Abstract
Aggression is one of the most frequently studied behavioural traits across a wide range of taxa; however, most studies evaluate aggressive behaviour in a social context, in which aggressive interactions between conspecifics are motivated by resource control (offensive or social aggression). However, in an antipredator context, the primary role of aggression is defence (defensive or antipredator aggression). Although the neuroendocrinology of antipredator aggression is often studied in domesticated and laboratory animals, how environment and individual state affect this behavioural trait in the wild is largely unknown. Here, by conducting a manipulative experiment, we tested whether (i) consistent between-individual differences (i.e. animal personality) are present in antipredator aggression in adult male Carpetan rock lizards (Iberolacerta cyreni) and (ii) short-term environmental changes (presence vs. absence of predator cues) and differences in individual state (body length, head size, hind limb length) affect individual mean behaviour (i.e. behavioural type). We found moderate-high repeatability in antipredator aggression (willingness to bite a human), indicating the presence of animal personality in this behavioural trait. Lizards were on average more defensive in the presence of predator cues; furthermore, short-legged males showed higher antipredator aggression than long-legged males in the presence of predator cues, probably as an attempt to balance their decreased escape speed. Larger (~ older) males were more defensive than smaller ones, probably due to their increased fighting ability. We conclude that antipredator aggression is an important part of an individual’s behavioural repertoire and its expression is driven by both environmental situation and individual state.
Significance statement
Antipredator/defensive aggression is not the primary antipredatory response; however, when other ways of escape are not possible, actually hurting the predator could be the only way of survival. While this behaviour obviously has substantial effects on fitness, it is severely understudied compared to social/offensive aggression. In a manipulative experiment, we found that there are consistent between-individual differences in antipredator aggression (i.e. willingness to bite during handling) of adult male Carpetan rock lizards (Iberolacerta cyreni), supporting the presence of animal personality and suggesting that this behavioural trait might respond to natural selection. Furthermore, short-term environmental variation (i.e. presence vs. absence of predator cues) in interaction with individual state affected antipredator aggression of individuals, emphasising the ecological and evolutionary relevance of this behaviour.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aggression is broadly defined as any overt fighting behaviour or signal of imminent behaviour with the capacity of harm (Moyer 1968; Huntingford 1976; Nelson 2006; Cain et al. 2011). Evolutionary behavioural ecology of consistent individual behavioural differences across time and ecological situations in a population (i.e. animal personality) became intensively studied during the last decades (for reviews and meta-analyses see Réale et al. 2007; Sih and Bell 2008; Bell et al. 2009; Sih et al. 2012; Garamszegi et al. 2013; Niemelä and Dingemanse 2018), and aggression is among the most frequently studied behavioural traits in this field across several taxa (Mafli et al. 2011; Sih et al. 2015; Santostefano et al. 2016; Michelangeli et al. 2017; Szász et al. 2019). However, aggression is usually evaluated in a social context, where it is motivated by resource control, including territorial and dominance disputes (often termed ‘offensive aggression’; see Nelson 2006). As members of the same species tend to utilise the same habitats and resources, the most common form of offensive aggression is the agonistic interaction between conspecifics (e.g. male-male combat; see McEvoy et al. 2013; Lee et al. 2014; Briffa et al. 2015); however, in a wider sense, the challenger does not necessarily has to be a conspecific (e.g. agonistic encounters between lions and hyenas over each other’s prey; Watts and Holekamp 2008). Importantly, offensive aggressive encounters rarely result in death and the outcome of the interactions highly depends on the fighting capabilities of the opponents (Nelson 2006). In contrast, aggression in an antipredator context is primarily and directly motivated by imminent danger of death (often termed as ‘defensive aggression’; see Nelson 2006; Cain et al. 2011). However, using the terms offensive vs. defensive aggression can be sometimes misleading, because (i) the weaker party in an offensive aggressive fight might actually defend itself via aggressive actions, while (ii) defensive aggression might include potentially dangerous prey behaving offensively towards a (yet) passive predator as a preventive measure. Therefore, we prefer and use the terms social (instead of offensive) and antipredator (instead of defensive) aggression in our paper. Antipredator aggression is typically triggered by near-contact proximity of a potential predator. Antipredator attack (e.g. bite) is not a primary antipredatory response, as it occurs only if fleeing is not possible, or defensive threats, such as vocalisation, defensive posture or displaying teeth and/or claws are ineffective and the only feasible way to escape is trying to actually hurt the predator (Cowlishaw 1994; Nelson 2006; Blanchard et al. 2008). Hence, antipredator aggression could be seen as a ‘last resort’ against predation, similarly to another, yet completely opposite antipredator behavioural trait, tonic immobility (i.e. the lack of movement; see Gallup and Rager 1996; Edelaar et al. 2012; Horváth et al. 2019).
