Abstract
Numerous studies have demonstrated adaptive behavioral responses of males and females to changes in operational sex ratio (the ratio of potentially receptive males to receptive females; OSR), and theory often assumes that animals have perfect instantaneous knowledge about the OSR. However, the role of sensory mechanisms in monitoring the local sex ratio by animals and whether animals can perceive local sex ratio in a manner consistent with model assumptions have not been well addressed. Here, we show that mating water striders Gerris gracilicornis respond to local sex ratio even when visual and physical contact with other individuals were experimentally prohibited. Our study shows that insects are able to estimate local population’s sex ratio and adjust their behavior based on nonvisual cues perceived at a distance or released to the habitat. Hence, the frequent theoretical assumption that individuals have knowledge about their local sex ratio regardless of their direct behavioral interactions may be an acceptable approximation of reality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animals adaptively respond to changes in operational sex ratio, the ratio of potentially receptive males to receptive females (OSR; Lawrence 1986; Jablonski and Vepsalainen 1995; Vepsalainen and Savolainen 1995; Weatherhead et al. 1995; Alonso-Pimentel and Papaj 1996), and theory often assumes that animals have perfect instantaneous knowledge about the OSR (Clutton-Brock and Parker 1992; Owens and Thompson 1994). However, the questions of whether animals can perceive local sex ratio in a manner consistent with model assumptions and how they do it have not been well addressed. It is possible that cues used to distinguish between sexes can also be used for monitoring the OSR. Animals can distinguish between sexes using visual (Rutowski 1977), acoustic (Miller 1979; Lind et al. 1996), chemical (Wyatt 2003), or vibrational signals (Wilcox 1979; Warren et al. 2009). For example, two-spotted ladybird beetle can discriminate sex by sex-specific behavioral cue (Hemptinne et al. 1998). Crickets (Hardy and Shaw 1983; Tregenza and Wedell 1997; Ryan and Sakaluk 2009) or beetles (Coleoptera; Peschke and Metzler 1987; Fukaya et al. 1996; Hemptinne et al. 1998; Ginzel et al. 2003; Zhang et al. 2003; Mutis et al. 2009) are able to recognize the sex with the chemical cue from the cuticular hydrocarbons. However, it is unknown whether these mechanisms are used by individuals to perceive and monitor the local OSR.
Water striders have been used as model organisms for evolutionary analyses of adaptive responses to the OSR. For example, researchers understand relatively well the evolutionary mechanisms responsible for the effect of OSR on female’s behavioral resistance to male copulation attempts or on the male postcopulatory mate-guarding behavior (Arnqvist 1992; Rowe 1992; Krupa and Sih 1993; Jablonski and Vepsalainen 1995; Vepsalainen and Savolainen 1995). The presence of such effects of OSR indicates that water striders are able to detect cues associated with OSR. Males should be able to do that even when they mate-guard on top of a female and their opportunities to monitor OSR through interactions with other individuals are limited (Jablonski and Vepsalainen 1995). Therefore, we hypothesized that, in addition to direct physical interactions, visual, chemical, or vibratory signals may serve as the cues for local OSR perception in water striders. For example, some water striders use sex-specific ripple signals to distinguish between the sexes (Wilcox 1979). Chemical cues are also used by Heteropteran insects to distinguish between sexes (Aldrich 1988) and the metathoracic scent gland is especially well developed in subfamily Gerrinae (Andersen 1982). This suggests that ripple signals and chemical cues may provide information about the local OSR to the water striders.
Before conducting experiments to determine which of the cues (from chemicals or vibratory signals), if any, are used by the water striders, we need to ascertain that these insects are able to detect OSR at a distance, without visual and tactile cues during direct interactions between individuals. The aim of this study was to determine whether the effect of OSR on mating behaviors can be observed when direct interactions between individuals and exchange of visual information are experimentally prohibited.
Material and methods
Study subjects
Males and females of Gerris gracilicornis were collected in Gwanak Mountain near Seoul National University, Seoul, South Korea, between 24 April and 14 May 2007. For at least 7 days prior to the test, we separated the water striders according to their sex (30 individuals per 30 × 40 cm container filled with water) in order to maintain a similar level of sperm storage in males before the experiment and in order to avoid variation among females in the amount of sperm already received through recent copulations. Frozen crickets (Verlarifictorus asperses) were given as the food, and pieces of floating Styrofoam were provided as the resting sites. In order to minimize the effects of body size on the mating behavior, we used individuals of similar body size, between the first and third quartiles of body length distribution (male, 11.9–12.6 mm; female, 14.1–14.8 mm).
