Abstract
Limited attention constrains predators from engaging in cognitively demanding tasks such as searching for cryptic prey at the same time as remaining vigilant towards threats. Since finite attention can result in negative correlations between foraging and vigilance, the tendency of individual predators to focus attention on searching for cryptic prey may be correlated with other behavioural traits which reflect risk-reward trade-offs, such as consistent inter-individual variation in boldness (a personality trait describing risk-taking, defined in this study as the time taken to leave a refuge). We investigated the importance of personality in prey detection by comparing inter-individual variation in the response of three-spined sticklebacks (Gasterosteus aculeatus) to conspicuous and cryptic prey. Fish were slower to attack cryptic prey than conspicuous prey, consistent with cryptic prey being harder to detect. Despite the greater challenge involved in detecting cryptic prey, inter-individual variation in the time taken to detect prey was similar in the cryptic and conspicuous prey treatments, and was uncorrelated with boldness, which was repeatable between individuals. We also observed a positive association between the rate of attack on conspicuous prey and whether individual fish attacked cryptic prey in other trials. Our findings suggest that boldness is not related to prey detection or attention in this context. Instead, consistent differences in motivation once exploration has begun between individual predators may explain inter-individual variation in the time taken to attack both prey cryptic and conspicuous prey.
Significance statement
Using an experimental approach to manipulate the conspicuousness of prey, we show that individual fish consistently differ in their rates of attacking prey. This demonstrates that fish show “personality variation” in predatory behaviour, but these inter-individual differences were not related to the boldness of each fish (their tendency to engage in risky behaviours).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Consistent differences in behaviour between individual predators are an important factor influencing predation risk, with the potential to shape ecological communities and maintain variation within prey populations (McGhee et al. 2013; Start and Gilbert 2017). Most previous research on this personality variation in predators has concentrated on exploring the relationship between inter-individual differences in predator activity or boldness (i.e. tendency to task risks) and encounter rates with prey or the rate of prey consumption (Ioannou et al. 2008a; Pruitt et al. 2012; Michalko and Řežucha 2018). Predator-prey encounters are defined as occurring when predators (or prey) have approached within a range which enables them to detect prey (or predators) (Lima and Dill 1990). After encountering prey, predators must therefore first detect potential targets before they can approach, attack, and eventually capture prey. Consequently, prey have evolved a range of camouflage strategies which allow them to evade detection by exploiting the sensory or cognitive systems of their predators (Skelhorn and Rowe 2016). One of the most widespread camouflage strategies is background matching, which increases prey survival by achieving a close resemblance between the hue, brightness, and pattern of a prey animal’s body and a sample of the surrounding habitat (Endler 1978; Troscianko et al. 2016), minimizing the signal produced by the animal relative to noise from the background (Merilaita et al. 2017). Inter-specific variation in predator visual systems has previously been linked to the effectiveness of different forms of cryptic colouration in prey (Stuart-Fox et al. 2008). Within populations of the same predator species, individuals also differ in characteristics relevant to the detection of cryptic prey, such as their experience of searching for differently camouflaged prey types, their capacity to learn from experience, and their motivation to gather additional information about the profitability of certain prey types (Ehlinger 1989; Sherratt 2011). However, despite the importance of consistent inter-individual behavioural variation as a factor influencing predation risk, little is known about the relationship between personality in predators and prey defences such as crypsis.
Boldness is a frequently studied personality trait which indicates a tendency to prioritize rewards over risks (Réale et al. 2007). Consistent inter-individual differences in boldness have been shown to affect the tendency to venture beyond the safety of cover (Pearish et al. 2013) and disperse more widely (Fraser et al. 2001), and are often highly correlated with other widely studied personality traits such as activity, the tendency to explore a novel environment, or aggressiveness (Bell and Sih 2007; Sih and Bell 2008; Quinn et al. 2012). This suggests that individual predators are likely to vary in the proportion of time they spend actively searching for prey, and the rate at which they encounter prey in their environment. In agreement with the expected effects of predator personality on encounter rates, experiments in controlled mesocosms have also demonstrated that stable differences in the activity of individual predators can determine prey survival (Pruitt et al. 2012). Although this study highlights the importance of predator activity levels and boldness in predicting the threat posed to prey, the relevance of such traits beyond the initial encounter stage remains unclear. While there is some evidence that predators exhibit consistent inter-individual differences in the speed with which they attack and their ability to capture prey (Exnerová et al. 2010; Smith and Blumstein 2010; McGhee et al. 2013), relatively little attention has been directed towards the possibility that individual predators differ in aspects of their behaviour which are relevant to other important stages of the predation sequence, including prey detection (Lima and Dill 1990).