Neuroendocrine control of antipredator aggression and its relationship with stress is well studied, although mainly in domesticated and laboratory animals (e.g. rodents; see Rodgers and Depaulis 1982; Popova 2005; Pesce et al. 2011), which are often selected to show relatively little defence to handling. In contrast, how environment and individual state affect this behavioural trait in the wild is less studied (Cury de Barros et al. 2010; Baxter-Gilbert and Riley 2018). In behavioural ecology, an individual’s state reflects any feature that affects the cost and benefits of its behavioural actions (Houston and McNamara 1999). These features may involve both inherently stable (e.g. sex differences, morphology) and labile features (e.g. energy reserves, health state, reproductive value). In addition, labile differences may include not only internal characteristics but the social environment (e.g. local density, behaviour of social partners) and ecological environment (e.g. predator, competitors, parasites) as well (see Sih et al. 2015). As mentioned above, predatory imminence is the main stimulus activating antipredator aggression; however, some stimuli may represent different range of imminence of threat. For instance, presence of active predators usually triggers high level of defensive behaviour, while passive predators do not (Blanchard et al. 2008). Similarly, one would expect that stimuli representing high level of risk (e.g. visual) should elicit stronger antipredator responses and defensiveness than cues representing the potential presence of predators without immediate risk (e.g. chemical cues) (Amo et al. 2006). Nonetheless, predator chemical cues (kairomones) were repeatedly shown to be enough to trigger glucocorticoid stress hormone release (e.g. corticosterone; Cockrem and Silverin 2002; Thomas et al. 2006; Trompeter and Langkilde 2011), and even short-term exposure to predator odour affects antipredator behaviour substantially (de Paula et al. 2005; Teyssier et al. 2014; Lloren et al. 2019) and may trigger defensive behaviour (Kalynchuk et al. 2004). Additionally, individual differences in antipredator aggression depending on labile intrinsic state variables are expected to change fast, as the underlying mechanisms are highly variable in time. For instance, in aggressive encounters between conspecifics, the outcome of aggressive interactions is often affected by body size and weight (see Rowland 1989; Nowbahari et al. 1999), and thus larger prey individuals might rely more on antipredator aggression than smaller ones. Furthermore, weaponry can be a predictor of fight success as well. In lizards, head size is an important proxy of fighting ability (Gvozdik and Van Damme 2003), and thus large-headed individuals likely defend themselves more effectively from predators than small-headed conspecifics. On the other hand, individuals better at escaping might employ an antipredator strategy where defensiveness is less prominent. In lizards, relative limb length was shown to positively predict locomotor performance (Bauwens et al. 1995); hence, individuals with relatively shorter limbs might rely more on antipredator aggression than their conspecifics with longer limbs.
Here, we tested (i) whether antipredator aggression personality is present in adult male Carpetan rock lizards (Iberolacerta cyreni), and (ii) whether short-term environmental changes (presence vs. absence of predator cues) and differences in individual state (body size, head size, and hind limb length) affect antipredator aggression behavioural type (individual mean behaviour). To this end, we performed a manipulative experiment in which we repeatedly tested each individual’s defensiveness both in the presence and absence of predator chemical cues (i.e. scent of smooth snake, Coronella austriaca). As detecting predator cues connotes a stressful situation, we predicted lizards to show stronger antipredator aggression after being exposed to smooth snake scent. According to the state-dependent safety principle, individuals with higher state are expected to show higher behavioural activity for its benefits, because their high state allows them to deal with the increased costs (Luttbeg and Sih 2010). Hence, we expected males with higher state regarding antipredator aggression (larger individuals with larger heads; Gvozdik and Van Damme 2003) to be more defensive than lower-state conspecifics. We also expected males with higher state regarding escape performance (individuals with longer limbs; Bauwens et al. 1995; Vanhooydonck et al. 2001) to be less defensive than males with lower expected escape abilities.
Materials and methods
Study animals
We noosed 25 adult male I. cyreni between 15 and 17 May of 2016 (coinciding with the peak of the mating period of this species) at the ‘Alto del Telégrafo’ peak (Sierra de Guadarrama, Madrid prov., Spain, 1900 m asl). The animals were transported to the ‘El Ventorrillo’ field station (MNCN-CSIC), approximately 5 km from the capture site, where they were housed individually outdoor in grey opaque boxes (57 cm × 37 cm × 30 cm; length, width, height, respectively). In the boxes, we used a layer of coconut fibres as substrate (2–3 cm thick, approximately), and we provided shelters (one per box) made of fibreboards (20 cm × 15 cm × 1 cm; length, width, height, respectively). The shelters were made without bottom and were open from the front, providing a suitable hiding place for the lizards, but the shelters could be also quickly removed with minimal disturbance to the animals. Between the experimental trials, we covered the boxes with fine metal mesh to protect animals from bird predators. Individuals spent 10–12 days in the boxes used for the experiment to acclimate before the treatments started. Water and food (house crickets, Acheta domestica) were provided ad libitum. At the end of the experiment, all lizards were released at their original capture sites, without any sign of injury.
Individual traits
We measured snout-vent length (SVL; 67.39 ± 3.66; mean ± standard deviation (SD)), head size, and the length of the limbs of lizards using a digital calliper to the nearest 0.01 mm. To characterise head size, we ran a principal component analysis on four head measures (head length, head width, jaw width and head height) that produced a single principal component with strong positive loadings (61% variation explained; all factor loadings > 0.67), which we used in our analyses as a head size variable. To characterise limb length, we measured the left and right femurs and tibias, and then summing the mean femur (19.97 ± 2.06; mean ± SD) and tibia lengths (18.19 ± 1.19; mean ± SD) for every individual. Because during the experiment all animals received food ad libitum, we did not analyse body weight. Note that adding body weight measured at various stages during the experiment to our models never changed the results qualitatively (data not shown).