Experimental setup
We set four experimental treatments. Twenty containers (22 × 15 cm) were used for the male-biased sex ratio treatment (male/female = 4:1), and 18 containers were used for the female-biased sex ratio (male/female = 1:4). In ten containers for each treatment, we put two parallel partitions across the middle of each container just above the water surface (about 3 mm above the surface) to allow transmission of ripples on the surface of water across the partition. Also, the space between two partitions (2 cm) was created to prevent physical contact between the focal mating pair and the “background” three males or three females (depending on the treatment) located on the opposite side of the partition. Hence, we conducted ten replicates of male-biased partition-present, male-biased partition-absent, and female-biased partition-present treatments and eight replicates of female-biased partition-absent treatment. Each individual was used only once in the experiments.
Behavioral variables and statistical methods
In this species, a mating interaction lasts many hours, with repeated copulation and guarding phases while the male remains on the female (Han et al. 2010). Therefore, any subsequent mating behaviors, except the very initial copulation, may be equally affected by the OSR as well as the preceding interactions within a pair. Considering this possibility, we chose to analyze the behavior of individuals only at the initial stage of a mating interaction. We chose two simple behavioral variables known to be affected by OSR in water striders (Rowe 1992; Krupa and Sih 1993; Jablonski and Vepsalainen 1995; Vepsalainen and Savolainen 1995): the female resistance to mating attempts and the copulation duration. Female G. gracilicornis “resists” to male’s copulation attempts by pushing away the male with her legs or by jumping. We recorded the presence or absence of female resistance during the mating initiation phase (i.e., from the time the focal pair started to interact until the copulation proper started as indicated by the extension of female genitalia; Han and Jablonski 2009). Exact logistic test (PROC LOGISTIC; SAS Institute 2002), which can handle contingency tables with zero cells (Stokes et al. 2000), was used to investigate the effect of two categorical independent variables, the OSR treatment (male-biased or female-biased) and the presence of partition (present or absent), on the presence of female resistance.
After copulation (intromission) started, we measured the duration (in seconds) of the first copulation bout (after which a male remains on a female in a guarding position). Since the copulation of G. gracilicornis was usually not terminated by females’ resistance but by the genitalia detachment by males mounted on the female (Han, personal observation), we regarded that the copulation duration is determined by the males. We missed the copulation termination of one male. Analysis of variance (ANOVA) was used to examine the effect of OSR and the presence of partition on the duration of the first copulation.
Results
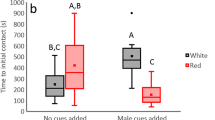
More females resisted copulation in the female-biased than in the male-biased sex ratio (Table 1; test score = 8.90, p = 0.005) regardless of the presence or absence of the partition (interaction term not significant in Table 1). The results suggest that females recognized cues related to the OSR even in the partition-present treatment when no physical or visual contact was possible. Although the interaction was not significant, the graph (Fig. 1a) suggests that females might have reacted to the OSR in a more extreme manner when no physical interactions were allowed with other individuals in the population.
Copulation duration, determined by a male withdrawing his genitalia, was significantly affected by the OSR regardless of the presence of a partition (Table 2; Fig. 1b). Males copulated longer in female-biased than in the male-biased sex ratio treatment.
Discussion
We showed that insects respond to local OSR in the absence of direct physical interactions or visual cues. The observed consistent behavioral responses to OSR regardless of the presence of the experimental partition clearly indicate that direct physical and visual cues are not needed to perceive the local OSR of a local population. While the proximate mechanism to recognize others’ sex at a distance has been reported in many insect taxa (Hardy and Shaw 1983; Peschke and Metzler 1987; Fukaya et al. 1996; Tregenza and Wedell 1997; Hemptinne et al. 1998; Ginzel et al. 2003; Zhang et al. 2003; Mutis et al. 2009; Ryan and Sakaluk 2009), the mechanism to monitor OSR was unclear because previous studies have not investigated whether individuals could monitor local OSR through such sex-specific cues.
