Abstract
Strategies to modify the gut microbiome in cancer patients using fecal microbiota transplantation (FMT) have gained momentum as a therapeutic intervention. However, how FMT impacts innate-like, antimicrobial T lymphocytes is unclear. In this study, we assessed peripheral blood (PB) mucosa-associated invariant T (MAIT) cell frequencies and functions in patients with metastatic renal cell carcinoma (mRCC) before and seven days after they received FMT as part of a clinical trial. We found comparable MAIT cell frequencies in healthy controls and mRCC patients. In contrast, γδ T cells exhibited a numerical decline in mRCC, which was partially reversed by FMT. We also found a significant increase in the PB CD4+ MAIT cell compartment of mRCC patients with or without FMT. Paired sample analyses revealed CD69 upregulation on MAIT cells accompanied by decreased PD-1 levels post-FMT. These changes were unique to MAIT cells as non-MAIT T lymphocytes showed either no trend or a trend in the opposite direction. Importantly, FMT did not render MAIT cells exhausted as also judged by their stable expression of TIM-3, LAG-3, BTLA, CTLA-4, TIGIT and VISTA. These findings were corroborated in functional assays in which MAIT cells were stimulated with MR1 ligands or with a combination of IL-12 and IL-18 to produce inflammatory cytokines and granzyme B. Indeed, when stimulated ex vivo with IL-12 and IL-18, MAIT cells mounted a more rigorous TNF-α response post-FMT. In conclusion, FMT improves MAIT cell functions, which should serve patients well in subsequent microbial challenges in the face of cancer-elicited immunosuppression. Trial Registration: https://clinicaltrials.gov/Identifier: NCT04163289 (registration date: November 14, 2019).
Similar content being viewed by others
Introduction
Renal cell carcinoma (RCC), the malignant transformation of tubular epithelial cells, is the most common type of kidney cancer and the most deadly urological neoplasm. RCC is more frequent in men than in women, and accounts for more than 2% of all oncological diagnoses in the middle-aged and elderly populations worldwide [1]. A considerable proportion of RCC patients present with evidence of metastatic disease at diagnosis, which is associated with poor overall survival. Apart from partial or radical nephrectomy, the systemic treatment of RCC includes radiotherapy, targeted therapy with tyrosine kinase inhibitors, and immunotherapy. Immune checkpoint inhibitors (ICIs) have been used in patients with metastatic RCC (mRCC) and shown promise. However, ICIs may exert systemic toxicity and immune-related adverse effects, resulting in their discontinuation [2]. Also importantly, a sizable fraction of mRCC patients do not favorably respond to ICIs. Therefore, more tolerable and more effective treatment strategies for mRCC are urgently needed.
Fecal microbiota transplantation (FMT) is a therapeutic intervention in which fecal matter from a healthy donor is introduced to alter the intestinal microbiota of a recipient. FMT has been successfully used for the management of recurrent Clostridioides difficile-associated diarrhea [3], and may be efficacious in certain metabolic and autoimmune disorders [4,5,6]. The potential clinical benefits of FMT have also been proposed or recognized in oncology, with the possibility of alleviating the side effects of radiotherapy, chemotherapy and immunotherapy [7,8,9,10,11].
Mucosa-associated invariant T (MAIT) cells constitute a subset of innate-like T lymphocytes with prominent antimicrobial activities, effector memory-like characteristics and notable homing to mucosal barriers, including the gut [12]. They are also relatively abundant in the peripheral blood (PB) where they comprise up to 10% of circulating T cells. The invariant T cell receptor (iTCR) of MAIT cells detects microbial metabolites of the riboflavin biosynthesis pathway presented by the monomorphic antigen (Ag)-presenting molecule MHC-related protein 1 (MR1) [13, 14]. MAIT cells can also be activated by select inflammatory cytokines, including type I interferons (IFNs), interleukin (IL)-7, IL-12, IL-15 and IL-18, which are produced during many infections. Upon stimulation, MAIT cells swiftly secrete immunomodulatory cytokines of their own [e.g., IFN-γ, tumor necrosis factor (TNF)-α, IL-4, IL-5, IL-13, IL-17A and/or IL-22] and destroy infected cells that display MR1 ligands [15]. Furthermore, there is growing appreciation for MAIT cell roles in tissue repair [16], which may contribute to the healing process after infections.
In addition to their crucial roles in anti-pathogen immunity, MAIT cells are implicated in mucosal homeostasis and sense the presence of commensal microbes [17]. In fact, commensal flora and their metabolites control MAIT cells’ intrathymic development and extrathymic expansion in the gut lamina propria [17, 18]. Certain commensal species belonging to the Bacteroidetes and Proteobacteria phyla are among the most potent producers of riboflavin and the most powerful stimulators of MAIT cells [19].
Although tumor-derived MR1 ligands that may serve as MAIT cell Ags remain obscure, anticancer, pro-tumorigenic and pro-metastatic properties have been reported or envisaged for MAIT cells, largely due to their ability to modify the biological behaviors of downstream effector and regulatory cells [20, 21]. How MAIT cell responses to microbes and their products during natural infections or in therapeutic settings (e.g., FMT) influence clinical outcomes is unclear. Such responses may alter the cellular landscape of tumor microenvironments (TEMs) and bodily macroenvironments to patients’ benefit or detriment. On the other hand, excessive exposure to bacterial products or inflammatory cytokines may overwhelm MAIT cells and impede their ability to face and overcome subsequent microbial challenges [22]. This is particularly important in cancer patients whose underlying malignancy and the treatments they receive may result in immunosuppression.