Behavioural assays
Behavioural assays took place on five consecutive days (between May 28th and June 2nd; we skipped an observation on 29 May because of thick cloud cover and rain). Individuals were tested in their home cages, as this approach more likely resembles how animals react in their natural home ranges (Beckmann and Biro 2013). We aimed to test individual behaviour in two, ecologically relevant situations. Thus, we placed a filter paper (4 cm × 22 cm) impregnated with the scent of a smooth snake (Coronella austriaca) inside the terraria of half of the lizards (Van Damme and Quick 2001; Amo et al. 2006). This snake is a saurophagous specialist, the main predator of I. cyreni (Martín 2015). The donor snake came from the same area where we had captured the lizards. Impregnated filter papers were obtained by placing them in the bottom of the snake’s terrarium for 24 h before the experiments. Conversely, we placed a scentless clean filter paper of the same size into the terraria of the remaining half of lizards. Individuals were repeatedly tested in both situations (on different days). The order of exposure to the treatments was semi-randomised such that each individual received a sequence of treatments (Briffa 2013) (see Supplementary Text for more information). Filter papers were placed at 9.00 am (UTC + 2.00) and at the same time, shelters were removed. Tests for antipredator aggression took place the same day at 12.00 pm. We note that defensive responses of lizards are strongly dependent on temperature (Hertz et al. 1982; Crowley and Pietruszka 1983). As all behavioural data reported here were obtained on low wind, sunny days, and environmental variation between cages should have been minimal and random, we are certain that all experimental lizards could reach their preferred body temperature. Therefore, observed differences in the experiment should reflect individual differences in antipredator aggression rather than temperature effects. Following a similar standardised procedure in all trials, the same experimenter (GeH) caught the lizard by hand and recorded individual attempts of antipredator defensive bites while being held in the hand for 1 min. We treated antipredator aggression as a binomial variable, giving score 1 to individuals that attempt to bite the experimenter and score 0 to those lizards that did not. We note here that besides antipredator aggression, we also tested for social aggression of each individual towards an ‘intruder’ male placed into the focal (resident) male’s home cage between 10.30 and 11.00 am. Results from these tests are not going to be discussed in this paper. However, we controlled for the potential effects of these tests on the antipredator aggression tests discussed here (see ‘Statistical analyses’). Behavioural assessment was not blind regarding the test animals’ identity; however, it does not pose a problem since the subjectivity in our methods was minimal.
Statistical analyses
To estimate repeatability for the binomial antipredator aggression data, we applied a generalised linear mixed model (GLMM) with a binomial distribution and logit link function on the pooled sample. In this model, antipredator aggression was the response variable. We also built separate GLMMs to examine the effect of treatments on repeatability. We used the rptR add-on package following the methods of Nakagawa and Schielzeth (2010). This method uses a multiplicative overdispersion GLMM with a logit link and using penalised quasi likelihood (PQL) estimation for repeatability on the original scale. Significance was estimated by randomisation tests. However, we report repeatabilities estimated on the underlying latent (link) scale as most original-scale repeatabilities are conditional for non-Gaussian data (Nakagawa and Schielzeth 2010). To control for variance explained by size differences between focal males, we added SVL as fixed effect to GLMMs otherwise similar to the previous ones and calculated enhanced agreement repetabilities (see Stoffel et al. 2017). Variance explained by the fixed effects are calculated by the variance on the link scale.
To test whether the treatments affected the mean antipredator aggression, we ran a binomial GLMM with logit link. In our model, antipredator aggression was the response variable, while predation treatment, SVL, relative head length, relative hind limb length and the two-way interactions of treatment and all morphology traits as fixed effects. ‘Relative’ length variables were residuals from trait—SVL linear regressions. We used them instead of the original raw variables to avoid multicollinearity. In a pilot GLMM, we tested the potential effects of preceding social aggression tests on antipredator aggression. Due to logistic constraints, we had to use intruder males (used only as stimuli) both smaller and larger than the focal individuals in the social aggression tests. Therefore, to test for the effects of social aggression tests, we added the SVL difference between resident and intruder males (integer variable, negative numbers indicating large intruder while positive numbers indicating small intruder males) as a correction variable; however, as this variable did not affect antipredator aggression (see Supplementary Table 1), we excluded this variable from our final model. Fixed effects were tested by Wald’s chi-square tests, while random effects were estimated using likelihood ratio tests (LRT). The models were run with the lme4 package (Bates et al. 2015). We tested potential habituation effects by including the z-transformed (standardised to mean = 0, standard deviation = 1) order of trials (hereafter: time) both as a single-fixed effect and random slopes (i.e. the interaction with individual) in our mixed-effect models. Random-intercept (random effect: individual) and random slope (random effects: individual, individual × time) models were compared; we decided to leave the random slope term in the final model only if it improved the model fit. We report marginal and conditional R2 estimations for our models based on the method of Nakagawa and Schielzeth (2013). All analyses were performed using R 4.0.2 (R Developmental Core Team 2020).
Results
Behavioural repeatability
Randomisation tests indicated significant repeatability of antipredator aggression across all assays (Table 1). As randomisation test gives robust measures of statistical significance in the case of non-Gaussian data (Nakagawa and Schielzeth 2010), we consider antipredator aggression personality being present in all treatments and also in the pooled sample. Size differences explained a substantial amount of phenotypic variation (Table 1), reducing the relative contribution of individual differences. Nevertheless, there was still significant moderate-high repeatability present at the individual level regarding both treatments (Table 1). Since confidence intervals highly overlapped between the treatments, the difference between repeatability estimates cannot be considered as significant.
Behavioural type
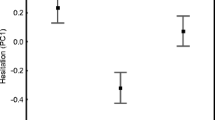
The GLMM indicated significant effect of perceived predation risk on antipredator aggression (Table 2, Fig. 1): lizards were on average more defensive in the presence of predator cues. Furthermore, we found significant predator treatment effect and predator treatment × relative hind limb length interaction (see Table 2). To interpret the interaction, we ran two separate GLMMs for the presence and absence of predator treatments. We found a negative relationship between relative hind limb length and antipredator aggression in the presence (χ2 = 3.92, df = 1, P = 0.047; Fig. 2a), but not in the absence of predator (χ2 = 0.72, df = 1, P = 0.39; Fig. 2b). Moreover, SVL had a significant effect on antipredator aggression (Table 2, Fig. 3): larger males were more defensive than smaller conspecifics. Time effect was also significant (Table 2), individuals became more defensive by time; however, individual trends did not differ (Table 2). The fixed effects explained 54%, while the whole model 84% of the total variance, which can be seen as good explanatory power for behavioural variables.