Detecting prey can be cognitively challenging when prey closely match the visual properties of the background. Locating prey in these conditions can involve intensive search effort, as demonstrated by evidence that predators concentrate their attention on particular prey types by forming search images for specific prey features (Langley et al. 1996; Bond and Kamil 1999). Due to constraints on the capacity of animals to process information at any given instant, attention is regarded as a finite cognitive resource (Dukas 2002). As a result, predators are less able to detect alternative prey when their attention is divided between searching for distinct prey types (Dukas and Kamil 2001), or detect unexpected peripheral stimuli, such as an approaching predator, during difficult search tasks (Dukas and Kamil 2000). Limited attention is also thought to underpin the widely reported trade-off between foraging and anti-predator vigilance, which occurs when these activities cannot be performed simultaneously (Godin and Smith 1988; Krause and Godin 1996). Prey detection might therefore be influenced by the tendency of individual predators to redirect attention away from their own anti-predator vigilance and towards their efforts to locate prey, as well as factors affecting search tactics, such as the extent to which individuals relax their anti-predator vigilance as their search progresses (Ioannou et al. 2008b). Since widely studied axes of behavioural variation such as the bold-shy continuum also reflect the priority given to resource acquisition over risk avoidance, personality traits such as boldness may be related to differences between individual predators in prey detection (Sih and Del Giudice 2012).
Once predators are in the vicinity of prey, after an encounter has occurred, the capacity of individual predators to direct their attention towards the detection of prey is likely to be an important factor determining detection times. Conversely, for conspicuous prey which can be readily detected without much difficulty, attention should be less important and should play a reduced role. If driven by limited attention, any consistent differences between individual predators in prey detection should therefore only be apparent when prey are cryptic and not when prey are conspicuous against the background. Since variation in boldness reflects a continuum ranging from risk-prone (bold) to risk-averse (shy) behaviour, boldness may also be correlated with the tendency to re-focus attention away from anti-predator vigilance and towards the search for prey. We therefore expected bold predators to detect cryptic prey more rapidly than shy individuals, but to observe no, or reduced, differences between bold and shy fish when fish were presented with conspicuous prey. We also predicted that there would be greater differences between individual predators in the time taken to detect cryptic prey than when detecting conspicuous prey.
To test these hypotheses, we experimentally manipulated the visual conspicuousness of prey relative to the background and repeatedly presented three-spined stickleback (Gasterosteus aculeatus) predators with conspicuous or cryptic prey items over the course of multiple trials. This experimental approach has previously been shown to drastically reduce the probability of visual detection for cryptic prey by three-spined sticklebacks when fish were given a relatively short period of time to search for prey (Ioannou and Krause 2009). We adapted this approach by allowing fish to search for prey over a longer period and by repeatedly testing the same individuals, enabling us to compare the degree of inter-individual variation in the time taken to detect prey between trials with cryptic prey and trials with conspicuous prey. This experimental design also allowed us to test whether boldness explained inter-individual differences in the response of predators to cryptic and conspicuous prey. We chose to quantify boldness by measuring the time taken for individual fish to leave a refuge, as this measure has previously been shown to be repeatable in three-spined sticklebacks (Ioannou et al. 2008a; Harcourt et al. 2009a; Ioannou and Dall 2016), is correlated with other measures of risk-taking (Wilson et al. 2011), and, most importantly, has direct impact on foraging and predation risk (Orrock et al. 2013; Hulthén et al. 2017; Balaban-Feld et al. 2019).
Methods
Subjects and housing
Three-spined sticklebacks used in the study were caught from the River Cary, Somerset, UK, on a single date in early November 2017, using large hand-nets dragged through vegetation. Of 275 fish initially caught for use in different behavioural experiments, 54 individuals were tested in this experiment (mean standard body length, 30.8 mm; standard deviation, 4.63 mm), and were haphazardly caught from this larger total. In the lab, fish were housed in glass tanks (width = 40 cm, length = 70 cm, height = 34 cm) with daily 13:11 dark:light cycle and water temperature was maintained at 16 °C (± 0.5 °C). As these lighting and temperature conditions prevented the sticklebacks from entering a reproductive state, it was not possible to non-invasively sex the fish used in the experiment (Borg et al. 2004; Harcourt et al. 2009a; Ioannou and Dall 2016). Throughout non-experimental periods, fish were fed defrosted bloodworms (Chironomidae larvae) once a day.
Experimental trials took place in November and December 2017, with a minimum of 5 days between fish being caught and the first fish being tested. During experimental periods, pairs of fish were transferred to breeding nets (width = 12 cm, length = 16 cm, height = 13 cm) consisting of a fine-meshed fish net material supported by a plastic frame. Breeding nets contained an artificial plant as a refuge and were positioned within one of the stock tanks. To ensure individual fish could be easily identified, fish were paired on the basis of differences in body size. To standardize hunger levels across individuals, fish were fed one bloodworm per day over the course of the experimental period after testing on that day. Once fed, fish did not have an opportunity to feed until the next trial, and as trials were performed one day apart, this is likely to have allowed sufficient hunger levels to build up between consecutive trials for fish to be motivated to search for prey when tested (Heller and Milinski 1979; Salvanes and Hart 1998; Harcourt et al. 2009b). After being tested in the experiment, fish were returned a separate glass tank (width = 40 cm, length = 70 cm, height = 34 cm) and kept isolated from untested fish to avoid retesting of the same individuals.
Experimental procedure
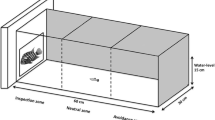
Experimental trials were conducted in four identical arenas (width = 40 cm, length = 60 cm, height = 18 cm; Fig. 1), filled with aged water to a depth of 10 cm. Each arena was divided into two compartments: a covered refuge, and an uncovered zone which contained a feeding patch (a grid of alternating red and white squares). The covered refuge was separated from the uncovered zone by a white plastic barrier, with a retractable door situated within the barrier. Trials were filmed using a GoPro Hero 5 video camera mounted above the arenas. The group of four experimental arenas were surrounded by white PVC sheeting, preventing disturbance to the fish during experimental trials.