FMT has been employed in recent preclinical studies and clinical trials to improve the efficacy of programmed cell death-1 (PD-1)-based ICIs in cancer [8, 9, 23]. However, whether and how FMT alters MAIT cell functions is essentially unexplored. In this study, we have used paired peripheral blood mononuclear cell (PBMC) samples from RCC patients before and after FMT, along with samples from a healthy control cohort, to assess MAIT cell frequencies and responses to a panel of iTCR-dependent and -independent stimuli. We demonstrate, for the first time, that FMT improves certain aspects of MAIT cell functions, including their cytokine production capacities. Therefore, MAIT cells should effectively fulfill their cognate and cytokine-mediated roles in host defense or may operate more optimally post-FMT.
Materials and methods
Subjects
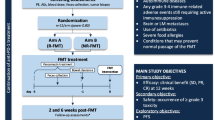
Ten consenting patients, two females and eight males (age range: 47–71) (Supplementary Table 1), with intermediate- or high-risk mRCC, without prior immunotherapy for their advanced disease, were enrolled. This study was approved by the Western University Research Ethics Board for Health Sciences Research Involving Human Subjects (HSREB protocol #114962). Ten closely age- and sex-matched healthy blood donors (Supplementary Table 1) were consented and recruited using HSREB protocol #5545, which was approved by the same institutional entity.
All patients had received a histologically confirmed diagnosis of advanced or metastatic (stage IV) RCC based on the American Joint Committee on Cancer staging system. Nine had clear cell RCC, and one was diagnosed with sarcomatoid RCC. Subjects younger than 18 or older than 100 were excluded. Other exclusion criteria were pregnancy, psychiatric illness, immunodeficiency, active systemic infections, active autoimmune disorders, inflammatory bowel disease, immunosuppressive therapy, radiation therapy within a four-week timeframe before the study commencement date, and the use of antibiotics within a two-week period prior to FMT. A more detailed list of inclusion and exclusion criteria can be found at ClinicalTrials.gov (Trial Identifier: NCT04163289).
To select fecal transplant donors, subjects with disorders and conditions listed in Table 1 were excluded [24, 25].
Blood processing and PBMC isolation and storage
PB from RCC patients was drawn into sodium heparin-coated tubes (BD Biosciences) before and seven days after FMT. Healthy controls (HCs) provided a single PB sample.
To isolate PBMCs, anticoagulated PB was diluted with sterile PBS and transferred into SepMate-50 tubes (STEMCELL Technologies) containing Ficoll-Paque PLUS (GE Healthcare) and subjected to density gradient centrifugation at 1200 × g for 10 min. PBMCs were then washed and resuspended at 107/mL in a freezing medium consisting of 12.5% human serum albumin, 30 µg/mL gentamicin and 10% dimethyl sulfoxide (DMSO) in RPMI 1640. Cryovials containing PBMC aliquots (5–10 × 106 cells/vial) were transferred into a container filled with 200 mL of isopropanol and placed inside a − 80 °C freezer to enable a gradual drop in temperature at a rate of ~ 1 °C/minute. After 24–48 h, cryovials were moved into a − 150 °C freezer where they were kept until use.
Fecal donor screening and FMT
Donors were screened for known transmissible pathogens following our established protocol [24, 25]. Each recipient was given approximately 40 oral capsules cumulatively containing 80–100 g of starting fecal material from a qualified donor (Table 1). FMT was performed seven days before recipients started dual immunotherapy with ipilimumab [an anti-cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) monoclonal antibody (mAb)] and nivolumab (an anti-PD-1 mAb) for mRCC as part of an ongoing clinical trial (Trial Identifier: NCT04163289).
Ex vivo PB MAIT cell stimulation
Escherichia coli (strain DH5α) lysate was prepared from bacterial cultures, stored at − 80 °C and used as we previously described [22]. To generate the MR1 ligand 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil (5-OP-RU), equimolar concentrations of 5-amino-6-D-ribitylaminouracil, generously provided by Dr. Olivier Lantz (Institut Curie), and methylglyoxal were mixed in DMSO for 24 h at room temperature.
Frozen PBMCs were thawed at room temperature before they were washed and resuspended in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 0.1 mM nonessential amino acids, 2 mM GlutaMAX-I, 1 mM sodium pyruvate, 10 mM HEPES and 100 U/mL penicillin/streptomycin, which we will refer to as complete medium. Typically, more than 80% of thawed PBMCs were viable as judged by trypan blue dye exclusion. Paired pre- and post-FMT PBMC samples were thawed, stimulated (as applicable), stained and analyzed on the same day by the same experimentalist.
PBMCs were seeded at 5 × 105 cells/well in U-bottom microplates and either left untreated or stimulated with clear E. coli lysate (diluted 1:10 in complete medium), with human B lymphoblastoid C1R cells (pulsed with 2 nM 5-OP-RU and used at a 1:10 C1R:PBMC ratio), or with a combination of recombinant human IL-12 (rhIL-12) p70 (PeproTech) and rhIL-18 (MBL International) (5 ng/mL each). Cultures were incubated for 24 h at 37 °C and 6% CO2. Brefeldin A (BFA) (Sigma-Aldrich) and monensin (eBioscience) were added to cultures at 10 µg/mL and 2 μM, respectively, for the final 5 h.
In several experiments, PBMCs were stimulated for 4 h with 50 ng/mL phorbol 12-myristate 13-acetate (PMA) and 500 ng/mL ionomycin in the presence of BFA and monensin.
Cytofluorimetric enumeration and immunophenotyping of conventional and innate-like T cells
Unstimulated and stimulated PBMCs were washed and resuspended in a cold buffer (PBS containing 2% FBS and 2 mM ethylenediaminetetraacetic acid) before they were stained at room temperature with fluorochrome-conjugated tetramers, antibodies and/or control reagents (Supplementary Table 2).
Dead cells were excluded using either 7-AAD (BD Biosciences) or Fixable Viability Dye (eBioscience). After surface staining, cells were fixed and permeabilized using a Forkhead box P3 (FoxP3)/Transcription Factor Staining Buffer Set (eBioscience) to detect intracellular cytokines, transcription factors and granzyme B (GZM B) as indicated.