What specific cues did the water striders use to determine the OSR? We considered that, in our experimental situation, males and females might have recognized the OSR using either the ripples on the water surface or chemical cues from other individuals. Water striders perceive ripples of the water surface through tarsal vibration receptors on their legs (Murphey 1971; Lawry 1973; Goodwyn et al. 2009). Females, even in the mating position, can receive cues from both the surface ripples and chemicals from other individuals in the population (through air or water as the medium). Males, however, have fewer opportunities than females to perceive ripple signals when they are in a mating position because mounted males’ forelegs and middle legs do not touch the water surface and their hindlegs are often out of touch with the water surface. Both sexes did not receive sex-specific ripple signals from other individuals in the experimental container because the sex-specific ripple signals, described in some water striders (Wilcox 1979), are not known in our study species G. gracilicornis (Han and Jablonski 2009). Furthermore, because G. gracilicornis males produce courtship signals at the very initial phase of a mating attempt (before intromission; Han and Jablonski 2009, 2010) and no additional mating pair (source of such signals) was present in the experiment, males and females could not have used the courtship ripples to estimate local OSR. The focal pair was the only mating pair in the experimental container and the only ripples on the water surface produced by the other experimental water striders (other than the focal pair) were due to their movements.
Because it seems unlikely that mating water striders might have perceived some information about OSR from simple ripples produced by moving water striders, we believe that they used chemical cues. The hypothetical role of chemicals in OSR monitoring is more strongly suggested by the fact that family Gerridae has the metathoracic scent gland, which is especially well developed in subfamily Gerrinae (Andersen 1982). These glands, located in the ventral part of the thorax, were suggested to have a role in sexual activity rather than in waterproofing of legs (Staddon 1979). Additionally, females of the marine water striders Halobates hawaiiensis were attracted to the extract from males consisting of male-specific palmitic and oleic acids, which disperse through the water surface rather than through the air (Tsoukatou et al. 2001). Therefore, we hypothesize that the scent glands in the genus Gerris may help in sex recognition and, thus, in perception of local OSR. Hence, our results not only suggest the need for future experiments to precisely determine the cues used by water striders in perceiving local OSR, but they also strongly indicate that chemical rather than vibrational cues are involved.
The pattern of water striders’ mating behavior in sex-biased conditions was consistent with our prediction and results from previous studies. Less resistant females in the male-biased sex ratio condition can be explained by “convenience polyandry” to avoid males’ harassment (Thornhill and Alcock 1983; Wilcox 1984; Rowe 1992). According to this mechanism, the cost associated with resistance and harassment from males is larger than the cost associated with multiple mating, and water strider females are less resistant to mating in male-biased sex ratio.
However, it is intriguing that water strider males copulated longer in the female-biased than in the male-biased sex ratio treatment. It has been known that males guard females longer in male-biased OSR (Clark 1988; Arnqvist 1992; Rowe 1992; Krupa and Sih 1993; Jablonski and Vepsalainen 1995; Vepsalainen and Savolainen 1995). Because of the prolonged mating in our study species, we could not measure guarding duration or any consecutive behavioral interactions precisely. But since G. gracilicornis males control copulation duration by withdrawing their genitalia (Han, personal observation), we could use the first copulation as the first behavioral response of a male to local sex ratio. Here, we propose a hypothesis that is consistent with the results. In the reproductive period, G. gracilicornis males are very aggressive and responsive to single females. Males frequently mount even on mating pairs, harass them, and sometimes disrupt sperm transfer of the mounting male and take over the female. We suspect that aggressive harassment of single males may disrupt the transfer of sperm of the mounting males by interrupting mounting male positioning on the female. Thus, because of the takeover or disruption of efficient sperm transfer, males in male-biased sex ratio may decrease the amount of sperm transfer and finish the copulation in a shorter duration.
We also considered an alternative explanation of why males copulated for longer in the female-biased OSR. Females were more resistant in this treatment. If stronger precopulatory resistance affects the efficiency of sperm transfer during the consecutive copulation, then in female-biased treatments, it might have taken longer for males to transfer sperm. However, we regard this hypothesis unlikely because a significant difference in female resistance between partition-present and partition-absent treatments in the male-biased condition (G = 4.7, df = 1, p = 0.03; Fig. 1) was not associated by a corresponding difference in male copulation duration (Tukey’s honestly significant difference (HSD) test, p = 0.97; Fig. 1).
Our results confirmed that adult insects detect population sex ratio without tactile or visual cues from other individuals. This brings the idea that juveniles may also be able to recognize demographic factors from nonvisual cues, such as sex-specific chemical compounds. The recognition of demographic factors such as sex ratio or density at a distance is very important for developing juvenile insects. Perceiving the social environment during development, juveniles can allocate the resources into traits suitable for the environment. For example, in a wing-dimorphic insect species, juvenile males in a male-biased condition may develop wings and disperse to a female-biased environment where males can encounter more females. However, because of the predation risk and limited mobility, juveniles have a difficulty to collect information on the local sex ratio by tactile or visual cues. If juveniles are able to infer the density or sex ratio of the population from chemical cues, they could use this information to modify their developmental pathways and to allocate their resources into traits that will maximize their fitness in specific conditions (Kasumovic and Brooks 2011). Hence, future studies may show that chemicals are reliable cues even for juvenile insects to gain information on demographic factors and to change their allocation strategy (Kasumovic and Andrade 2006; Kasumovic et al. 2009).
