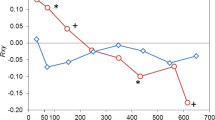
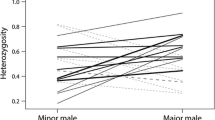
Abstract
Inbreeding depression is a relative decline in fitness in offspring of related parents. The magnitude of inbreeding costs varies among taxa and may increase under stressful conditions. Inbreeding tolerance is expected to be low and selection for inbreeding avoidance intense when both sexes invest substantially in shared offspring like in nuptial gift-giving butterflies. This is especially true for increasing mating rate for inbreeding avoidance as nuptial feeding decreases net costs of mating for females. We explored implications of inbreeding in the nuptial gift-giving green-veined white butterfly, Pieris napi. Compared to outbred ones, partially inbred (F = 0.25) eggs and neonate larvae had 25% lower hatching success and 30% lower survival until adult eclosion, respectively. Inbreeding was also associated with small size. Yet, the magnitude of inbreeding depression was independent of larval conditions. A lack of assortative mating and mating durations independent of mating type suggest that neither females nor males discriminate close relatives (r = 0.5) as mates. Indicative of a postcopulatory mechanism to avoid inbreeding, female remating intervals decreased following incestuous matings. Such a plastic response may affect the level of postcopulatory sexual selection as female remating interval (time between successive matings) is necessarily negatively correlated with mating rate (matings per unit time) and mating frequency (lifetime number of matings), and precopulatory mate choice appeared insignificant. Moreover, incest-induced shift in the phenotype towards the adaptive peak may contribute to the evolution of female mating rates, although alternative explanations for polyandry besides material benefits have rarely been invoked when nuptial feeding is involved.



Similar content being viewed by others
References
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Armbruster P, Reed DH (2005) Inbreeding depression in benign and stressful environments. Heredity 95:235–242
Armbruster P, Hutchinson RA, Linvell T (2000) Equivalent inbreeding depression under laboratory and field conditions in a tree-hole-breeding mosquito. Proc R Soc Lond B 267:1939–1945
Arnqvist G, Nilsson T (2000) The evolution of polyandry: multiple mating and female fitness in insects. Anim Behav 60:145–164
Bateman AJ (1948) Intrasexual selection in Drosophila. Heredity 2:349–368
Bates D, Maechler M (2009) lme4: linear mixed-effects models using S4 classes. R Package, version 0.999375-32. Available at http://cran.r-project.org/. Accessed 1 February 2010
Bergström J, Wiklund C (2002) Effects of size and nuptial gifts on butterfly reproduction: can females compensate for a smaller size through male-derived nutrients? Behav Ecol Sociobiol 52:296–302
Bergström J, Wiklund C (2005) No effect of male courtship intensity on female remating in the butterfly Pieris napi. J Insect Behav 18:479–489
Bergström J, Wiklund C, Kaitala A (2002) Natural variation in female mating frequency in a polyandrous butterfly: effects of size and age. Anim Behav 64:49–54
Birkhead TR, Møller AP (1998) Sperm competition and sexual selection. Academic, London
Bissoondath CJ, Wiklund C (1996) Effect of male history and body size on ejaculate size and quality in two polyandrous butterflies, Pieris napi and Pieris rapae (Lepidoptera: Pieridae). Funct Ecol 10:457–464
Bissoondath CJ, Wiklund C (1997) Effect of male body size on sperm precedence in the polyandrous butterfly Pieris napi L. (Lepidoptera: Pieridae). Behav Ecol 8:518–523
Bretman A, Wedell N, Tregenza T (2004) Molecular evidence of post-copulatory inbreeding avoidance in the field cricket Gryllus bimaculatus. Proc R Soc Lond B 271:159–164
Carrasco D, Kaitala A (2009) Egg-laying tactic in Phyllomorpha laciniata in the presence of parasitoids. Ent Exp Appl 131:300–307
Charlesworth D, Charlesworth B (1987) Inbreeding depression and its evolutionary consequences. Ann Rev Ecolog Syst 18:237–268
Colegrave N, Kotiaho JS, Tomkins JL (2002) Mate choice or polyandry: reconciling genetic compatibility and good genes sexual selection. Evol Ecol Res 4:911–917
Cornell SJ, Tregenza T (2007) A new theory for the evolution of polyandry as a means of inbreeding avoidance. Proc R Soc B 274:2873–2879
Crnokrak P, Roff DA (1999) Inbreeding depression in the wild. Heredity 83:260–270