After dead cell and doublet exclusions, a lymphocyte gate was drawn based on forward and side scatter characteristics before MAIT cells were identified as CD3+ cells that stained positively with 5-OP-RU-loaded MR1 tetramers. CD3+MR1 tetramer− cells were considered non-MAIT T cells. Our gating strategy is illustrated in Supplementary Fig. 1. Invariant natural killer T (iNKT) cells were defined as CD3+PBS-57-loaded CD1d tetramer+ cells. We used 6-formylpterin (6-FP)-loaded MR1 tetramers (Supplementary Fig. 1) and empty CD1d tetramers as negative staining controls, as appropriate. All tetramer reagents were supplied by the NIH Tetramer Core Facility.
A BD FACSCanto II cytometer and FlowJo software (Tree Star) were used for data acquisition and analysis, respectively.
Statistical analyses
GraphPad Prism 8 was used for all statistical analyses. Normal distribution of data sets was assessed by the D’Agostino–Pearson test. Comparisons were made using the statistical tests identified in figure legends, and differences with p ≤ 0.05 were considered significant. Data are presented as mean ± standard error of the mean (SEM) when applicable.
Results
Unlike γδ T cells, MAIT cells maintain their PB frequencies in RCC patients before and after FMT
Numerical declines in the PB MAIT cell compartment of cancer patients have been observed [26,27,28,29]. In addition, infections and other microbial challenges often lower PB MAIT cell frequencies, potentially due to activation-induced cell death or altered migratory patterns [30, 31]. In this study, we found comparable MAIT cell percentages between RCC patients and age/sex-matched HCs (Fig. 1). This was clear when MAIT cells were enumerated among either bulk PBMCs (Fig. 1A) or CD3+ T lymphocytes (Fig. 1C). Moreover, our paired sample analyses revealed no reduction in PB MAIT cell abundance after FMT (Fig. 1B and D). In fact, we found a moderate trend toward increased, rather than decreased, MAIT cell frequencies among CD3+ events post-FMT (Fig. 1D).
PB MAIT cell frequencies remain stable following FMT for RCC. MAIT cell frequencies were determined by 5-OP-RU-loaded MR1 tetramer staining among live PBMCs (A and B) or CD3+ events (C and D) in 10 healthy controls (HCs) (A and C) and 10 renal cell carcinoma (RCC) patients before and seven days after FMT (A–D). Box-and-Whisker plots (A and C) illustrate MAIT cell percentages in the three cohorts. The results of pre- and post-FMT paired sample analyses are also depicted (B and D). Each circle represents an individual sample. Kruskal–Wallis tests were followed by Dunn’s post hoc comparisons (A and C), and Wilcoxon signed-rank tests were used for matched pair analyses (B and D)
Most PB MAIT cells in healthy subjects express CD8, a coreceptor that binds MR1 and enhances cytokine production by MAIT cells [32]. Therefore, the functional plasticity of MAIT cell subsets may be partially due to their CD8 expression, or lack thereof. As anticipated, the vast majority of PB MAIT cells in our HC cohort expressed CD8 (Fig. 2A). However, we found significantly higher CD4+CD8− MAIT cell levels in RCC patients than in HCs (Fig. 2A), regardless of their FMT treatment status (Fig. 2A–B). This was exclusive to MAIT cells since non-MAIT T lymphocytes from HC and RCC cohorts exhibited a similar coreceptor expression pattern (Supplementary Fig. 2A), which also remained stable after FMT in RCC patients (Supplementary Fig. 2B).
CD4+CD8− MAIT cell frequencies are increased in the PB of RCC patients. The percentages of PB CD4+CD8−, CD4−CD8+, CD4+CD8+ and CD4−CD8− MAIT cell subsets were determined by flow cytometry among CD3+ 5-OP-RU-loaded MR1 tetramer+ cells in 10 healthy controls (HCs) and 10 RCC patients before and after FMT. Horizontal slice charts (A) illustrate the averaged distribution of MAIT cells expressing CD4 and/or CD8, or not. The exact mean and SEM values are also shown, and * denotes a statistically significant difference with p ≤ 0.05 using Mann–Whitney U tests (A). Wilcoxon signed-rank tests were employed for matched pair analyses comparing pre- and post-FMT samples (B)
Innate-like T lymphocytes other than MAIT cells, such as iNKT and γδ T cells, comprise a minute fraction of non-MAIT T cells. However, they can play important roles in antimicrobial defense and in host responses to cancer [33]. Therefore, it was of interest to also enumerate these cell types in our cohorts. While circulating at low frequencies in both HCs and RCC patients, PB iNKT cell levels appeared to form a larger component of CD3+ T cells in patients (with or without FMT) compared with HCs (Supplementary Fig. 3A). By contrast, there was a strong trend toward reduced γδ T cell percentages among PB CD3+ T cells in RCC, which were partially restored following FMT (Supplementary Fig. 3B). FMT was similarly effective in resolving γδ T cell deficits when the frequencies of these cells were determined among total PBMCs (1.73 ± 0.48% and 2.13 ± 0.58% in pre- and post-FMT samples, respectively, p < 0.01).
The above results indicate that circulating MAIT cell pools are retained and γδ T cell frequencies recover after FMT for RCC.