Discussion
Antipredator aggression can be seen as prey’s last resort when escape from the predators failed and prey is being caught. This behaviour is obviously affecting prey’s fitness; however, it is severely understudied in natural populations compared to social aggression (but see Cury de Barros et al. 2010; Markó et al. 2013; Baxter-Gilbert and Riley 2018). Here, by conducting a manipulative experiment with adult male I. cyreni, we asked whether animal personality (consistent between-individual difference) is present in antipredator aggression and also tested whether ecological conditions (presence vs. absence of perceived predation risk before the trials) and individual state (body size, head size, limb length) affect the expression of this behaviour. We found strong indication for antipredator aggression personality, both in the presence and absence of perceived predation risk before the mimicked attack, emphasising that this trait is an important aspect of the behavioural repertoire of I. cyreni and might be under natural selection. We found lizards exposed to chemical stimuli from their snake predator, and larger males in general to show increased antipredator aggression. We also found an environment-dependent individual state effect: individuals with relatively short legs were more defensive compared to their long-legged conspecifics, but only after exposure to snake predator cues. Individuals became more defensive by time; however, habituation is assumed to reduce unnecessary antipredator responses (i.e. probability of bites) (Rodríguez-Prieto et al. 2010, 2011; Vincze et al. 2016); thus, our pattern suggests a reverse response. Such response is known as sensitization: an internal mechanism that intensifies behavioural response to constant stimulation (Bee 2001; Martin and Réale 2008; Stamps et al. 2012; Osborn and Briffa 2017). Below, we discuss first the relevance and consequences of animal personality being present in antipredator aggression, and second, the effects of external environment and internal state on this behaviour.
Behavioural repeatability
Antipredator aggression was significantly repeatable within individuals. We are aware that the wide confidence intervals of our repeatability estimates indicate that our sample size is somewhat low, especially in terms of repeated measures. However, we note that all of the enhanced agreement repeatability estimate presented here can be seen as moderate-high (see Bell et al. 2009); hence, we think that the pattern we present regarding the presence of behavioural repeatability (a statistical test for animal personality) is robust. While body size variation explained a substantial proportion of behavioural variation (larger lizards showing higher antipredator aggression), there was still significant between-individual behavioural difference present in the examined population after controlling for body size. Thus, the most important question our result raises is what are the costs of antipredator aggression, i.e. why do some individuals not defend themselves actively in a supposedly life-threatening situation? Unarguably, being captured by a predator rarely holds any positive outcomes for the prey regardless its behaviour; yet, if survival is on the stake, a defensive attack may initially provide some advantage (Blanchard et al. 2008). However, there are other passive alternative antipredator strategies in many animals. In particular, tonic immobility, when the prey remains motionless when captured by a predator, or in other situations of extreme fear is also widespread (Gallup and Rager 1996; Miyatake et al. 2008). It has been argued that tonic immobility can increase prey survival because the lack of movement makes the predator believe it already killed the prey and stop the attack, releasing the prey to start ingestion, allowing the prey to escape (Thompson et al. 1981). Even though we did not quantify ‘struggling’, we observed that lizards either vigorously struggled and bit or they were passive. Therefore, the absence of defensive behaviour in some of the lizards in our experiment might be considered as a sign of tonic immobility. Tonic immobility strategy has been previously considered in personality studies (Edelaar et al. 2012; Santos et al. 2015; Horváth et al. 2019). Our present findings hint that between-individual differences in antipredatory last resort behavioural traits might be interpreted along a tonic immobility-antipredator aggression continuum. This hypothesis warrants further studies.
Behavioural type
In line with our expectations, lizards exposed to chemical cues from their snake predator were more defensive in general than males from the control treatment. Previous experimental results show that olfactory predator cues are able to induce strong antipredator behavioural responses in various amphibian and reptile taxa (e.g. Rana dalmatina tadpoles, see Hettyey et al. 2015; Urszán et al. 2015a, b, 2018; R. latastei tadpoles, see Scribano et al. 2020; Anguis fragilis, see Cabido et al. 2004; Podarcis muralis, see Amo et al. 2006; P. tliguerta, P. sicula and Lacerta bedriagae, see Van Damme and Quick 2001); nevertheless, studies rarely test the effect of these treatments on antipredator attacks. Similar to our present findings, López and Martín (2001) reported the fossorial amphisbaenian Blanus cinereus to show increased willingness to bite towards predator-scent (Coronella girondica and Scolopendra sp.) impregnated swabs than control stimuli. These results suggest that presence of chemical cues from predators connotes a stressful situation which leads to increased antipredator aggression. However, as predator presence vs. absence was changed daily in our experiment, the detected pattern here could be seen as short-term responses, representing a form of activational behavioural plasticity (see Snell-Rood 2013). Furthermore, partly in line with our expectations, males with shorter hind legs showed increased antipredator aggression, but only after exposure to snake predator cues. Limb length was showed to be strongly positively correlated with sprint speed (i.e. locomotor performance; Bauwens et al. 1995; Vanhooydonck et al. 2001), which is an important component of life history trade-offs and suitable proxy of individual quality (Garland 1984; Le Galliard et al. 2004; Irschick et al. 2008; Husak et al. 2016; Winchell et al. 2018). Hence, males with longer hind legs could be seen as higher-, while short-legged males as lower-state individuals. A comparable pattern was reported by Hertz et al. (1982), who found a negative relationship between body temperature (high body temperature increases locomotor performance) and defensiveness in the lizards Agama savignyi and A. pallida. In line with these previous results, it is plausible that longer-legged males have higher chance to outrun and out-manoeuver potential predators, thus rely less on defensive attacks to escape. Furthermore, as predator cues in our experiment indicate the presence of an ambush snake predator, an active escape might trigger a quick predator attack. As the ability to escape highly depends on sprint speed, short-legged males are at a disadvantage compared to long-legged conspecifics, thus, holding their ground and confronting the predator actively might be the best strategy for them. Increased antipredator aggression under constrained locomotor performance in tegu lizards (Tupinambis merianae) was shown previously by Cury de Barros et al. (2010). Also in line with the predictions, larger (~ older) males were more defensive in general than smaller (~ younger) conspecifics. In a previous study, large I. cyreni individuals were found to be more prone to flight (i.e. more risk-averse) than smaller ones (Martín and López 2003). This pattern corresponds to state-dependent safety principle (Luttbeg and Sih 2010): although larger lizards are more conspicuous to predators and thus they flee earlier than smaller ones (Martín and López 2003; Baxter-Gilbert and Riley 2018), when cornered, they can confront the predator with higher chance than smaller conspecifics due to their more powerful bites (Gvozdik and Van Damme 2003; Cury de Barros et al. 2010).