Top-down view of the set-up used in the experiment. A retractable door situated within the barrier (dotted line) was used to habituate the fish in the refuge zone before allowing them access after the door was opened remotely. An additional barrier was positioned immediately in front of the door opening, preventing visual contact between the refuge and the feeding grid
During experimental trials, the visual conspicuousness of prey was manipulated by placing four bloodworms on either a single white grid square (conspicuous treatment) or a single red grid square (cryptic treatment) before the start of each trial. In previous experiments using this methodology in which trials lasted 15 min, cryptic prey were almost never detected by three-spined sticklebacks when fish were given a relatively short period of time to search for prey (Ioannou and Krause 2009). While olfactory cues are important in alerting sticklebacks to the presence of prey, experimental evidence suggests that visual cues are used to pinpoint the exact location of prey (Johannesen et al. 2012). Trials commenced when fish were transferred to the refuge and left to acclimatize for 5 min; after which, the door to the uncovered zone containing the grid was remotely opened, and left open for the remainder of the trial (40 min).
Over the course of the experiment, individual fish were repeatedly tested: two trials with conspicuous prey and two trials with cryptic prey, resulting in four trials per individual. Trials were conducted once per day over a 5-day period, with a day-long gap separating blocks of two trials occurring on the first (trials 1 and 2) and last 2 days (trials 3 and 4). Treatments were pseudo-randomly assigned within 2-day blocks such that each treatment occurred only once within trials 1 and 2, and only once in trials 3 and 4. When determining where to place prey items within each treatment, the choice of grid square was also pseudo-randomized: in trials 1 and 2, a grid square was randomly chosen from the row nearest to the refuge, and in trials 3 and 4, a grid square was randomly chosen from the row furthest from the refuge.
Video analysis
Three behavioural variables were extracted from video recordings of each experimental trial. The time taken to first leave the refuge was recorded as a measure of risk-taking tendency (i.e. boldness). Encounters with prey were defined as occurring when fish swam over the feeding grid, and the time taken to first encounter prey was the time difference between the instant the fish left the refuge and the point at which it first swam over the feeding grid. Attacks on prey occurred when fish consumed prey, and the time taken to attack was calculated in two different ways: either as the difference between the first encounter with prey and the point at which prey were first consumed, or the time difference between the fish leaving the refuge and when the fish began to consume prey. Two separate definitions of the time taken to attack were used in the analysis to test whether the results were sensitive to when an encounter with prey was considered to have occurred (visual contact with prey was possible from when fish first left the refuge, but prey detection was more likely when fish approached within a much closer range by directly swimming over the feeding grid). As similar results were obtained regardless of how the time taken to attack was defined, results based on the time taken to attack prey relative to when the fish first left the refuge are presented in the Supplementary Material. To minimize observer bias, blinded methods were used when behavioural data were extracted from videos.
Statistical analysis
All statistical analyses were conducted in R version 3.6.0 (R Development Core Team 2019). The degree to which individual fish differed consistently in their risk-taking tendency (time taken to first leave the refuge) was assessed by estimating the adjusted repeatability of this trait, using a generalized mixed-effects modelling approach contained within the rptR package in R (Stoffel et al. 2017). In this context, the adjusted repeatability represents the proportion of the total phenotypic variance that can be attributed to individuals, excluding variation explained by fixed effect explanatory variables of trial number (i.e. 1 to 4) and standard body length (Nakagawa and Schielzeth 2010). Trials in which individual fish failed to leave the refuge were disregarded from this stage of the analysis (22 out of 216 trials), in order to avoid influencing estimates of within-individual variation and thus affecting the resulting repeatability estimate (Stamps 2016). Statistical significance of the repeatability estimate was assessed using a combination of P values obtained via a likelihood ratio test and the overlap of 95% confidence intervals with zero computed through parametric bootstrapping.
Data on the time taken to first encounter and attack prey included multiple censored observations, in which fish failed to either encounter (10 out of 194 trials in which fish left the refuge; conspicuous treatment: 8 out of 99 trials; cryptic treatment: 2 out of 95 trials) or attack the prey before a trial ended (102 out of 184 trials in which fish encountered prey; conspicuous treatment: 41 out of 91 trials; cryptic treatment: 61 out of 93 trials). Cox proportional hazards (PH) models were therefore used to analyze the effect of prey treatment and other explanatory variables on the time taken to first encounter or attack prey, using the coxme package in R (Therneau 2018). This statistical approach is capable of handling truncated or censored observations, and enables the relationship between the explanatory variables and the hazard rate (in this context, the instantaneous rate at which prey are first encountered or attacked, given that the event has not yet occurred) to be examined, without making any assumptions about the precise shape of the baseline hazard function (Therneau and Grambsch 2000). Random effect terms can also be incorporated within Cox PH models in order to account for non-independence arising from repeated observations from the same groups (in this case, individuals), and to describe variation between groups (or individuals) in the relative impact of the explanatory variables on the baseline hazard function (Austin 2017). For all the Cox PH models, the proportional hazards assumption was checked by inspecting plots of the Schoenfeld residuals, and the functional form of continuous explanatory was assessed by examining plots of these variables against the Martingale residuals.