FMT does not disrupt the MAIT1-MAIT17-MAIT1/17 balance in the PB
The immunomodulatory activities of MAIT cells are governed by the type of transcription factors that they express. MAIT cells can be generally divided into MAIT1 and MAIT17 subsets based on their ability to express T-bet (T-box expressed in T cells) or RORγT (retinoic acid receptor-related orphan receptor γt), respectively [34]. However, these transcription factors are not mutually exclusive in human MAIT cells as “MAIT1/17” cells harbor both. We found T-bet+RORγt− (MAIT1), T-bet−RORγt+ (MAIT17), T-bet+RORγt+ (MAIT1/17) and T-bet−RORγt− (double-negative) cell frequencies to be comparable in HCs and RCC patients (Fig. 3A and Supplementary Fig. 4). Furthermore, FMT did not tip the balance in favor of any of the above-defined subsets (Fig. 3A–B) neither did it change the geometric mean fluorescence intensity (gMFI) values of T-bet and RORγt (Supplementary Fig. 5).
PB MAIT cells maintain their MAIT1-MAIT17-MAIT1/17 balance post-FMT. PBMCs from 10 healthy controls (HCs) and 10 RCC patients (before and seven days after FMT) were analyzed by flow cytometry for indicated transcription factors within the CD3+ 5-OP-RU-loaded MR1 tetramer+ MAIT cell population. (A) Pie charts depict the frequencies of T-bet+RORγt−, T-bet−RORγt+, T-bet+RORγt+ and T-bet−RORγt− subsets. (B) Box-and-Whisker plots illustrate the proportions of the above subsets in RCC patients pre- and post-FMT. Each circle represents an individual sample, and data are shown as mean ± SEM. Kruskal–Wallis tests, followed by Dunn’s post hoc tests, were employed for group comparisons (A), and Wilcoxon signed-rank tests were used for matched pair analyses (B)
Chronic stimulation of human PB MAIT cells in ex vivo settings reportedly promotes the late-onset production of T helper 2 (TH2)-type cytokines, especially IL-13 and IL-5 and to a lesser extent IL-4 [35]. Following FMT, the presence of myriad vitamin B2-producing bacteria within the fecal matter should result, at least in theory, in prolonged iTCR triggering and chronic MAIT cell stimulation. Therefore, we compared pre- and post-FMT samples for their GATA-3+ MAIT cell content. As expected, only a small proportion of MAIT cells expressed GATA-3 in our cohorts (Supplementary Fig. 6A). Furthermore, GATA-3+ MAIT cell frequencies within pre- and post-FMT PBMCs were statistically comparable (Supplementary Fig. 6B).
FMT upregulates CD69 and downregulates PD-1 on MAIT cells
Exposure to bacteria and their products activates MAIT cells, which may or may not be followed by cellular exhaustion and a failure to respond to subsequent microbial challenges [22]. Therefore, we examined the expression of activation and exhaustion markers by MAIT cells in RCC patients before and after FMT. Indeed, FMT upregulated CD69, an early activation marker, as judged both by higher CD69+ MAIT cell frequencies (Fig. 4A) and by the elevated gMFI of CD69 staining, indicative of increased CD69 expression on a per-cell basis (Fig. 4B). By comparison, non-MAIT T cells downregulated their CD69 expression post-FMT (Fig. 4C–D). Comparing MAIT and non-MAIT T cells for CD38, another activation marker, revealed no changes after FMT except for a slight, but still significant, increase in the gMFI of CD38 staining in non-MAIT T cells (Supplementary Fig. 7).
FMT leads to increased CD69 expression on MAIT cells, but not on non-MAIT T lymphocytes. PBMCs from 10 RCC patients, collected before to seven days after FMT, were stained and analyzed by flow cytometry for the surface expression of CD69 on CD3+ 5-OP-RU-loaded MR1 tetramer+ (MAIT) cells as well as CD3+ 5-OP-RU-loaded MR1 tetramer− (non-MAIT T) cells. CD69+ cell frequencies (A and C) and the geometric mean fluorescence intensity (gMFI) of CD69 staining (B and D) are shown. Each circle represents an individual RCC sample. Wilcoxon signed-rank tests were used to compare paired samples, * and ** denote statistically significant differences with p ≤ 0.05 and p ≤ 0.01, respectively
Next, we assayed for the expression of surface markers associated with cellular exhaustion or co-inhibitory functions, including B- and T-lymphocyte attenuator (BTLA), CTLA-4, lymphocyte activation gene 3 (LAG-3), PD-1, T cell immunoreceptor with immunoglobulin and ITIM domains (TIGIT), T cell immunoglobulin and mucin-3 (TIM-3), and V-domain immunoglobulin suppressor of T cell activation (VISTA). We detected higher levels of PD-1 and BTLA on MAIT cells in RCC patients compared with HCs (Fig. 5). Importantly, parallel sample analyses revealed a significant reduction in PD-1 expression by MAIT cells (Fig. 5). Also of note, we found increased percentages of BTLA+ and TIM-3+ non-MAIT T cells in RCC patients (Supplementary Fig. 8). However, FMT did not change the expression levels of any of the above markers in this compartment.
FMT results in decreased PD-1 expression on MAIT cells. Pre- and post-FMT PBMC samples from 10 RCC patients along with PBMCs from 10 healthy controls (HCs) were analyzed by flow cytometry for the surface expression of BTLA, CTLA-4, LAG-3, PD-1, TIGIT, TIM-3 and VISTA by MAIT cells. The percentages of cells expressing indicated exhaustion/co-inhibitory markers are illustrated (top and middle rows), and so is the gMFI of staining for each marker (bottom row). Each circle represents an individual sample. Group comparisons (top row) were carried out by one-way ANOVA followed by the Tukey’s Multiple Comparison test. For paired sample analyses (middle and bottom rows), Wilcoxon signed-rank tests were used. * and **** denote significant differences with p ≤ 0.05 and p ≤ 0.0001, respectively
Collectively, the above data indicate that FMT results in MAIT cell activation without rendering them overwhelmed and consequently exhausted. On the contrary, FMT lowers the expression of the classic exhaustion marker PD-1.