Conclusion
Taken together, we found antipredator aggression showing consistent between-individual differences in the studied population of I. cyreni males, suggesting that this behavioural trait (i) should be considered in animal personality studies and (ii) might respond to natural selection assuming that the between-individual variation has a genetic component. We are aware that significant repeatability during a short period cannot be seen as a sign of animal personality in the classical sense, as stable individual differences throughout the life or several years. However, we think that such differences are still informative in the given ecological context, i.e. the short and highly synchronised mating season (e.g. Horváth et al. 2016, 2017). Antipredator aggression was positively associated with the presence of predator cues and body size (regardless of the presence of predator cues), while relatively short-legged males showed increased defensiveness only when predator cues were present, probably as an antipredatory strategy compensating limited escape speed. Results of our experiment indicate that differences in inherently stable state variables (size, hind limb length) affect individual antipredator aggression, further emphasising the ecological and evolutionary relevance of this behaviour. Nevertheless, as other inherently labile traits, such as levels of testosterone are proved to substantially affect intraspecific social aggression in a various of taxa (Veiga et al. 1998; Weiss and Moore 2004; O’Connor et al. 2014; Szász et al. 2019), further studies are needed to reveal potential background mechanisms behind variation in antipredator aggression. Linking antipredator- and social aggression in a behavioural syndrome framework (Sih et al. 2004; Dingemanse et al. 2012; Dochtermann and Dingemanse 2013), as well as understanding the inheritance of antipredator aggression also warrants future studies.
Data availability
Data are available at Figshare: https://doi.org/10.6084/m9.figshare.13067024.
References
Amo L, López P, Martín J (2006) Can wall lizards combine chemical and visual cues to discriminate predatory from non-predatory snakes inside refuges? Ethology 112:478–484. https://doi.org/10.1111/j.1439-0310.2005.01170.x
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bauwens D, Garland T, Castilla A, Van Damme R (1995) Evolution of sprint speed in lacertid lizards: morphological, physiological and behavioural covariation. Evolution 49:848–863
Baxter-Gilbert J, Riley JL (2018) Runners and fighters: clutch effects and body size drive innate antipredator behaviour in hatchling lizards. Behav Ecol Sociobiol 72:97. https://doi.org/10.1007/s00265-018-2505-7
Beckmann C, Biro PA (2013) On the validity of a single (boldness) assay in personality research. Ethology 119:937–947. https://doi.org/10.1111/eth.12137
Bee MA (2001) Habituation and sensitization of aggression in bullfrogs (Rana catesbeiana): testing the dual-process theory of habituation. J Comp Psychol 115:307–316. https://doi.org/10.1037/0735-7036.115.3.307
Bell AM, Hankison SJ, Laskowski KL (2009) The repeatability of behaviour: a meta-analysis. Anim Behav 77:771–783. https://doi.org/10.1016/j.anbehav.2008.12.022
Blanchard RJ, Blanchard DC, Griebel G, Nutt DJ (eds) (2008) Handbook of anxiety and fear. Academic Press, London
Briffa M (2013) Plastic proteans: reduced predictability in the face of predation risk in hermit crabs. Biol Lett 9:20130592. https://doi.org/10.1098/rsbl.2013.0592
Briffa M, Sneddon LU, Wilson AJ (2015) Animal personality as a cause and consequence of contest behaviour. Biol Lett 11:20141007
Cabido C, Gonzalo A, Galán P, Martín J, López P (2004) Chemosensory predator recognition induces defensive behavior in the slow-worm (Anguis fragilis). Can J Zool 82:510–515. https://doi.org/10.1139/z04-023
Cain K, Rich M, Ainsworth K, Ketterson E (2011) Two sides of the same coin? Consistency in aggression to conspecifics and predators in a female songbird. Ethology 117:786–795. https://doi.org/10.1038/jid.2014.371
Cockrem JF, Silverin B (2002) Sight of a predator can stimulate a corticosterone response in the great tit (Parus major). Gen Comp Endocrinol 125:248–255. https://doi.org/10.1006/gcen.2001.7749
Cowlishaw G (1994) Vulnerability to predation in baboon populations. Behaviour 131:293–304
Crowley SR, Pietruszka RD (1983) Aggressiveness and vocalization in the leopard lizard (Gambelia wislizennii): the influence of temperature. Anim Behav 31:1055–1060. https://doi.org/10.1016/S0003-3472(83)80012-8
Cury de Barros F, Eduardo de Carvalho J, Abe AS, Kohlsdorf T (2010) Fight versus flight: the interaction of temperature and body size determines antipredator behaviour in tegu lizards. Anim Behav 79:83–88. https://doi.org/10.1016/j.anbehav.2009.10.006
de Paula HGM, Gouveia A, Vinícius De Almeida M, Hoshino K (2005) Anxiety levels and wild running susceptibility in rats: assessment with elevated plus maze test and predator odor exposure. Behav Process 68:135–144. https://doi.org/10.1016/j.beproc.2004.12.003
Dingemanse NJ, Dochtermann NA, Nakagawa S (2012) Defining behavioural syndromes and the role of “syndrome deviation” in understanding their evolution. Behav Ecol Sociobiol 66:1543–1548. https://doi.org/10.1007/s00265-012-1416-2
