistency, such that within a population, individuals consistently behave differently from one another (Lessells and Boag 1987). Behavioural repeatability between individuals may be low in two scenarios: either if within individual variation is high, or if between individual variation is low (Nakagawa and Schielzeth 2010). Our estimates for behavioural repeatability between individuals were all less than 0.5, which tentatively indicates that the measured behaviours show a degree of behavioural plasticity within individuals (Wilson 2018). Behaviours dependent upon an individual’s physiology or morphology, i.e. their resource allocation (Laskowski et al. 2020), are predicted to be consistent within an individual, but variable between individuals, and therefore repeatable (Bell et al. 2009). Repeatability will be lower for traits dependent on how an individual utilises the resources in their environment, i.e. on resource acquisition (Laskowski et al. 2020), such as behaviours associated with energetic needs, e.g. feeding, and the social environment, e.g. aggression (Bell et al. 2009). Surprisingly, aggression and feeding at the low resource site had the highest repeatability estimates in our study. However, if there is an interaction between an individual and its environment that contributes to between individual variation, for example, if individuals in the low resource site face greater fine-scale variation in the amount of food in their territory compared to individuals in the high resource site, repeatability estimates could be inflated (Nakagawa and Schielzeth 2010). We identified higher residual (mean-scaled) variation for feeding at the low resource site. There is, therefore, a significant amount of variation in trait expression that is a consequence of unmeasured sources of variation (Dochtermann and Royauté 2019). If this unmeasured source of variation is environmental, then an interaction between the environment and individuals at the low resource site is plausible. The high degree of unmeasured variation suggests that there is a degree of within territory context specificity that is influencing the behaviour of C. lunulatus that would not have been identified if we had only considered behaviour at the population level.
One potential cause of an interaction between individuals and the environment is habitat patchiness resulting in some individuals having greater access to resources than others. Variation in habitat patchiness can influence the behaviour of individuals to varying extents according to the specific area of habitat they occupy (Cattarino et al. 2015; van Leeuwen et al. 2017). Habitat patchiness may be driving variation in feeding and aggression in our study, masking the amount of variation that can be directly associated with intrinsic differences between individuals at low resource availability site. It is possible that a preference for specific species of coral as a food source could be driving this variation, rather than habitat patchiness directly. However, we did not quantify the resource availability to the coral species level within each territory due to the temporal scale and design of our study. Nonetheless, the time invested in feeding was significantly different between individuals at the low resource site. As C. lunulatus is an obligate corallivore, feeding is directly related to the amount of available live resource, which was significantly lower at the low resource site. A lower nutritional gain at the low resource site also makes energy investment in aggression too costly compared to investing energy in foraging for what little resources are available (Berumen and Pratchett 2006). Therefore, aggression is also directly linked to the availability of live resource and will also be influenced by habitat patchiness across different territories. C. lunulatus individuals at the low resource site may change their behavioural strategy according to the quality of their territory and the distribution of food availability within the territory (Righton and Mills 2007). An influence from the spatial configuration of habitat would lead to higher consistency within individuals, and therefore the higher-than-expected estimate of repeatability for aggression and feeding at the low resource site.
We have offered insight into how behaviour at both the population and individual level influences the response of C. lunulatus individuals to different levels of resource availability, which suggests that loss of cover through environmental disturbance could result in shifts in behaviour at a population level. By looking at behavioural change at both individual and population levels through observation in a natural setting, we have tentatively highlighted the potential for individual behavioural strategies to be masked by mean effects. Our work also highlights the need to both identify the threshold at which behavioural changes occur and identify the relative roles of behavioural plasticity and behavioural consistency, or ‘personalities’. This could be achieved by observing the behaviours of the same individuals across different contexts (varying resource) within sites. To further understand behaviour within sites, our results suggest that context-specific factors such as environmental variation within territories are drivers of behaviour at the individual level, and should be considered and quantified in the future. This will enable a more thorough understanding of the distribution, adaptation and persistence of important reef indicator species, which in turn can inform future management efforts.
Data availability
Data and code for this work are available through the Figshare repository (https://doi.org/10.6084/m9.figshare.17378384).