MAIT cells remain responsive to MR1 ligands in FMT recipients
To assess the iTCR-coupled MAIT cell activation pathway, we stimulated PBMCs with 5-OP-RU, a potent MR1 ligand [14], which was pulsed on C1R cells to allow for stable Ag presentation to MAIT cells [36]. As illustrated in Fig. 6A–B, exposure to 5-OP-RU reduced the frequency of detectable MAIT cells in cultures containing PBMCs from HCs and RCC patients (pre- and post-FMT), due likely to activation-induced iTCR internalization.
MAIT cells maintain their capacity to respond to MR1/iTCR-dependent stimuli after FMT for RCC. Pre- and post-FMT PBMC samples from RCC patients (n = 10) and PBMCs from 10 healthy controls (HCs) were co-incubated for 24 h with C1R cells in the absence or presence of 5-OP-RU. The frequencies of detectable MAIT cells were subsequently determined by flow cytometry (A–B). Representative plots illustrating the remaining MAIT cells (A), and summary data depicting MAIT cell frequencies, relative to unstimulated cultures (B), are provided. In parallel cultures, PBMCs were stimulated for 24 h with E. coli lysate before MAIT cells capable of producing indicated cytokines and GZM B were enumerated (C). Background cytokine and GZM B levels were obtained from unstimulated cultures and subtracted for each mediator. Each circle represents an individual sample (C). Paired Student’s t tests (B) and two-way ANOVA (followed by the Tukey’s Multiple Comparison test) (C) were performed to compute statistically significant differences, or lack thereof. ** and *** denote p ≤ 0.01 and p ≤ 0.0001, respectively
To confirm MAIT cells’ ability to mount iTCR-dependent responses, we added E. coli lysate, a crude source of bacterial MR1 ligands, to PBMC cultures. This stimulation mode enabled detection of a substantial number of MAIT cells with comparable levels of intracellular effector molecules, especially IFN-γ and TNF-α, across the three sample sets (Fig. 6C). Moreover, MAIT cell responses to E. coli were statistically similar in pre- and post-FMT cultures (Supplementary Fig. 9). Therefore, the MR1/iTCR-driven MAIT cell activation pathway remains operational after FMT.
MAIT cells elicit preserved or enhanced responses to TCR-independent stimuli after FMT
Many microbes, including those without an active riboflavin biosynthesis machinery that supplies MR1 ligands, can activate MAIT cells in iTCR-independent fashions, primarily through cytokines. To begin to evaluate MAIT cell responses under such circumstances, we stimulated PBMCs with PMA and ionomycin in short-term cultures enabling direct MAIT cell activation while avoiding or minimizing bystander responses. PMA activates protein kinase C, and ionomycin raises intracellular Ca++ levels, thus bypassing TCRs to induce T cell activation when used in combination. MAIT cells from RCC patients appeared more responsive than controls to PMA and ionomycin (Fig. 7A). In addition, MAIT cells in pre- and post-FMT paired samples were both able to produce IFN-γ, TNF-α, IL17A and GZM B (Fig. 7A).
MAIT cells launch equally vigorous or more vigorous responses to MR1/iTCR-independent stimuli after FMT for RCC. Pre- and post-FMT PBMC samples from 10 RCC patients and PBMCs from 10 healthy controls (HCs) were left untreated in medium, stimulated for 4 h with a combination of PMA and ionomycin (A), or stimulated for 24 h with a combination of rhIL-12 and rhIL-18 (B). The proportions of IFN-γ+, TNF-α+, IL-17A+ and GZM B+ MAIT cells were then determined by flow cytometry. Background cytokine and GZM B levels from unstimulated cultures were subtracted. Each circle represents an individual sample. Group comparisons were made using two-way ANOVA followed by the Tukey’s Multiple Comparison test. Paired dataset comparisons were made using paired t tests for IFN-γ, IL-17A and TNF-α, and Wilcoxon signed-rank tests for GZM B. * and ** denote differences with p ≤ 0.05 and p ≤ 0.01, respectively
Next, we stimulated PBMCs with rhIL-12 and rhIL-18, cytokines that are often released during microbial challenges and known to potently activate innate-like T lymphocytes [22, 37]. MAIT cells from HCs and RCC patients were equally capable, if not more capable, of producing cytokines and GZM B in these cultures (Fig. 7B). Of note, FMT significantly augmented the TNF-α production capacity of MAIT cells after FMT (Fig. 7B). Therefore, FMT does not compromise the functional competence of MAIT cells and may in fact improve certain aspects of their antimicrobial, and potentially anti-tumor, activities.
Discussion
The efficacy and tolerability of ICIs employed against carcinomas are influenced by the composition of patients’ microbiota, which may be manipulated therapeutically, for instance, through FMT, to improve clinical outcomes and to mitigate adverse side effects [7,8,9, 11, 38]. However, how FMT affects immune responses at the cellular level is far from clearly understood. This is particularly important in light of the robust antimicrobial properties of innate-like T cells, including MAIT cells, which also exhibit anti- and/or pro-tumor potentials [20]. MAIT cells need to stand sentinel to protect against opportunistic infections in cancer patients. While riboflavin-producing and other microbes within the transferred fecal matter should activate MAIT cells, their sheer number may also potentially cause MAIT cell exhaustion, thus making patients prone to a wide spectrum of bacterial and viral infections.
According to previous reports, PD-1+ MAIT cell frequencies are elevated in the PB and bone marrow of patients with multiple myeloma [39], and PD-1+TIM-3+ MAIT cells accumulate in the primary tumors of patients with colon cancer [40]. In our study, RCC patients had higher PD-1+ and BTLA+ PB MAIT cell percentages compared with HCs irrespective of their FMT treatment status.