Dochtermann NA, Dingemanse NJ (2013) Behavioral syndromes as evolutionary constraints. Behav Ecol 24:806–811. https://doi.org/10.1093/beheco/art002
Edelaar P, Serrano D, Carrete M, Blas J, Potti J, Tella JL (2012) Tonic immobility is a measure of boldness toward predators: an application of Bayesian structural equation modeling. Behav Ecol 23:619–626. https://doi.org/10.1093/beheco/ars006
Gallup G, Rager D (1996) Tonic immobility as a model of extreme states of behavioral inhibition. In: Sanberg P, Ossenkopp K-P, Kavaliers M (eds) Motor activity and movement disorders: re- search issues and applications. Humana Press, Totowa, NJ, pp 57–80
Garamszegi LZ, Markó G, Herczeg G (2013) A meta-analysis of correlated behaviors with implications for behavioral syndromes: relationships between particular behavioral traits. Behav Ecol 24:1068–1080. https://doi.org/10.1093/beheco/art033
Garland T (1984) Physiological correlates of locomotory performance in a lizard: an allometric approach. Am J Phys 247:R806–R815
Gvozdik L, Van Damme R (2003) Evolutionary maintenance of sexual dimorphism in head size in the lizard Zootoca vivipara: a test of two hypotheses. J Zool 259:7–13. https://doi.org/10.1017/s0952836902003308
Hertz PE, Huey RB, Nevo E (1982) Fight versus flight: body temperature influences defensive responses of lizards. Anim Behav 30:676–679. https://doi.org/10.1016/S0003-3472(82)80137-1
Hettyey A, Tóth Z, Thonhauser KE, Frommen JG, Penn DJ, Van Buskirk J (2015) The relative importance of prey-borne and predator-borne chemical cues for inducible antipredator responses in tadpoles. Oecologia 179:699–710. https://doi.org/10.1007/s00442-015-3382-7
Horváth G, Martín J, López P, Garamszegi LZ, Bertók P, Herczeg G (2016) Blood parasite infection intensity covaries with risk-taking personality in male Carpetan rock lizards (Iberolacerta cyreni). Ethology 122:355–363. https://doi.org/10.1111/eth.12475
Horváth G, Martín J, López P, Garamszegi LZ, Herczeg G (2017) Food and vitamin D3 availability affects lizard personalities: an experiment. Behav Ecol Sociobiol 71:27. https://doi.org/10.1007/s00265-016-2257-1
Horváth G, Garamszegi LZ, Bereczki J, Urszán TJ, Balázs G, Herczeg G (2019) Roll with the fear: environment and state dependence of pill bug (Armadillidium vulgare) personalities. Sci Nat 106:7. https://doi.org/10.1007/s00114-019-1602-4
Houston AI, McNamara JM (1999) Models of adaptive behaviour. Cambridge University Press, Cambridge
Huntingford FA (1976) The relationship between anti-predator behaviour and aggression among concspecifics in the three-spined stickleback, Gasterosteus aculeatus. Anim Behav 24:245–260
Husak JF, Ferguson HA, Lovern MB (2016) Trade-offs among locomotor performance, reproduction and immunity in lizards. Funct Ecol 30:1665–1674. https://doi.org/10.1111/1365-2435.12653
Irschick DJ, Meyers JJ, Husak JF, Le Galliard J-F (2008) How does selection operate on whole-organism functional performance capacities? A review and synthesis. Evol Ecol Res 10:177–196
Kalynchuk LE, Gregus A, Boudreau D, Perrot-Sinal TS (2004) Corticosterone increases depression-like behavior, with some effects on predator odor-induced defensive behavior, in male and female rats. Behav Neurosci 118:1365–1377. https://doi.org/10.1037/0735-7044.118.6.1365
Le Galliard J-F, Clobert J, Ferrière R (2004) Physical performance and Darwinian fitness in lizards. Nature 432:502–505. https://doi.org/10.1038/nature03057
Lee VE, Head ML, Carter MJ, Royle NJ (2014) Effects of age and experience on contest behavior in the burying beetle, Nicrophorus vespilloides. Behav Ecol 25:172–179. https://doi.org/10.1093/beheco/art101
Lloren JI, Davidson SM, Twardek WM, Elvidge CK (2019) Baseline activity and shoal type determine antipredator behaviors in bluegill from a southern Ontario lake. Behav Ecol Sociobiol 73:57. https://doi.org/10.1007/s00265-019-2669-9
López P, Martín J (2001) Chemosensory predator recognition induces specific defensive behaviours in a fossorial amphisbaenian. Anim Behav 62:259–264. https://doi.org/10.1006/anbe.2001.1762
Luttbeg B, Sih A (2010) Risk, resources and state-dependent adaptive behavioural syndromes. Philos Trans R Soc B 365:3977–3990. https://doi.org/10.1098/rstb.2010.0207
Mafli A, Wakamatsu K, Roulin A (2011) Melanin-based coloration predicts aggressiveness and boldness in captive eastern Hermann’s tortoises. Anim Behav 81:859–863. https://doi.org/10.1016/j.anbehav.2011.01.025
Markó G, Azcárate M, Hegyi G, Herczeg G, Laczi M, Nagy G, Señar JC, Török J, Garamszegi LZ (2013) Behavioural responses to handling stress in the great tit: within-individual consistency and the effect of age, sex and body condition. Ornis Hungarica 21:12–25. https://doi.org/10.2478/orhu-2013-0012
Martín J (2015) Lagartija carpetana–Iberolacerta cyreni (Müller y Hellmich, 1937). In: Marco A (ed) Salvador A. Enciclopedia Virtual de los Vertebrados Españoles, Madrid, pp 1–9
Martín J, López P (2003) Ontonegenetic variation in antipredator behavior of Iberian rock lizards (Lacerta monticola): effects of body-size-dependent thermal-exchange rates and costs of refuge use. Can J Zool 81:1131–1137. https://doi.org/10.1139/z03-094
Martin JGA, Réale D (2008) Temperament, risk assessment and habituation to novelty in eastern chipmunks, Tamias striatus. Anim Behav 75:309–318. https://doi.org/10.1016/j.anbehav.2007.05.026