The influence of prey treatment (cryptic vs. conspicuous) on the time taken to first encounter and the time taken to first attack prey was examined by fitting the Cox PH models to data from trials where the fish had left the refuge (194 trials), and data from trials where fish encountered prey (184 trials), respectively. Both models included prey treatment, the standard body length of each fish and the time taken for fish to first leave the refuge (during the same experimental trial) as explanatory variables and included an individual-level random effect. Since the inclusion of trial number as an explanatory variable was found to result in non-proportional hazards, both models were also stratified by trial number. While inclusion of a stratified factor does not allow the effect of trial number to be estimated, it does enable the effect of trial number to be controlled for. Throughout the analysis, the statistical significance of explanatory variables was evaluated by using likelihood ratio tests to compare the full model (including all possible factors) to a reduced model lacking the variable in question. Hazard ratios and their 95% confidence intervals were also obtained by taking the exponential of model parameter estimates for fixed effects, to provide an indication of the relative impact of each explanatory variable on the rate of encounter or attack. Cumulative event curves were plotted using the survminer R package and were based on the number of observed events (encounters or attacks) among individuals which had not yet encountered or attacked prey at each unique event time (Kassambra et al. 2019).
To investigate the potential influence of prey treatment on the degree of inter-individual variation in the time taken to attack prey, data from the conspicuous and cryptic treatments were analyzed independently in the two separate Cox PH models. Both models included standard body length and the time taken to first leave the refuge (in the same trial) as explanatory variables, trial number as a stratification factor, and an individual-level random effect. Standardized measures of inter-individual variation (e.g. the individual-level repeatability, or the proportion of behavioural variation that could be attributed to individuals) cannot be calculated from Cox PH models because the residual (i.e. within-individual) variance cannot be estimated. Instead, the Cox PH models were used to obtain estimates of the variance associated with the individual-level random effect term, to provide an indication of the extent of inter-individual differences in the time taken to attack prey. The statistical significance of the individual-level random effect was assessed using likelihood ratio tests comparing the integrated log-likelihood value (for a model with the random effect) to the partial log-likelihood of the model with the same fixed effect covariates but lacking the random effect term (Therneau and Grambsch 2000). The uncertainty surrounding each estimate was reflected in 95% confidence intervals, obtained using a profile likelihood method.
To investigate whether the ability of fish to detect cryptic prey was correlated with their response to conspicuous prey, an additional Cox PH model was constructed to examine the relationship between behaviour during cryptic treatment trials and the time taken to attack prey in conspicuous treatment trials. As only a limited number of individuals attacked prey in both cryptic and conspicuous treatment trials, we chose to represent the behavioural response of fish towards cryptic prey in these trials using a binary variable indicating whether or not individual fish attacked prey during any of the cryptic treatment trials. The model also included the standard body length of individual fish and the time taken for fish to first leave the refuge (in the same trial) as additional explanatory variables, trial number as a stratification factor, and an individual-level random effect. The model was fitted to data where fish encountered prey in at least one cryptic treatment trial and therefore had the opportunity to attack cryptic prey at least once (88 trials).
Results
Of the 54 fish tested, 53 individuals left the refuge in at least one experimental trial and 46 individual fish left the refuge in more than one experimental trial. In agreement with findings from previous studies on three-spined sticklebacks (Ioannou and Dall 2016), the time taken to first leave the refuge was moderately repeatable (R = 0.210; 95% confidence intervals, 0.055–0.346; P < 0.001), demonstrating that individual fish consistently differed in their risk-taking tendency (boldness).
Among the 53 fish which encountered prey during at least one experimental trial by swimming over the feeding grid, only 37 individuals attacked prey in either the conspicuous or cryptic treatments. There was no effect of treatment on the time taken for fish to encounter prey once they had left the refuge (Cox proportional hazards model (Cox PH), 184 observed encounters in 194 trials where fish left the refuge, χ2 = 0.115, P = 0.735; Fig. 2a), suggesting that the time taken to reach the prey was not affected by any differences in visual conspicuousness of prey between treatments. Fish were, however, significantly slower to attack cryptic prey compared with conspicuous prey (Cox PH, 82 observed attacks in 184 trials where fish encountered prey: χ2 = 19.1, P < 0.001; Fig. 2b). The rate at which cryptic prey were attacked was substantially reduced, approximately threefold, relative to the rate of attack in trials with conspicuous prey (hazard ratio (HR) = 0.300; 95% confidence intervals, 0.176–0.514). There was also evidence for inter-individual variation in the time taken by fish to attack prey, as demonstrated by the relatively poor performance of models lacking a random effect term for individual identity when compared with the full model (Cox PH, χ2 = 59.0, P < 0.001).