Importantly, we found CD69 upregulation and PD-1 downregulation by PB MAIT cells after FMT. This was not true for non-MAIT T cells, the vast majority of which are mainstream T lymphocytes that recognize peptide Ags, not riboflavin metabolites. Furthermore, MAIT cells are more sensitive than conventional T lymphocytes to cytokines like IL-12 and IL-18 [22, 37]. Finally, while MAIT cells occur in a poised effector position to respond quickly to microbial challenges [41], conventional T cells take longer to fully respond to cognate Ags. Head-to-head comparisons between innate-like and conventional T cells are not always meaningful or even possible in complex in vivo systems. However, potential differences in stimulation thresholds and response kinetics may dictate the divergent expression of activation and exhaustion markers by these cell types.
We found FMT not to raise the expression of BTLA, CTLA-4, LAG-3, TIM-3, TIGIT or VISTA. Our cytofluorimetric panel design also allowed us to determine the frequencies of PD-1+TIGIT+ and PD-1+BTLA+ double-expressors, which too remained stable post-FMT (data not shown).
Consistent with the above findings, MAIT cells were either equally or more capable of responding to iTCR/MR1-dependent and -independent stimuli. An enhanced TNF-α response to IL-12 and IL-18 was noteworthy in post-FMT PBMC cultures. A fortified IL-12/IL-18-TNF-α axis should boost immune surveillance against various pathogens, including viruses, which may cause severe infections in immunosuppressed patients.
Together, our results indicate that FMT activates MAIT cells without making them exhausted, a finding that provides additional justification for the usefulness of this therapeutic modality in human malignancies. The current study was focused on PB MAIT cells as part of a clinical trial for mRCC, and future investigations will address the impact of FMT on tissue-resident and tumor-infiltrating MAIT cells in primary sites of neoplastic transformation, including mucosal layers, and in metastatic tumor masses.
In an ex vivo lamina propria cell culture setting, Rodin et al. demonstrated stronger CD25 induction on colon tumor-infiltrating MAIT cells when pembrolizumab, a PD-1-blocking mAb, was present [40]. In our ongoing clinical trial, mRCC patients receive FMT before dual immunotherapy with ipilimumab and nivolumab, which block CTLA-4 and PD-1, respectively. We will assess the effect of this sequential treatment strategy on MAIT cell responses and their correlation with overall and progression-free survival among other clinical measures.
We found lower PB γδ T cell frequencies in our RCC cohort compared with HCs, which was partially corrected by FMT. The crosstalk between microbiota and γδ T cells controls mucosal tissue homeostasis and modulates immune responses in numerous conditions, including cancer. For instance, using the B16/F10 melanoma and Lewis lung carcinoma mouse models of pulmonary metastasis, Cheng et al. demonstrated that commensal bacteria contribute to anticancer immune surveillance through a mechanism that requires IL-17-producing γδ T cells [42]. We are currently investigating the phenotypic characteristics of γδ T cells as well as their TCR-dependent and -independent responses before and after FMT.
Another intriguing finding of this study was a drop in the CD8:CD4 MAIT cell ratio in RCC, which is reminiscent of previous reports in colorectal cancer (CRC) and melanoma cohorts [26, 29]. PB CD8+ and CD4+ MAIT cells from healthy donors appear to be transcriptionally distinct and likely dissimilar in terms of homeostatic, migratory, effector and regulatory functions [43]. For example, cytotoxicity-associated genes are predominantly expressed by the CD8+ subset of PB MAIT cells. In a recent study, a positive correlation was found between circulating CD8+ MAIT cell percentages and improved survival in patients with stage IV melanoma receiving anti-PD-1 therapy [29]. In contrast, elevated CD4+ MAIT cell frequencies were correlated with poor survival. Also of note, tumor-infiltrating CD4+ MAIT cells expressing FoxP3 have been previously reported in CRC patients [44]. Future studies on large patient cohorts will need to decipher the roles played by CD4+ and CD8+ MAIT cell subsets in various TEMs.
Innate-like T cells are not restricted by MHC and may, as such, be targeted in diverse patient populations. This is unlike mainstream T cell-based therapies tailored to patients’ unique genetic makeup due to polymorphism at the MHC super-locus. MR1 and CD1d, which present a more limited array of Ags to MAIT and iNKT cells, respectively, are monomorphic. An added advantage of MAIT cells is their resistance to chemotherapeutic agents, owed to their high expression levels of ABCB1 [12], which should permit intact responses to pathogens, and potentially to cancer, when/if FMT and/or immunotherapy is combined with chemotherapy. Finally, under optimal culture conditions, MAIT cells may be expanded in vitro and stored for future use as off-the-shelf therapeutics [45].
The findings of this body of work should lay the foundation for future studies in which the benefits of FMT in anti-pathogen and anti-tumor immunity are explored in the context of innate-like T cell responses.
Data availability
The data generated in this study are available within the article’s main body and supplementary files or may be requested from the corresponding authors. The materials we used in this investigation are available from the indicated sources or may be obtained from the corresponding authors upon reasonable request.