McEvoy J, While GM, Sinn DL, Wapstra E (2013) The role of size and aggression in intrasexual male competition in a social lizard species, Egernia whitii. Behav Ecol Sociobiol 67:79–90. https://doi.org/10.1007/s00265-012-1427-z
Michelangeli M, Smith CR, Wong BBM, Chapple DG (2017) Aggression mediates dispersal tendency in an invasive lizard. Anim Behav 133:29–34. https://doi.org/10.1016/j.anbehav.2017.08.027
Miyatake T, Tabuchi K, Sasaki K, Okada K, Katayama K, Moriya S (2008) Pleiotropic antipredator strategies, fleeing and feigning death, correlated with dopamine levels in Tribolium castaneum. Anim Behav 75:113–121. https://doi.org/10.1016/j.anbehav.2007.04.019
Moyer KE (1968) Kinds of aggression and their physiological basis. Commun Behav Biol 2:65–87
Nakagawa S, Schielzeth H (2010) Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev 85:935–956. https://doi.org/10.1111/j.1469-185X.2010.00141.x
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142. https://doi.org/10.1111/j.2041-210x.2012.00261.x
Nelson RJ (ed) (2006) Biology of aggression. Oxford University Press, Oxford
Niemelä PT, Dingemanse NJ (2018) On the usage of single measurements in behavioural ecology research on individual differences. Anim Behav 145:99–105. https://doi.org/10.1016/j.anbehav.2018.09.012
Nowbahari E, Fénéron R, Malherbe MC (1999) Effect of body size on aggression in the ant, Cataglyphis niger (Hymenoptera; Formicidae). Aggress Behav 25:369–379. https://doi.org/10.1002/(SICI)1098-2337(1999)25:5<369::AID-AB5>3.0.CO;2-C
O’Connor JL, McBrayer LD, Higham TE, Husak JF, Moore IT, Rostal DC (2014) Effects of training and testosterone on muscle fiber types and locomotor performance in male six-lined racerunners (Aspidoscelis sexlineata). Physiol Biochem Zool 84:394–405. https://doi.org/10.1086/660850
Osborn A, Briffa M (2017) Does repeatable behaviour in the laboratory represent behaviour under natural conditions? A formal comparison in sea anemones. Anim Behav 123:197–206. https://doi.org/10.1016/j.anbehav.2016.10.036
Pesce M, Speranza L, Franceschelli S, Ialenti V, Patruno A, Febo MA, De Lutiis MA, Felaco M, Grilli A (2011) Biological role of interleukin-1beta in defensive aggressive behavior. J Biol Reg Homeos Ag 25:323–329
Popova NK (2005) Brain serotonin in genetically defined defensive behaviour. In: Miller R, Ivanitsky AM, Balaban PM (eds) Complex brain functions. Conceptual Advances in Russian Neuroscience. Taylor & Francis e-Library, pp 309–321
R Developmental Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org
Réale D, Reader SM, Sol D, McDougal PT, Dingemanse NJ (2007) Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318. https://doi.org/10.1111/j.1469-185X.2007.00010.x
Rodgers RJ, Depaulis A (1982) GABAergic influences on defensive fighting in rats. Pharmacol Biochem Behav 17:451–456. https://doi.org/10.1016/0091-3057(82)90303-3
Rodríguez-Prieto I, Martín J, Fernández-Juricic E (2010) Habituation to low-risk predators improves body condition in lizards. Behav Ecol Sociobiol 64:1937–1945. https://doi.org/10.1007/s00265-010-1004-2
Rodríguez-Prieto I, Martín J, Fernández-Juricic E (2011) Individual variation in behavioural plasticity: direct and indirect effects of boldness, exploration and sociability on habituation to predators in lizards. Proc R Soc Lond B 278:266–273. https://doi.org/10.1098/rspb.2010.1194
Rowland WJ (1989) The effects of body size, aggression and nuptial coloration on competition for territories in male threespine sticklebacks, Gasterosteus aculeatus. Anim Behav 37:282–289. https://doi.org/10.1016/0003-3472(89)90117-6
Santos CD, Cramer JF, Pârâu LG, Miranda AC, Wikelski M, Dechmann DKN (2015) Personality and morphological traits affect pigeon survival from raptor attacks. Sci Rep 5:15490. https://doi.org/10.1038/srep15490
Santostefano F, Wilson AJ, Araya-Ajoy YG, Dingemanse NJ (2016) Interacting with the enemy: indirect effects of personality on conspecific aggression in crickets. Behav Ecol 27:1235–1246. https://doi.org/10.1093/beheco/arw037
Scribano G, Balestrieri A, Gazzola A, Pellitteri-Rosa D (2020) Strong behavioural defensive responses of endemic Rana latastei tadpoles induced by a native predator’s odour. Ethology 126:922–930. https://doi.org/10.1111/eth.13072
Sih A, Bell AM (2008) Insights for behavioral ecology from behavioral syndromes. Adv Study Behav 38:227–281. https://doi.org/10.1016/S0065-3454(08)00005-3
Sih A, Bell A, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378. https://doi.org/10.1016/j.tree.2004.04.009
Sih A, Cote J, Evans M, Fogarty S, Pruitt J (2012) Ecological implications of behavioural syndromes. Ecol Lett 15:278–289. https://doi.org/10.1111/j.1461-0248.2011.01731.x
Sih A, Mathot KJ, Moirón M, Montiglio PO, Wolf M, Dingemanse NJ (2015) Animal personality and state – behaviour feedbacks: a review and guide for empiricists. Trends Ecol Evol 30:50–60. https://doi.org/10.1016/j.tree.2014.11.004
Snell-Rood EC (2013) An overview of the evolutionary causes and consequences of behavioural plasticity. Anim Behav 85:1004–1011. https://doi.org/10.1016/j.anbehav.2012.12.031
Stamps JA, Briffa M, Biro PA (2012) Unpredictable animals: individual differences in intraindividual variability (IIV). Anim Behav 83:1325–1334. https://doi.org/10.1016/j.anbehav.2012.02.017