Cumulative event curves showing the effect of prey treatment (conspicuous vs. cryptic) on the probability that fish had encountered (a) or attacked prey (b) before a given time during an experimental trial. The time taken to first encounter was calculated with reference to emergence from the refuge (a), and the time taken to attack was calculated using the first encounter with prey as the starting point (b). Shading surrounding the cumulative event curves indicates 95% confidence intervals. Crosses indicate experimental trials which ended before prey were encountered or an attack was made
To determine whether the degree of inter-individual variation in the time taken to attack prey was dependent on the type of prey encountered, data from the conspicuous and cryptic treatments were analyzed separately. Including a random effect term for individual identity significantly improved the model fit to a similar extent for both conspicuous (Cox PH, 50 observed attacks in 91 trials where fish encountered prey, no. of individuals = 52, χ2 = 8.99, P = 0.003) and cryptic treatments (Cox PH, 32 observed attacks in 93 trials where fish encountered prey, no. of individuals = 51, χ2 = 11.6, P < 0.001). Model estimates of the variance associated with the individual-level random effect term suggested that variation between individuals in the time taken to attack conspicuous prey was lower (estimated variance of individual-level random effect, 1.61; 95% confidence intervals, 0.34–4.96) than variation between individuals in the response to cryptic prey (estimated variance of individual-level random effect, 5.41; 95% confidence intervals, 1.09–21.5). However, these estimates were associated with a high degree of uncertainty in both treatments as indicated by the wide and overlapping 95% confidence intervals, preventing any firm conclusions from being drawn on the question of whether inter-individual differences in the time taken to attack prey were more or less pronounced depending on treatment. Additionally, there was no significant effect of the time taken to first leave the refuge (boldness) on the time taken to attack relative to when prey were encountered in either treatment (conspicuous treatment, Cox PH χ2 = 0.73, P = 0.393; cryptic treatment, Cox PH χ2 = 2.96, P = 0.085).
To explore the relationship between the behaviour of individual fish towards conspicuous prey and their response to cryptic prey, we also examined the effect of whether or not prey were attacked in any of the cryptic treatment trials on the time taken to attack conspicuous prey. Fish which attacked cryptic prey in at least one experimental trial were significantly quicker to attack conspicuous prey than those which never attacked the prey in any of the cryptic treatment trials (Cox PH, 49 attacks in 88 trials, χ2 = 26.4, P < 0.001; Fig. 3). This is consistent with the interpretation that inter-individual variation in the time taken to attack prey was correlated across the two treatments. Throughout the analysis, similar results were obtained when the time taken to attack prey was defined relative to when the fish left the refuge, instead the first encounter with prey (see Supplementary Material, Fig. S1-S2).
Cumulative event curve showing how the probability of attacking conspicuous prey before a given time during an experimental trial is influenced by whether or not fish had attacked prey during trials with cryptic prey. The blue and purple curves represent fish which attacked cryptic prey in at least one experimental trial (blue), and those which did not attack cryptic prey at all (red), respectively. The time taken to attack prey was calculated using the first encounter with prey as the starting point. Shading surrounding the cumulative event curve indicates 95% confidence intervals. Crosses indicate experimental trials which ended before prey were encountered or an attack occurred
Discussion
In our experiment, fish were slower to attack cryptic prey than conspicuous prey, confirming that prey matching their background were in fact cryptic by being harder to detect (Ioannou and Krause 2009). While individual sticklebacks consistently differed in the time taken to attack both cryptic and conspicuous prey, the degree of inter-individual behavioural variation was similar in the two treatments. If differences between individual predators had been driven by a trade-off between focusing limited attention on the search for prey as opposed to anti-predator vigilance, we would have expected to observe greater variation between individual fish in the time taken to attack cryptic prey than in the response to conspicuous prey. Contrary to our initial expectations based on previous work (Dukas and Kamil 2001; Ioannou et al. 2008a), there was also no evidence for a correlation between an individual’s boldness (the time taken to first leave the refuge) and the time taken to attack either cryptic or conspicuous prey. These findings suggest that boldness (i.e. risk-taking) is not correlated with prey detection in this context and therefore may not be linked to attention or perceptual abilities. Instead of an effect of boldness, we found that the rate of attack on conspicuous prey was positively associated with whether individual fish attacked cryptic prey in other trials. The link between the behaviour of fish in trials with cryptic prey and the time taken to attack conspicuous prey suggests that variation in another unidentified individual-level trait may be more important in determining the behavioural response to both prey types.
One possible explanation for the absence of a relationship between boldness and the time taken to attack prey is that individual risk-taking tendencies determine how long it takes for a fish to leave a refuge, but the behaviour of a fish once it has left the refuge is uncorrelated with risk-taking. Instead of risk-taking, consistent differences in motivation between individuals might explain the behaviour of fish outside of the refuge and could underpin inter-individual variation in the response of sticklebacks to both prey types (cryptic and conspicuous). Importantly, while differences in motivation between individuals can potentially account for inter-individual variation in the time taken to attack prey, motivation is unlikely to explain the longer time taken to attack cryptic compared with conspicuous prey. This is because average motivation levels should be the same in both treatments, as the two prey types only differed in their visual conspicuousness against a background. Other factors with the potential to affect risk-taking or the motivation to search for prey, such as the olfactory cues generated by prey, would also be expected to be the same in both treatments and to remain constant between trials. If motivation is driving inter-individual variation in the response to prey, our results contrast with findings from previous work in which motivation was found to be correlated with boldness (Webster et al. 2009; Carter et al. 2010; McDonald et al. 2016). These findings are also unexpected because of the widespread evidence that inter-individual differences in measures of risk-taking such as the time taken to leave a refuge are related to other personality traits which are relevant to behaviour outside of a refuge (Sih and Bell 2008). In many ecological contexts, widely studied personality traits such as boldness might not always be the most relevant axes of variation (Koski 2014), and it may be important to consider factors such as motivation when attempting to use boldness to predict how individual predators will respond to prey. Differences in motivation between individuals could be driven by a range of factors, including physiological differences between individual fish, such as variation in metabolic rates. Although the pace-of-life syndrome hypothesis proposes that risk-taking is positively associated with higher metabolic rates and a fast life-history, the evidence for this relationship is mixed (Royauté et al. 2018; Hansen et al. 2020), suggesting that there are many contexts in which boldness will not be correlated with metabolic traits (Montiglio et al. 2018).