In summary, the frequent theoretical assumption that individuals have knowledge about their local OSR regardless of their direct behavioral interactions may be an acceptable approximation of reality. The results showed that water striders can perceive information about local OSR using cues that do not involve direct interactions, sex-specific vibratory signals, or visual information. Instead, they may use the cues that are released to the habitat (most likely sex-specific chemicals). Future experiments to precisely determine the hypothetical chemical cues used by those insects are needed.
References
Aldrich J (1988) Chemical ecology of the Heteroptera. Annu Rev Entomol 33(1):211–238
Alonso-Pimentel H, Papaj DR (1996) Operational sex ratio versus gender density as determinants of copulation duration in the walnut fly, Rhagoletis juglandis (Diptera: Tephritidae). Behav Ecol Sociobiol 39(3):171–180
Andersen NM (1982) The semiaquatic bugs (Hemiptera, Gerromorpha): phylogeny, adaptations, biogeography and classification. Entomonograph 3. Scandinavian Science Press, Klampenborg
Arnqvist G (1992) The effects of operational sex ratio on the relative mating success of extreme male phenotypes in the water strider Gerris odontogaster (Zett.)(Heteroptera; Gerridae). Anim Behav 43:681–683
Clark S (1988) The effects of operational sex ratio and food deprivation on copulation duration in the water strider (Gerris remigis Say). Behav Ecol Sociobiol 23(5):317–322
Clutton-Brock TH, Parker GA (1992) Potential reproductive rates and the operation of sexual selection. Q Rev Biol 67:437–456
Fukaya M, Yasuda T, Wakamura S, Honda H (1996) Reproductive biology of the yellow-spotted longicorn beetle, Psacothea hilaris (Pascoe) (Coleoptera: Cerambycidae). III. Identification of contact sex pheromone on female body surface. J Chem Ecol 22(2):259–270
Ginzel MD, Blomquist GJ, Millar JG, Hanks LM (2003) Role of contact pheromones in mate recognition in Xylotrechus colonus. J Chem Ecol 29(3):533–545
Goodwyn PP, Katsumata-Wada A, Okada K (2009) Morphology and neurophysiology of tarsal vibration receptors in the water strider Aquarius paludum (Heteroptera: Gerridae). J Insect Physiol 55(9):855–861
Han CS, Jablonski PG (2009) Female genitalia concealment promotes intimate male courtship in a water strider. PLoS One 4(6):e5793
Han CS, Jablonski PG (2010) Male water striders attract predators to intimidate females into copulation. Nat Commun 1(5):52
Han CS, Jablonski PG, Kim B, Park F (2010) Size-assortative mating and sexual size dimorphism are predictable from simple mechanics of mate-grasping behavior. BMC Evol Biol 10(1):359
Hardy TN, Shaw KC (1983) The role of chemoreception in sex recognition by male crickets: Acheta domesticus and Teleogryllus oceanicus. Physiol Entomol 8(2):151–166
Hemptinne JL, Lognay G, Dixon A (1998) Mate recognition in the two-spot ladybird beetle, Adalia bipunctata: role of chemical and behavioural cues. J Insect Physiol 44(12):1163–1171
SAS Institute (2002) PROC user’s manual, version 9.1. SAS Institute, Cary
Jablonski P, Vepsalainen K (1995) Conflict between sexes in the water strider, Gerris lacustris: a test of two hypotheses for male guarding behavior. Behav Ecol 6(4):388–392
Kasumovic MM, Andrade M (2006) Male development tracks rapidly shifting sexual versus natural selection pressures. Curr Biol 16(7):R242–R243
Kasumovic MM, Brooks RC (2011) It’s all who you know: the evolution of socially cued anticipatory plasticity as a mating strategy. Q Rev Biol 86(3):181–197
Kasumovic MM, Bruce MJ, Herberstein ME, Andrade MCB (2009) Evidence for developmental plasticity in response to demographic variation in nature. Ecology 90(8):2287–2296
Krupa JJ, Sih A (1993) Experimental studies on water strider mating dynamics: spatial variation in density and sex ratio. Behav Ecol Sociobiol 33(2):107–120
Lawrence W (1986) Male choice and competition in Tetraopes tetraophthalmus: effects of local sex ratio variation. Behav Ecol Sociobiol 18(4):289–296