Stoffel MA, Nakagawa S, Schielzeth H (2017) rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol Evol 8:1639–1644. https://doi.org/10.1111/2041-210X.12797
Szász E, Markó G, Hegyi G, Török J, Garamszegi LZ, Rosivall B (2019) Nest-site defence aggression during courtship does not predict nestling provisioning in male collared flycatchers. Behav Ecol Sociobiol 73:62. https://doi.org/10.1007/s00265-019-2672-1
Teyssier A, Bestion E, Richard M, Cote J (2014) Partners’ personality types and mate preferences: predation risk matters. Behav Ecol 25:723–733. https://doi.org/10.1093/beheco/aru049
Thomas RM, Urban JH, Peterson DA (2006) Acute exposure to predator odor elicits a robust increase in corticosterone and a decrease in activity without altering proliferation in the adult rat hippocampus. Exp Neurol 201:308–315. https://doi.org/10.1016/j.expneurol.2006.04.010
Thompson RKR, Foltin RW, Boylan RJ, Sweet A, Graves CA, Lowitz CE (1981) Tonic immobility in Japanese quail can reduce. Anim Learn Behav 9:145–149
Trompeter WP, Langkilde T (2011) Invader danger: lizards faced with novel predators exhibit an altered behavioral response to stress. Horm Behav 60:152–158. https://doi.org/10.1016/j.yhbeh.2011.04.001
Urszán T, Garamszegi L, Nagy G, Hettyey A, Török J, Herczeg G (2015a) No personality without experience? A test on Rana dalmatina tadpoles. Ecol Evol 5:5847–5856. https://doi.org/10.1002/ece3.1804
Urszán T, Török J, Hettyey A, Garamszegi LZ, Herczeg G (2015b) Behavioural consistency and life history of Rana dalmatina tadpoles. Oecologia 178:129–140. https://doi.org/10.1007/s00442-014-3207-0
Urszán T, Garamszegi L, Nagy G, Hettyey A, Török J, Herczeg G (2018) Experience during development triggers between-individual variation in behavioural plasticity. J Anim Ecol 87:1264–1273. https://doi.org/10.1111/1365-2656.12847
Van Damme R, Quick K (2001) Use of predator chemical cues by three species of lacertid lizards (Lacerta bedriagae, Podarcis tiliguerta, Podarcis sicula). J Herpetol 35:27–36
Vanhooydonck B, Van Damme R, Aerts P (2001) Speed and stamina trade-off in lacertid lizards. Evolution 55:1040–1048. https://doi.org/10.1111/j.0014-3820.2001.tb00620.x
Veiga JP, Salvador A, Merino S, Puerta M (1998) Reproductive effort affects immune response and parasite infection in a lizard: a phenotypic manipulation using testosterone. Oikos 82:313. https://doi.org/10.2307/3546971
Vincze E, Papp S, Preiszner B, Seress G, Bókony V, Liker A (2016) Habituation to human disturbance is faster in urban than rural house sparrows. Behav Ecol 27:1304–1313. https://doi.org/10.1093/beheco/arw047
Watts HE, Holekamp KE (2008) Interspecific competition influences reproduction in spotted hyenas. J Zool 276:402–410. https://doi.org/10.1111/j.1469-7998.2008.00506.x
Weiss SL, Moore MC (2004) Activation of aggressive behavior by progesterone and testosterone in male tree lizards, Urosaurus ornatus. Gen Comp Endocrinol 136:282–288. https://doi.org/10.1016/j.ygcen.2004.01.001
Winchell K, Maayan I, Fredette J, Revell L (2018) Linking locomotor performance to morphological shifts in urban lizards. Proc R Soc B 285:20180229
Acknowledgements
We are thankful for two anonymous reviewers whose comments lead to improvements of our paper. Our sincere thank goes to Gina Bíró and Gonzalo Rodríguez-Ruiz for their assistance during fieldwork. We are grateful to ‘El Ventorrillo’ Field Station (MNCN-CSIC) for use of their facilities.
Funding
Open access funding provided by Eötvös Loránd University. Financial support was provided by the projects Hungarian Scientific Research Fund (OTKA-K 105517; for GáH), the Ministerio de Economía y Competitividad project MINECO CGL2014-53523-P (for JM and PL), the National Research, Development and Innovation Fund for international cooperation (SNN 125627; for GáH), the Postdoctoral research grant of the National Research, Development and Innovation Fund (NKFIH, PD 132041; for GeH) and the János Bólyai Research Scholarship of the Hungarian Academy of Sciences (for GáH).
Author information
Authors and Affiliations
Contributions
All authors designed the study; GeH collected data and performed the experiments; GeH analysed the data and wrote the manuscript with the substantial contribution of GáH, JM and PL; all authors reviewed the manuscript and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. No lizard was injured during the handling related to the study and after completion of the experiments, animals were returned in good health at the exact site of capture. The study was performed under license (permit number: 10/056780.9/16) from the Environmental Agency of Madrid Government (“Consejería de Medio Ambiente de la Comunidad de Madrid”, Spain), and in accordance with the national animal welfare standards and protocols supervised by the Bioethical Committee of the Spanish Research Council (CSIC).
Additional information
Communicated by S. Joy Downes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Horváth, G., Martín, J., López, P. et al. Ain’t going down without a fight: state-and environment-dependence of antipredator defensive aggressive personalities in Carpetan rock lizard. Behav Ecol Sociobiol 74, 139 (2020). https://doi.org/10.1007/s00265-020-02922-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-020-02922-0