Although there were clear differences in detectability between cryptic and conspicuous prey, it is possible that we did not observe an effect of boldness because the task of detecting cryptic prey might not have placed sufficient demands on attention (Dukas and Kamil 2001). For example, locating cryptic prey might not have been challenging enough for trade-offs in the allocation of attention between foraging and vigilance to become influential. Additionally, an effect of boldness on the response of predators to cryptic prey may only become apparent when predators have an opportunity to form search images over successive encounters with prey, or are forced to divide their attention by searching for multiple distinct prey types (Dukas and Kamil 2000). Similarly, an effect of limited attention on prey detection may only emerge when predators perceive themselves to be under greater threat from their own predators, although in our experiment fish displayed consistent inter-individual differences in the time taken to leave the refuge, suggesting that the uncovered zone of the experimental arena was perceived as risky.
The impact of intra-specific variation in predator behaviour on diversity in prey visual defences has previously been explored in greater depth in the context of warning colours. For predators which are exposed to aposematic prey, learned associations between prey colour patterns and toxicity are thought to be maintained more readily for more abundant prey phenotypes, resulting in positive frequency-dependent selection which favours monomorphic aposematic prey populations (Ruxton et al. 2004). However, spatial variation in the composition of predator communities has been identified as a factor contributing to heterogeneity in selection pressures, and promoting polymorphism within aposematic species (Endler and Mappes 2004; Nokelainen et al. 2014). Personality variation in avian predators has also been shown to affect the degree of initial wariness displayed towards newly encountered aposematic prey, as well as the rate at which predators learned to avoid unpalatable prey types (Exnerová et al. 2010). If the expression of personality differences within predator populations is affected by local ecological conditions such as predation risk (Bell and Sih 2007), any resulting differences in the distribution of personality types within predator populations might also lead to variability in selection on prey. By contrast, negative frequency-dependent selection by visual predators is recognized as having the potential to promote polymorphism in populations of cryptic prey, because predator search images provide a survival advantage to rare prey types (Bond and Kamil 2002). Further work is required to clarify how variation in the tendency of individual predators to attack prey would affect patterns of variation within prey populations.
Beyond background matching, limited attention in predators may have implications for the strength of selection on a number of other prey traits, including other forms of defensive colouration and traits which influence collective behaviour. There is evidence to suggest that some camouflage strategies, such as disruptive colouration, are more effective at preventing improvements in prey detection with increasing experience than others which rely to a greater extent on preventing initial detection by naïve observers (Troscianko et al. 2018). Since selective attention plays a pivotal role in the formation of search images over repeated encounters with the same prey type, one untested possibility is that consistent differences between individual predators might have a greater impact on prey survival for some types of camouflage than others. Limits on attention in predators may also contribute to variability in how predators select for traits which influence both the composition and collective behaviour of prey groups. For predators which hunt groups of prey, successfully capturing a single individual from within the group can be challenging because it involves processing spatial information from multiple targets within the predator’s visual field (Krakauer 1995). If the demands of tracking multiple prey exceed a predator’s limited capacity to process information, the resulting confusion effect can lead to a reduction in attack rates and a decline in the accuracy with which predators target individual prey (Ioannou et al. 2008c). Crucially, predators’ ability to overcome the confusion effect by focusing their attention on prey will depend on the costs associated with a reduction in their own anti-predator vigilance (Milinski and Heller 1978; Milinski 1984). If relaxing their own anti-predator vigilance is too costly, predators may switch to less cognitively demanding ways of countering confusion. These strategies might include concentrating attacks on prey close to the edge of the group where prey density is likely to be lower (Duffield and Ioannou 2017), or preferentially targeting phenotypically dissimilar or “odd” prey within groups (Penry-Williams et al. 2018). Future research should examine whether individual predators vary consistently in their response to camouflaged prey over successive encounters or differ in how they target individual prey within groups.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Austin PC (2017) A tutorial on multilevel survival analysis: methods, models and applications. Int Stat Rev 85:185–203