References
Anholt BR (1997) How should we test for the role of behaviour in population dynamics? Evol Ecol 11:633–640. https://doi.org/10.1023/A:1018478117159
Barbasch TA, Buston PM (2018) Plasticity and personality of parental care in the clown anemonefish. Anim Behav 136:65–73. https://doi.org/10.1016/j.anbehav.2017.12.002
Bartlett MS (1951) The effect of standardization on a chi-square approximation in factor analysis. Biometrika 38:337–344. https://doi.org/10.1111/j.2044-8317.1951.tb00299.x
Bell AM, Hankison SJ, Laskowski KL (2009) The repeatability of behaviour: a meta-analysis. Anim Behav 77:771–783. https://doi.org/10.1016/j.anbehav.2008.12.022
Berumen ML, Pratchett MS (2006) Effects of resource availability on the competitive behaviour of butterflyfishes (Chaetodontidae). Proceedings 10th International Coral Reef Symposium, Okinawa, Japan, pp 644–650
Blowes SA, Pratchett MS, Connolly SR (2013) Heterospecific aggression and dominance in a guild of coral-feeding fishes: the roles of dietary ecology and phylogeny. Am Nat 182:157–168. https://doi.org/10.1086/670821
Boström-Einarsson L, Bonin MC, Munday PL, Jones GP (2018) Loss of live coral compromises predator-avoidance behaviour in coral reef damselfish. Sci Rep 8:7795. https://doi.org/10.1038/s41598-018-26090-4
Cattarino L, Mcalpine CA, Rhodes JR (2015) Spatial scale and movement behaviour traits control the impacts of habitat fragmentation on individual fitness. J Anim Ecol 85:168–177. https://doi.org/10.1111/1365-2656.12427
Chandler JF, Burn D, Berggren P, Sweet MJ (2016) Influence of resource availability on the foraging strategies of the triangle butterflyfish Chaetodon triangulum in the Maldives. PLoS One 11:e0151923. https://doi.org/10.1371/journal.pone.0151923
Clifton J, Unsworth R, Smith D (2010) Introduction. In: Clifton J, Unsworth R, Smith D (eds) Marine research and conservation in the Coral Triangle: the Wakatobi National Park. Nova Science Publishers, New York, pp 1–9
Cole AJ, Pratchett MS, Jones GP (2008) Diversity and functional importance of coral-feeding fishes on tropical coral reefs. Fish Fish 9:286–307. https://doi.org/10.1111/j.1467-2979.2008.00290.x
Dall SRX, Houston AI, McNamara JM (2004) The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecology 7:734–739. https://doi.org/10.1111/j.1461-0248.2004.00618.x
Davies NB, Hartley IR (1996) Food patchiness, territory overlap and social systems: an experiment with unnocks Prunella modularis. J Anim Ecol 65:837–846. https://doi.org/10.2307/5681
Delarue EMP, Kerr SE, Lee-Rymer T (2015) Habitat complexity, environmental change and personality: a tropical perspective. Behav Process 120:101–110. https://doi.org/10.1016/j.beproc.2015.09.006
Dingemanse NJ, Kazem AJN, Réale D, Wright J (2010) Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol 25(2):81–89. https://doi.org/10.1016/j.tree.2009.07.013
Dingemanse NJ, Wolf M (2010) Recent models for adaptive personality differences: a review. Phil Trans R Soc B 365:3947–3958. https://doi.org/10.1098/rstb.2010.0221
Dixson DL, Abrego D, Hay ME (2014) Chemically mediated behaviour of recruiting corals and fishes: a tipping point that may limit reef recovery. Science 345:892–897. https://doi.org/10.1126/science.1255057
Dochtermann NA, Royauté R (2019) The mean matters: going beyond repeatability to interpret behavioural variation. Anim Behav 153:147–150. https://doi.org/10.1016/j.anbehav.2019.05.012
Early R, Keith SA (2018) Geographically variable biotic interactions and implications for species ranges. Glob Ecol 28:42–53. https://doi.org/10.1111/geb.12861
Graham NAJ, Pascale C, Richard DE, Simon J, Yves L, MacNeil MA, McClanahan T, Ohman M, Polunin N, Wilson SK (2011) Extinction vulnerability of coral reef fishes. Ecology 14:341–348. https://doi.org/10.1111/j.1461-0248.2011.01592.x
Gunn RL, Hartley IR, Algar AC, Niemelä PT, Keith SA (2021) Understanding behavioural responses to human-induced rapid environmental change: a meta-analysis. Oikos published online. https://doi.org/10.1111/oik.08366
Hayes JP, Jenkins SH (1997) Individual variation in mammals. J Mammal 78:274–293. https://doi.org/10.2307/1382882
Justino DG, Maruyama PK, Oliveira PE (2012) Floral resource availability and hummingbird territorial behaviour on a Neotropical savanna shrub. J Ornithol 153:189–197. https://doi.org/10.1007/s10336-011-0726-x
Kaiser HF, Rice J (1974) Little jiffy, mark IV. Educ Psychol Meas 34:111–117. https://doi.org/10.1177/001316447403400115