R Development Core Team (2009) The R project for statistical computing, R version 2.10.1. Available at http://www.r-project.org/. Accessed 15 December 2009
Eberhard WG (1996) Female control: sexual selection by cryptic female choice. Princeton University Press, Princeton
Eimes JA, Parker PG, Brown JL, Brown WR (2005) Extrapair fertilization and genetic similarity of social mates in the Mexican Jay. Behav Ecol 16:456–460
Fox CW, Scheibly KL (2006) Variation in inbreeding depression among populations of the seed beetle, Stator limbatus. Ent Exp Appl 121:137–144
Ghalambor CK, McKay JK, Carroll SP, Reznick DN (2007) Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol 21:394–407
Haikola S, Fortelius W, O’Hara RB, Kuussaari M, Wahlberg N, Saccheri IJ, Singer MC, Hanski I (2001) Inbreeding depression and the maintenance of genetic load in Melitaea cinxia metapopulations. Cons Genet 2:325–335
Henter HJ (1993) Inbreeding depression and haplodiploidy: experimental measures in a parasitoid and comparisons across diploid and haplodiploid insect taxa. Evolution 57:1793–1803
Hosken DJ, Blanckenhorn WU (1999) Female multiple mating, inbreeding avoidance, and fitness: it is not only the magnitude of cost and benefits that counts. Behav Ecol 10:462–464
Husband BC, Schemske DW (1996) Evolution of the magnitude and timing of inbreeding depression in plants. Evolution 50:54–70
Jennions MD, Petrie M (2000) Why do females mate multiply? A review of the genetic benefits. Biol Rev 75:21–64
Johnstone RA (1995) Sexual selection, honest advertisement and the handicap principle: reviewing the evidence. Biol Rev 70:1–65
Joron M, Brakefield PM (2003) Captivity masks inbreeding effects on male mating success in butterflies. Nature 424:191–194
Kaitala A, Wiklund C (1994) Polyandrous female butterflies forage for matings. Behav Ecol Sociobiol 35:385–388
Keller LF, Waller DM (2002) Inbreeding effects in wild populations. Trends Ecol Evol 17:230–241
Kivelä SM, Välimäki P (2008) Competition between larvae in a butterfly, Pieris napi, and maintenance of different life history strategies. J Anim Ecol 77:529–539
Kivelä SM, Välimäki P, Oksanen J, Kaitala A, Kaitala V (2009) Seasonal clines of evolutionarily stable reproductive effort in insects. Am Nat 174:526–536
Kokko H, Mappes J (2005) Sexual selection when fertilization is not guaranteed. Evolution 59:1876–1885
Kokko H, Ots I (2006) When not to avoid inbreeding. Evolution 60:467–475
Larsdotter Mellström H, Wiklund C (2009) Male use sex pheromone assessment to tailor ejaculates to risk of sperm competition in a butterfly. Behav Ecol 20:1147–1151
Larsdotter Mellström H, Wiklund C (2010) What affects mating rate? Polyandry is higher in the directly developing generation of the butterfly Pieris napi. Anim Behav 80:413–418
Markow TA (1997) Assortative fertilization in Drosophila. Proc Nat Acad Sci 94:7756–7760
Morjan WE, Obrycki JJ, Krafsur ES (1999) Inbreeding effects on Propylea quatuordecimpunctata (Coleoptera: Coccinellidae). Ann Entomol Soc Am 92:260–268
Nieminen M, Singer MC, Fortelius W, Schöps K, Hanski I (2001) Experimental confirmation that inbreeding depression increases extinction risk in butterfly populations. Am Nat 157:237–244
Parker GA (1970) Sperm competition and its evolutionary consequences in the insects. Biol Rev 45:525–567
Parker GA (1979) Sexual selection and sexual conflict. In: Blum MS, Blum NA (eds) Sexual selection and reproductive competition in insects. Academic, New York, pp 123–166
Parker GA (2006) Sexual conflict over mating and fertilization: an overview. Phil Trans R Soc B 361:235–259
Pinheiro J, Bates D, DebRoy S, Sarkar D (2008) nlme: linear and nonlinear mixed effects models. R Package, version 3.1-89. Available at http://cran.r-project.org/. Accessed 20 January 2009
Price TD, Qvarnström A, Irwin DE (2003) The role of phenotypic plasticity in driving genetic evolution. Proc R Soc Lond B 270:1433–1440
Pusey A, Wolf M (1996) Inbreeding avoidance in animals. Trends Ecol Evol 11:201–206
Reale D, Roff DA (2003) Inbreeding, developmental stability, and canalization in the sand cricket Gryllus firmus. Evolution 57:597–605
Roff DA (1998) Effects of inbreeding on morphological and life history traits of the sand cricket, Gryllus firmus. Heredity 81:28–37
Roff DA (2002) Inbreeding depression: tests of the overdominance and partial dominance hypotheses. Evolution 56:768–775
Roff DA, DeRose MA (2001) The evolution of trade-offs: effects of inbreeding on fecundity relationships in the cricket Gryllus firmus. Evolution 55:111–121