Balaban-Feld J, Mitchell WA, Kotler BP, Vijayan S, Tov Elem LT, Rosenzweig ML, Abramsky Z (2019) Individual willingness to leave a refuge and trade-off and safety: a test with social fish. Proc R Soc B 286:20190826
Bell AM, Sih A (2007) Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus). Ecol Lett 10:828–834
Bond AB, Kamil AC (1999) Searching image in blue jays: facilitation and interference in sequential priming. Anim Learn Behav 27:461–471
Bond AB, Kamil AC (2002) Visual predators select for crypticity and polymorphism in virtual prey. Nature 415:609–613
Borg B, Bornestaf C, Hellqvist A, Schmitz M, Mayer I (2004) Mechanisms in the photoperiodic control of reproduction in the stickleback. Behaviour 141:1521–1530
Carter AJ, Goldizen AW, Tromp SA (2010) Agamas exhibit behavioral syndromes: bolder males bask and feed more but may suffer higher predation. Behav Ecol 21:655–661
Duffield C, Ioannou CC (2017) Marginal predation: do encounter or confusion effects explain the targeting of prey group edges? Behav Ecol 28:1283–1292
Dukas R (2002) Behavioural and ecological consequences of limited attention. Philos Trans R Soc B 357:1539–1547
Dukas R, Kamil AC (2000) The cost of limited attention in blue jays. Behav Ecol 11:502–506
Dukas R, Kamil AC (2001) Limited attention: the constraint underlying search image. Behav Ecol 12:192–199
Ehlinger TJ (1989) Learning and individual variation in bluegill foraging: habitat-specific techniques. Anim Behav 38:643–658
Endler JA (1978) A predator’s view of animal color patterns. In: Hecht MK, Steere WC, Wallace B (eds) Evolutionary biology, vol 11. Springer, Boston, pp 319–364
Endler JA, Mappes J (2004) Predator mixes and the conspicuousness of aposematic signals. Am Nat 163:532–547
Exnerová A, Svádová KH, Fučíková E, Drent P, Štys P (2010) Personality matters: individual variation in reactions of naive bird predators to aposematic prey. Proc R Soc B 277:723–728
Fraser DF, Gilliam JF, Daley MJ, Le AN, Skalski GT (2001) Explaining leptokurtic movement distributions: intrapopulation variation in boldness and exploration. Am Nat 158:124–135
Godin J-GJ, Smith SA (1988) A fitness cost of foraging in the guppy. Nature 333:69–71
Hansen MJ, Ligocki IY, Zillig KE, Steel AE, Todgham AE, Fangue NA (2020) Risk-taking and locomotion in foraging threespine sticklebacks (Gasterosteus aculeatus): the effect of nutritional stress is dependent on social context. Behav Ecol Sociobiol 74:12
Harcourt JL, Ang TZ, Sweetman G, Johnstone RA, Manica A (2009a) Social feedback and the emergence of leaders and followers. Curr Biol 19:248–252
Harcourt JL, Sweetman G, Johnstone RA, Manica A (2009b) Personality counts: the effect of boldness on shoal choice in three-spined sticklebacks. Anim Behav 77:1501–1505
Heller R, Milinski M (1979) Optimal foraging of sticklebacks on swarming prey. Anim Behav 27:1127–1141
Hulthén K, Chapman BB, Nilsson PA, Hansson LA, Skov C, Brodersen J, Vinterstare J, Brönmark C (2017) A predation cost to bold fish in the wild. Sci Rep 7:1239
Ioannou CC, Dall SRX (2016) Individuals that are consistent in risk-taking benefit during collective foraging. Sci Rep 6:33991
Ioannou CC, Krause J (2009) Interactions between background matching and motion during visual detection can explain why cryptic animals keep still. Biol Lett 5:191–193
Ioannou CC, Payne M, Krause J (2008a) Ecological consequences of the bold-shy continuum: the effect of predator boldness on prey risk. Oecologia 157:177–182
Ioannou CC, Ruxton GD, Krause J (2008b) Search rate, attack probability, and the relationship between prey density and prey encounter rate. Behav Ecol 19:842–846
Ioannou CC, Tosh CR, Neville L, Krause J (2008c) The confusion effect - from neural networks to reduced predation risk. Behav Ecol 19:126–130
Johannesen A, Dunn AM, Morrell LJ (2012) Olfactory cue use by three-spined sticklebacks foraging in turbid water: prey detection or prey location? Anim Behav 84:151–158
Kassambra A, Kosinski M, Biecek P (2019) Survminer: drawing survival curves using ‘ggplot2’. R package version 0.4.5, https://cran.r-project.org/web/packages/survminer
Koski SE (2014) Broader horizons for animal personality research. Front Ecol Evol 2:70
Krakauer DC (1995) Groups confuse predators by exploiting perceptual bottlenecks: a connectionist model of the confusion effect. Behav Ecol Sociobiol 36:421–429
Krause J, Godin J-GJ (1996) Influence of prey foraging posture on flight behavior and predation risk: predators take advantage of unwary prey. Behav Ecol 7:264–271
Langley CM, Riley DA, Bond AB, Goel N (1996) Visual search for natural grains in pigeons (Columba livia): search images and selective attention. J Exp Psychol Anim B 22:139–151
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
McDonald ND, Rands SA, Hill F, Elder C, Ioannou CC (2016) Consensus and experience trump leadership, suppressing individual personality during social foraging. Sci Adv 2:e1600892
McGhee KE, Pintor LM, Bell AM (2013) Reciprocal behavioral plasticity and behavioral types during predator-prey interactions. Am Nat 182:704–717
Merilaita S, Scott-Samuel NE, Cuthill IC (2017) How camouflage works. Philos Trans R Soc B 372:20160341