Keith SA, Baird AH, Hobbs JA, Woolsey ES, Hoey AS, Fadli N, Sanders NJ (2018) Synchronous behavioural shifts in reef fishes linked to mass coral bleaching. Nat Clim 8:986–991. https://doi.org/10.1038/s41558-018-0314-7
Keith SA, Bull JW (2017) Animal culture impacts species’ capacity to realise climate-driven range shifts. Ecography 40(2):296–304. https://doi.org/10.1111/ecog.02481
Koolhaas JM, Korte SM, de Boer SF, van der Vegt BJ, van Reenen CG, Hopster H, de Jong IC, Ruis MAW, Blokhuis HJ (1999) Coping style in animals: current status in behaviour and stress-physiology. Neurosci Biobehav Rev 23:925–935. https://doi.org/10.1016/S0149-7634(99)00026-3
Kralj-Fišer S, Schneider JM (2012) Individual behavioural consistency and plasticity in an urban spider. Anim Behav 84:197–204. https://doi.org/10.1016/j.anbehav.2012.04.032
Laskowski KL, Moiron M, Niemelä P (2020) Integrating behaviour in life-history theory : allocation versus acquisition? Trends Ecol Evol 36:132–138. https://doi.org/10.1016/j.tree.2020.10.017
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121. https://doi.org/10.2307/4087240
MacArthur RH, Pianka ER (1966) On optimal use of a patchy environment. Am Nat 100:603–609. https://doi.org/10.1086/282454
Mathot KJ, Wright J, Kempenaers B, Dingemanse NJ (2012) Adaptive strategies for managing uncertainty may explain personality-related differences in behavioural plasticity. Oikos 121:1009–1020. https://doi.org/10.1111/j.1600-0706.2012.20339.x
Maynard Smith J (1982) Evolution and the theory of games. Cambridge University Press, Cambridge
Maynard Smith JM, Price G (1973) The logic of animal conflict. Nature 246:15–18. https://doi.org/10.1038/246015a0
Mazzamuto MV, Merrick MJ, Bisi F, Koprowski JL, Wauters L, Martinoli A (2020) Timing of resource availability drives divergent social systems and home range dynamics in ecologically similar tree squirrels. Front Ecol Evol 8:174. https://doi.org/10.3389/fevo.2020.00174
Moiron M, Laskowski KL, Niemelä PT (2020) Individual differences in behaviour explain variation in survival: a meta-analysis. Ecology 23:399–408. https://doi.org/10.1111/ele.13438
Nagelkerken I, Munday PL (2016) Animal behaviour shapes the ecological effects of ocean acidification and warming: moving from individual to community-level responses. Glob Change Biol 22:974–989. https://doi.org/10.1111/gcb.13167
Nakagawa S, Schielzeth H (2010) Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev 85:935–956. https://doi.org/10.1111/j.1469-185X.2010.00141.x
Nash KL, Graham NAJ, Januchowski-Hartley FA, Bellwood DR (2012) Influence of habitat condition and competition on foraging behaviour of parrotfishes. Mar Ecol Prog Ser 457:113–124. https://doi.org/10.3354/meps09742
Niemelä PT, Dingemanse NJ (2018) On the usage of single measurements in behavioural ecology research on individual differences. Anim Behav 145:99–105. https://doi.org/10.1016/j.anbehav.2018.09.012
Nowicki JP, Walker SPW, Coker DJ, Hoey AS, Nicolet KJ, Pratchett MS (2018) Pair bond endurance promotes cooperative food defense and inhibits conflict in coral reef butterflyfish. Sci Rep 8(1):1–11. https://doi.org/10.1038/s41598-018-24412-0
Nussey DH, Wilson AJ, Brommer JE (2007) The evolutionary ecology of individual phenotypic plasticity in wild populations. J Evol Biol 20:831–844. https://doi.org/10.1111/j.1420-9101.2007.01300.x
Peiman K, Robinson B (2010) Ecology and evolution of resource-related heterospecific aggression. Q Rev Biol 85:133–158. https://doi.org/10.1086/652374
Powell A, Jones T, Smith DJ, Jompa J, Bell JJ (2015) Spongivory in the Wakatobi Marine National Park, Southeast Sulawesi, Indonesia. Pac Sci 69:487–508. https://doi.org/10.2984/69.4.5
Pratchett MS, Berumen ML, Kapoor B (2014) Biology of Butterflyfishes. CRC Press, Boca Raton. https://doi.org/10.1201/b15458
Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ (2007) Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318. https://doi.org/10.1111/j.1469-185X.2007.00010.x
Reese ES (1975) A comparative field study of the social behaviour and related ecology of reef fishes of the family Chaetodontidae. Z Tierpsychol 37:37–61. https://doi.org/10.1111/j.1439-0310.1975.tb01126.x
R Core Team (2019) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Righton D, Mills C (2007) Application of GIS to investigate the use of space in coral reef fish: a comparison of territorial behaviour in two Red Sea butterflyfishes. Int J Geogr Inf Syst 20:215–232. https://doi.org/10.1080/13658810500399159