Abbreviations
- Ag:
-
Antigen
- BFA:
-
Brefeldin A
- BTLA:
-
B- and T-lymphocyte attenuator
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated antigen-4
- CRC:
-
Colorectal cancer
- DMSO:
-
Dimethyl sulfoxide
- FBS:
-
Fetal bovine serum
- FMT:
-
Fecal microbiota transplantation
- FoxP3:
-
Forkhead box P3
- 6-FP:
-
6-Formylpterin
- GATA-3:
-
GATA binding protein 3
- gMFI:
-
Geometric mean fluorescence intensity
- GZM:
-
Granzyme
- HC:
-
Healthy control
- HLA:
-
Human leukocyte antigen
- ICI:
-
Immune checkpoint inhibitor
- IFN:
-
Interferon
- IL:
-
Interleukin
- iNKT:
-
Invariant natural killer T [cell]
- iTCR:
-
Invariant T cell receptor
- LAG-3:
-
Lymphocyte activation gene 3
- mAb:
-
Monoclonal antibody
- MAIT:
-
Mucosa-associated invariant T [cell]
- MHC:
-
Major histocompatibility complex
- MR1:
-
MHC-related protein 1
- mRCC:
-
Metastatic renal cell carcinoma
- 5-OP-RU:
-
5-(2-Oxopropylideneamino)-6-D-ribitylaminouracil
- PB:
-
Peripheral blood
- PBMC(s):
-
Peripheral blood mononuclear cell(s)
- PBS:
-
Phosphate-buffered saline
- PD-1:
-
Programmed cell death-1
- PMA:
-
Phorbol 12-myristate 13-acetate
- RCC:
-
Renal cell carcinoma
- RORγt:
-
Retinoic acid receptor-related orphan receptor γt
- T-bet:
-
T-box expressed in T cells
- TH2:
-
T helper 2 [cell]
- TIGIT:
-
T cell immunoreceptor with immunoglobulin and ITIM domains
- TIM-3:
-
T cell immunoglobulin and mucin-3
- TME(s):
-
Tumor microenvironment(s)
- TNF:
-
Tumor necrosis factor
- VISTA:
-
V-domain immunoglobulin suppressor of T cell activation
References
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70:7–30. https://doi.org/10.3322/caac.21590
Stellato M, Procopio G, De Giorgi U et al (2021) Clinical outcome of renal cancer patients who early interrupted immunotherapy due to serious immune-related adverse events. Meet-Uro 13 trial on behalf of the MeetUro investigators. J Transl Med 19:328. https://doi.org/10.1186/s12967-021-03008-9
Ianiro G, Maida M, Burisch J, Simonelli C, Hold G, Ventimiglia M, Gasbarrini A, Cammarota G (2018) Efficacy of different faecal microbiota transplantation protocols for clostridium difficile infection: a systematic review and meta-analysis. United Eur Gastroenterol J 6:1232–1244. https://doi.org/10.1177/2050640618780762
Kootte RS, Levin E, Salojärvi J et al (2017) Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab 26:611-619.e6. https://doi.org/10.1016/j.cmet.2017.09.008
Kong L, Lloyd-Price J, Vatanen T, Seksik P, Beaugerie L, Simon T, Vlamakis H, Sokol H, Xavier RJ (2020) Linking strain engraftment in fecal microbiota transplantation with maintenance of remission in crohn’s disease. Gastroenterology 159:2193-2202.e5. https://doi.org/10.1053/j.gastro.2020.08.045
Makkawi S, Camara-Lemarroy C, Metz L (2018) Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurol Neuroimmunol Neuroinflamm 5:e459. https://doi.org/10.1212/NXI.0000000000000459
Su Z, Lu L, Chen F, Chen J, Chen X (2021) Gut microbiota and sunitinib-induced diarrhea in metastatic renal cell carcinoma: a pilot study. Cancer Manag Res 13:8663–8672. https://doi.org/10.2147/CMAR.S328451
Routy B, Le Chatelier E, Derosa L et al (2018) Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359:91–97. https://doi.org/10.1126/science.aan3706
Davar D, Dzutsev AK, McCulloch JA et al (2021) Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 371:595–602. https://doi.org/10.1126/science.abf3363
Ianiro G, Rossi E, Thomas AM et al (2020) Faecal microbiota transplantation for the treatment of diarrhoea induced by tyrosine-kinase inhibitors in patients with metastatic renal cell carcinoma. Nat Commun 11:4333. https://doi.org/10.1038/s41467-020-18127-y
McQuade JL, Ologun GO, Arora R, Wargo JA (2020) Gut microbiome modulation via fecal microbiota transplant to augment immunotherapy in patients with melanoma or other cancers. Curr Oncol Rep 22:74. https://doi.org/10.1007/s11912-020-00913-y
Dusseaux M, Martin E, Serriari N et al (2011) Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 117:1250–1259. https://doi.org/10.1182/blood-2010-08-303339
Kjer-Nielsen L, Patel O, Corbett AJ et al (2012) MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 491:717–723. https://doi.org/10.1038/nature11605
Corbett AJ, Eckle SB, Birkinshaw RW et al (2014) T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature 509:361–365. https://doi.org/10.1038/nature13160
Rudak PT, Choi J, Haeryfar SMM (2018) MAIT cell-mediated cytotoxicity: roles in host defense and therapeutic potentials in infectious diseases and cancer. J Leukoc Biol 104:473–486. https://doi.org/10.1002/JLB.4RI0118-023R
Salou M, Lantz O (2019) A TCR-dependent tissue repair potential of MAIT cells. Trends Immunol 40:975–977. https://doi.org/10.1016/j.it.2019.09.001
Legoux F, Bellet D, Daviaud C et al (2019) Microbial metabolites control the thymic development of mucosal-associated invariant T cells. Science 366:494–499. https://doi.org/10.1126/science.aaw2719
Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O (2003) Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 422:164–169. https://doi.org/10.1038/nature01433
Tastan C, Karhan E, Zhou W et al (2018) Tuning of human MAIT cell activation by commensal bacteria species and MR1-dependent T-cell presentation. Mucosal Immunol 11:1591–1605. https://doi.org/10.1038/s41385-018-0072-x