Lawry JV Jr (1973) A scanning electron microscopic study of mechanoreceptors in the walking legs of the water strider, Gerris remigis. J Anat 116(Pt 1):25
Lind H, Dabelsteen T, McGregor PK (1996) Female great tits can identify mates by song. Anim Behav 52:667–671
Miller DB (1979) The acoustic basis of mate recognition by female zebra finches (Taeniopygia guttata). Anim Behav 27:376–380
Murphey R (1971) Sensory aspects of the control of orientation to prey by the waterstrider, Gerris remigis. J Comp Physiol Neuroethol, Sens, Neural, Behav Physiol 72(2):168–185
Mutis A, Parra L, Palma R, Pardo F, Perich F, Quiroz A (2009) Evidence of contact pheromone use in mating behavior of the raspberry weevil (Coleoptera: Curculionidae). Environ Entomol 38(1):192–197
Owens IPF, Thompson DBA (1994) Sex differences, sex ratios and sex roles. Proc R Soc Lond B 258:93–99
Peschke K, Metzler M (1987) Cuticular hydrocarbons and female sex pheromones of the rove beetle, Aleochara curtula (Goeze) (Coleoptera: Staphylinidae). Insect Biochem 17(1):167–178
Rowe L (1992) Convenience polyandry in a water strider: foraging conflicts and female control of copulation frequency and guarding duration. Anim Behav 44(2):189–202
Rutowski RL (1977) The use of visual cues in sexual and species discrimination by males of the small sulphur butterfly Eurema lisa (Lepidoptera, Pieridae). J Comp Physiol Neuroethol, Sens, Neural, Behav Physiol 115(1):61–74
Ryan KM, Sakaluk SK (2009) Dulling the senses: the role of the antennae in mate recognition, copulation and mate guarding in decorated crickets. Anim Behav 77(5):1345–1350
Staddon BW (1979) The scent glands of Heteroptera. Adv Insect Physiol 14:351–418
Stokes ME, Davis CS, Koch GG (2000) Categorical data analysis using the SAS system. SAS Institute, Cary
Thornhill R, Alcock J (1983) The evolution of insect mating systems. Harvard University Press, Cambridge
Tregenza T, Wedell N (1997) Definitive evidence for cuticular pheromones in a cricket. Anim Behav 54(4):979–984
Tsoukatou M, Cheng L, Vagias C, Roussis V (2001) Chemical composition and behavioral responses of the marine insect Halobates hawaiiensis (Heteroptera: Gerridae). Z Naturforsch C 56(7/8):597–602
Vepsalainen K, Savolainen R (1995) Operational sex ratios and mating conflict between the sexes in the water strider Gerris lacustris. Am Nat 146:869–880
Warren B, Gibson G, Russell IJ (2009) Sex recognition through midflight mating duets in Culex mosquitoes is mediated by acoustic distortion. Curr Biol 19(6):485–491
Weatherhead PJ, Barry FE, Brown GP, Forbes MRL (1995) Sex ratios, mating behavior and sexual size dimorphism of the northern water snake, Nerodia sipedon. Behav Ecol Sociobiol 36(5):301–311
Wilcox RS (1979) Sex discrimination in Gerris remigis: role of a surface wave signal. Science 206(4424):1325–1327
Wilcox RS (1984) Male copulatory guarding enhances female foraging in a water strider. Behav Ecol Sociobiol 15(3):171–174
Wyatt TD (2003) Pheromones and animal behaviour: communication by smell and taste. Cambridge Univ Pr, Cambridge
Zhang A, Oliver JE, Chauhan K, Zhao B, Xia L, Xu Z (2003) Evidence for contact sex recognition pheromone of the Asian longhorned beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae). Naturwissenschaften 90(9):410–413
Acknowledgments
We thank the Korean Research Foundation (grant KRF-2007-313-C00747), National Research Foundation (grants 2009-0082824; 2010-0025546) and Developing Nations Research Grant from the Animal Behavior Society to CH for supporting this research. This research was part of the ecology class taught by P.G.J. at SNU.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. Gwynne
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Han, C.S., Kang, CK., Shin, HS. et al. Insects perceive local sex ratio in the absence of tactile or visual sex-specific cues. Behav Ecol Sociobiol 66, 1285–1290 (2012). https://doi.org/10.1007/s00265-012-1382-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-012-1382-8