Righton D, Miller M, Ormond R (1998) Correlates of territory size in the butterflyfish Chaetodon austriacus. J Exp Mar Biol Ecol 226:183–193. https://doi.org/10.1016/S0022-0981(97)00235-9
Schlaepfer MA, Runge MC, Sherman PW (2002) Ecological and evolutionary traps. Trends Ecol Evol 17:474–480. https://doi.org/10.1016/S0169-5347(02)02580-6
Sih A, Bell A, Johnson JC (2004) Behavioural syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378. https://doi.org/10.1016/j.tree.2004.04.009
Stimson J (1973) The role of the territory in the ecology of the intertidal limpet Lottia gigantea (Gray). Ecology 54:1020–1030. https://doi.org/10.2307/1935568
Thompson CA, Matthews S, Hoey AS, Pratchett MS (2019) Changes in sociality of butterflyfishes linked to population declines and coral loss. Coral Reefs 38:527–537. https://doi.org/10.1007/s00338-019-01792-x
Tricas T (1989) Determinants of feeding territory size in the coralivorous butterflyfish. Anim Behav 37:830–841. https://doi.org/10.1016/0003-3472(89)90067-5
Tuomainen U, Candolin U (2011) Behavioural responses to human-induced environmental change. Biol Rev 86:640–657. https://doi.org/10.1111/j.1469-185X.2010.00164.x
van Leeuwen CHA, Dalen K, Museth J, Junge C, Vøllestad LA (2017) Habitat fragmentation has interactive effects on the population genetic diversity and individual behaviour of a freshwater salmonid fish. River Res Appl 34:60–68. https://doi.org/10.1002/rra.3226
Wilson AJ (2018) How should we interpret estimates of individual repeatability? Evol Lett 2:4–8. https://doi.org/10.1002/evl3.40
Wolak ME, Fairbairn DJ, Paulsen YR (2012) Guidelines for estimating repeatability. Methods Ecol Evol 3:129–137. https://doi.org/10.1111/j.2041-210X.2011.00125.x
Wong BBM, Candolin U (2015) Behavioural responses to changing environments. Behav Ecol 26:665–673. https://doi.org/10.1093/beheco/aru183
Wyman RL, Hotaling L (1988) A test of the model of the economic defendability of a resource and territoriality using young Etroplus maculatus and Pelmatochromis subocellatus kribensis. Environ Biol Fish 21:69–76. https://doi.org/10.1007/BF02984444
Yabuta S (1999) Behaviors in agonistic interaction of the butterflyfish (Chaetodon lunulatus). J Ethol 18:11–15
Yabuta S (2008) Signal function of tail-up posture in the monogamous butterflyfish (Chaetodon lunulatus): dummy experiments in the field. Ichthyol Res 55:299–302. https://doi.org/10.1007/s10228-007-0027-9
Yabuta S, Berumen ML (2014) Social structure and spawning behaviour of Chaetodon butterflyfishes. In: Pratchett MS, Berumen ML, Kapoor BG (eds) Biology of butterflyfishes. CRC Press, Boca Raton, pp 200–225
Zambre AM, Arthur R (2018) Foraging plasticity in obligate corallivorous Melon butterflyfish across three recently bleached reefs. Ethology 124:302–310. https://doi.org/10.1111/eth.12733
Acknowledgements
The authors wish to thank Operation Wallacea and the staff of Hoga Island Marine Research Station for their assistance in this work, and for their guidance in the research permit application process. The authors would also like to thank Kayleigh Svensson, Afryan Maris and the student volunteers that assisted with the data collection. We would also like to thank the three anonymous reviewers for taking the time to read the manuscript and for their constructive suggestions.
Funding
This work was supported by the Natural Environment Research Council (SAK, grant number NE/S00050X/1 and RLG, grant number NE/L002604/1), with RLG’s studentship through the Envision Doctoral Training Partnership.
Author information
Authors and Affiliations
Contributions
RLG, IRH, ACA and SAK conceived the ideas. RLG designed the methodology, collected the data with support from NN and analysed the data. RLG led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Ethics approval
This is an observational study and as such ethical approval was not required. All research was conducted following applicable international guidelines for the use of animals. This work was conducted in partnership with Hasanuddin University, Makassar, with permission from RISTEK (research permit number: 171/E5/E5.4/SIP/2019).
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by J. Lindström
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gunn, R.L., Hartley, I.R., Algar, A.C. et al. Variation in the behaviour of an obligate corallivore is influenced by resource availability. Behav Ecol Sociobiol 76, 24 (2022). https://doi.org/10.1007/s00265-022-03132-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-022-03132-6