Michalko R, Řežucha R (2018) Top predator’s aggressiveness and meso-predator’s risk-aversion additively determine probability of predation. Behav Ecol Sociobiol 72:105
Milinski M (1984) A predator’s costs of overcoming the confusion-effect of swarming prey. Anim Behav 32:1157–1162
Milinski M, Heller R (1978) Influence of a predator on the optimal foraging behaviour of sticklebacks (Gasterosteus aculeatus L.). Nature 275:642–644
Montiglio PO, Dammhahn M, Messier GD, Réale D (2018) The pace-of-life syndrome revisited: the role of ecological conditions and natural history on the slow-fast continuum. Behav Ecol Sociobiol 72:116
Nakagawa S, Schielzeth H (2010) Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev 85:935–956
Nokelainen O, Valkonen J, Lindstedt C, Mappes J (2014) Changes in predator community structure shifts the efficacy of two warning signals in Arctiid moths. J Anim Ecol 83:598–605
Orrock JL, Preisser EL, Grabowski JH, Trussell GC (2013) The cost of safety: refuges increase the impact of predation risk in aquatic systems. Ecology 94:573–579
Pearish S, Hostert L, Bell AM (2013) Behavioral type–environment correlations in the field: a study of three-spined stickleback. Behav Ecol Sociobiol 67:765–774
Penry-Williams IL, Ioannou CC, Taylor MI (2018) The oddity effect drives prey choice but not necessarily attack time. Ethology 124:496–503
Pruitt JN, Stachowicz JJ, Sih A (2012) Behavioral types of predator and prey jointly determine prey survival: potential implications for the maintenance of within-species behavioral variation. Am Nat 179:217–227
Quinn JL, Cole EF, Bates J, Payne RW, Cresswell W (2012) Personality predicts individual responsiveness to the risks of starvation and predation. Proc R Soc B 279:1919–1926
R Development Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna http://www.R-project.org
Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ (2007) Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318
Royauté R, Berdal MA, Garrison CR, Dochtermann NA (2018) Paceless life? A meta-analysis of the pace-of-life syndrome hypothesis. Behav Ecol Sociobiol 72:64
Ruxton GD, Sherratt TN, Speed MP (2004) Avoiding attack: the evolutionary ecology of crypsis, warning signals and mimicry. Oxford University Press, Oxford
Salvanes AGV, Hart PJB (1998) Individual variability in state-dependent feeding behaviour in three-spined sticklebacks. Anim Behav 55:1349–1359
Sherratt TN (2011) The optimal sampling strategy for unfamiliar prey. Evolution 65:2014–2025
Sih A, Bell A (2008) Insights for behavioral ecology from behavioral syndromes. Adv Study Behav 38:227–281
Sih A, Del Giudice M (2012) Linking behavioural syndromes and cognition: a behavioural ecology perspective. Philos Trans R Soc B 367:2762–2772
Skelhorn J, Rowe C (2016) Cognition and the evolution of camouflage. Proc R Soc B 283:20152890
Smith BR, Blumstein DT (2010) Behavioral types as predictors of survival in Trinidadian guppies (Poecilia reticulata). Behav Ecol 21:919–926
Stamps JA (2016) Individual differences in behavioural plasticities. Biol Rev 91:534–567
Start D, Gilbert B (2017) Predator personality structures prey communities and trophic cascades. Ecol Lett 20:366–374
Stoffel MA, Nakagawa S, Schielzeth H (2017) rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol Evol 8:1639–1644
Stuart-Fox D, Moussalli A, Whiting MJ (2008) Predator-specific camouflage in chameleons. Biol Lett 4:326–329
Therneau TM (2018) Coxme: mixed effects Cox models. R package version 2.2–7. https://cran.r-project.org/web/packages/coxme
Therneau TM, Grambsch PM (2000) Modelling survival data: extending the Cox model. Springer-Verlag, New York
Troscianko J, Wilson-Aggarwal J, Stevens M, Spottiswoode CN (2016) Camouflage predicts survival in ground-nesting birds. Sci Rep 6:19966
Troscianko J, Skelhorn J, Stevens M (2018) Camouflage strategies interfere differently with observer search images. Proc R Soc B 285:20181386
Webster MM, Ward AJW, Hart PJB (2009) Individual boldness affects interspecific interactions in sticklebacks. Behav Ecol Sociobiol 63:511–520
Wilson ADM, Binder TR, McGrath KP, Cooke SJ, Godin J-GJ (2011) Capture technique and fish personality: angling targets timid bluegill sunfish, Lepomis macrochirus. Can J Fish Aquat Sci 68:749–757
Acknowledgements
We are very grateful to the two anonymous reviewers for helpful and constructive suggestions, which helped to improve the manuscript.
Funding
This work was supported by a NERC GW4+ Doctoral Training Partnership studentship from the Natural Environment Research Council (NE/L002434/1) awarded to ASC, and a Natural Environment Research Council Fellowship (NE/K009370/1) and a Leverhulme Trust grant (RPG-2017-041 V) awarded to CCI.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Ethical approval was obtained through the University of Bristol (project number UB/16/047).
Additional information
Communicated by J. G. Frommen
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Szopa-Comley, A.W., Donald, W.G. & Ioannou, C.C. Predator personality and prey detection: inter-individual variation in responses to cryptic and conspicuous prey. Behav Ecol Sociobiol 74, 70 (2020). https://doi.org/10.1007/s00265-020-02854-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-020-02854-9