Saccheri IJ, Brakefield PM, Nichols RA (1996) Severe inbreeding depression and rapid fitness rebound in the butterfly Bicyclus anynana (Satyridae). Evolution 50:2000–2013
Saccheri IJ, Lloyd HD, Helyar SJ, Brakefield PM (2005) Inbreeding uncovers fundamental differences in the genetic load affecting male and female fertility in a butterfly. Proc R Soc Lond B 272:39–46
Scoble MJ (1992) The Lepidoptera—form, function and diversity. The Natural History Museum and Oxford University Press, New York
Simmons LW (1989) Kin recognition and its influence on mating preferences of the field cricket, Gryllus bimaculatus (de Geer). Anim Behav 38:68–77
Simmons L (2001) Sperm competition and its evolutionary consequences in insects. Princeton University Press, Princeton
Simmons LW, Thomas ML (2008) No postcopulatory response to inbreeding by male crickets. Biol Lett 4:183–185
Stockley P (1999) Sperm selection and genetic incompatibility: does relatedness of mates affect male success in sperm competition? Proc R Soc B 266:1663–1669
Stockley P, Searle JB, MacDonald DW, Jones CS (1993) Female multiple mating behavior in the common shrew as a strategy to reduce inbreeding. Proc R Soc Lond B 254:173–179
Sugawara T (1979) Stretch reception in the bursa copulatrix of the buttefly, Pieris rapae crucivora, and its role in behaviour. J Comp Physiol 130:191–199
Svärd L, Wiklund C (1989) Mass and production rate of ejaculates in relation to monandry/polyandry in butterflies. Behav Ecol Sociobiol 24:395–402
Tammaru T, Esperk T (2007) Growth allometry in immature insects: larvae do not grow exponentially. Funct Ecol 21:1099–1105
Tregenza T, Wedell N (2000) Genetic compatibility, mate choice and patterns of parentage: invited review. Mol Ecol 9:1013–1027
Tregenza T, Wedell N (2002) Polyandrous females avoid costs of inbreeding. Nature 415:71–73
Välimäki P, Kaitala A (2006) Does a lack of mating opportunities explain monandry in the green-veined white butterfly (Pieris napi)? Oikos 115:110–116
Välimäki P, Kaitala A (2007) Life history trade-offs in relation to the degree of polyandry and developmental pathway in Pieris napi (Lepidoptera, Pieridae). Oikos 116:1569–1580
Välimäki P, Kaitala A (2010) Properties of male ejaculates do not generate geographic variation in female mating tactics in a butterfly Pieris napi. Anim Behav 79:1173–1179
Välimäki P, Kaitala A, Kokko H (2006) Temporal patterns in reproduction may explain variation in mating frequencies in the green-veined white butterfly Pieris napi. Behav Ecol Sociobiol 61:99–107
van Oosterhout C, Zijlska WG, van Heuven MK, Brakefield P (2000) Inbreeding depression and genetic load in laboratory metapopulations of the butterfly Bicyclus anynana. Evolution 54:218–225
Waser NM (1993) Population structure, optimal outbreeding, and assortative mating in angiosperms. In: Thornhill NW (ed) The natural history of inbreeding and outbreeding. The University of Chicago Press, Chicago, pp 173–199
Wedell N, Wiklund C, Cook PA (2002) Monandry and polyandry as alternative lifestyles in a butterfly. Behav Ecol 13:450–455
Wiklund C, Kaitala A, Lindfors V, Abenius J (1993) Polyandry and its effect on female reproduction in the green-veined white butterfly (Pieris napi L.). Behav Ecol Sociobiol 33:25–33
Zeh JA, Zeh DW (1996) The evolution of polyandry I: intragenomic conflict and genetic incompatibility. Proc R Soc Lond B 263:1711–1717
Zeh JA, Zeh DW (1997) The evolution of polyandry II: post-copulatory defenses against genetic incompatibility. Proc R Soc Lond B 264:69–75
Acknowledgments
We thank M. Mutanen, H. Pöykkö, and two anonymous referees for the helpful comments on an earlier draft of the manuscript. This study was partly financed by the Finnish cultural foundation (grant to P.V.) and the Jenny and Antti Wihuri foundation (S.M.K.). The authors declare that they have no conflict of interest. All of Finland’s guidelines and legal requirements for the use of animals in research were followed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by N. Wedell
Rights and permissions
About this article
Cite this article
Välimäki, P., Kivelä, S.M. & Mäenpää, M.I. Mating with a kin decreases female remating interval: a possible example of inbreeding avoidance. Behav Ecol Sociobiol 65, 2037–2047 (2011). https://doi.org/10.1007/s00265-011-1213-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-011-1213-3