Haeryfar SMM, Shaler CR, Rudak PT (2018) Mucosa-associated invariant T cells in malignancies: a faithful friend or formidable foe? Cancer Immunol Immunother 67:1885–1896. https://doi.org/10.1007/s00262-018-2132-1
Yao T, Shooshtari P, Haeryfar SMM (2020) Leveraging public single-cell and bulk transcriptomic datasets to delineate MAIT cell roles and phenotypic characteristics in human malignancies. Front Immunol 11:1691. https://doi.org/10.3389/fimmu.2020.01691
Shaler CR, Choi J, Rudak PT et al (2017) MAIT cells launch a rapid, robust and distinct hyperinflammatory response to bacterial superantigens and quickly acquire an anergic phenotype that impedes their cognate antimicrobial function: defining a novel mechanism of superantigen-induced immunopathology and immunosuppression. PLoS Biol 15:e2001930. https://doi.org/10.1371/journal.pbio.2001930
Baruch EN, Youngster I, Ben-Betzalel G et al (2021) Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 371:602–609. https://doi.org/10.1126/science.abb5920
Craven LJ, Nair Parvathy S, Tat-Ko J, Burton JP, Silverman MS (2017) Extended screening costs associated with selecting donors for fecal microbiota transplantation for treatment of metabolic syndrome-associated diseases. Open Forum Infect Dis 4:ofx243. https://doi.org/10.1093/ofid/ofx243
Parvathy SN, Lenehan JG, Fernandes R, Poutanen SM, Hota S, Maleki Vareki S, Silverman M (2021) Enhanced donor screening for faecal microbial transplantation during COVID-19. Gut 70:2219–2220. https://doi.org/10.1136/gutjnl-2021-324593
Ling L, Lin Y, Zheng W et al (2016) Circulating and tumor-infiltrating mucosal associated invariant T (MAIT) cells in colorectal cancer patients. Sci Rep 6:20358. https://doi.org/10.1038/srep20358
Duan M, Goswami S, Shi JY et al (2019) Activated and exhausted MAIT cells foster disease progression and indicate poor outcome in hepatocellular carcinoma. Clin Cancer Res 25:3304–3316. https://doi.org/10.1158/1078-0432.CCR-18-3040
Melo AM, O’Brien AM, Phelan JJ et al (2019) Mucosal-associated invariant T cells display diminished effector capacity in oesophageal adenocarcinoma. Front Immunol 10:1580. https://doi.org/10.3389/fimmu.2019.01580
Vorwald VM, Davis DM, Van Gulick RJ et al (2022) Circulating CD8+ mucosal-associated invariant T cells correlate with improved treatment responses and overall survival in anti-PD-1-treated melanoma patients. Clin Transl Immunology. 11:e1367. https://doi.org/10.1002/cti2.1367
Szabo PA, Anantha RV, Shaler CR, McCormick JK, Haeryfar SM (2015) CD1d- and MR1-restricted T cells in sepsis. Front Immunol 6:401. https://doi.org/10.3389/fimmu.2015.00401
Haeryfar SM, Mallevaey T (2015) Editorial: CD1- and MR1-restricted T cells in antimicrobial immunity. Front Immunol 6:611. https://doi.org/10.3389/fimmu.2015.00611
Souter MNT, Awad W, Li S et al (2022) CD8 coreceptor engagement of MR1 enhances antigen responsiveness by human MAIT and other MR1-reactive T cells. J Exp Med 219:e20210828. https://doi.org/10.1084/jem.20210828
Stolk D, van der Vliet HJ, de Gruijl TD, van Kooyk Y, Exley MA (2018) Positive & negative roles of innate effector cells in controlling cancer progression. Front Immunol 9:1990. https://doi.org/10.3389/fimmu.2018.01990
Gherardin NA, Souter MN, Koay HF et al (2018) Human blood MAIT cell subsets defined using MR1 tetramers. Immunol Cell Biol 96:507–525. https://doi.org/10.1111/imcb.12021
Kelly J, Minoda Y, Meredith T et al (2019) Chronically stimulated human MAIT cells are unexpectedly potent IL-13 producers. Immunol Cell Biol 97:689–699. https://doi.org/10.1111/imcb.12281
Yao T, Rudak PT, Laumont CM et al (2022) MAIT cells accumulate in ovarian cancer-elicited ascites where they retain their capacity to respond to MR1 ligands and cytokine cues. Cancer Immunol Immunother 71:1259–1273. https://doi.org/10.1007/s00262-021-03118-9
Ussher JE, Bilton M, Attwod E et al (2014) CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur J Immunol 44:195–203. https://doi.org/10.1002/eji.201343509
Dizman N, Meza L, Bergerot P et al (2022) Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: a randomized phase 1 trial. Nat Med 28:704–712. https://doi.org/10.1038/s41591-022-01694-6
Favreau M, Venken K, Faict S et al (2017) Both mucosal-associated invariant and natural killer T-cell deficiency in multiple myeloma can be countered by PD-1 inhibition. Haematologica 102:e266–e270. https://doi.org/10.3324/haematol.2017.163758
Rodin W, Sundström P, Ahlmanner F, Szeponik L, Zajt KK, Wettergren Y, Bexe Lindskog E, Quiding Järbrink M (2021) Exhaustion in tumor-infiltrating Mucosal-Associated Invariant T (MAIT) cells from colon cancer patients. Cancer Immunol Immunother 70:3461–3475. https://doi.org/10.1007/s00262-021-02939-y
Gutierrez-Arcelus M, Teslovich N, Mola AR et al (2019) Lymphocyte innateness defined by transcriptional states reflects a balance between proliferation and effector functions. Nat Commun 10:687. https://doi.org/10.1038/s41467-019-08604-4
Cheng M, Qian L, Shen G, Bian G, Xu T, Xu W, Hu S (2014) Microbiota modulate tumoral immune surveillance in lung through a γδT17 immune cell-dependent mechanism. Cancer Res 74:4030–4041. https://doi.org/10.1158/0008-5472.CAN-13-2462
Vorkas CK, Krishna C, Li K, Aubé J, Fitzgerald DW, Mazutis L, Leslie CS, Glickman MS (2022) Single-cell transcriptional profiling reveals signatures of helper, effector, and regulatory MAIT cells during homeostasis and activation. J Immunol 208:1042–1056. https://doi.org/10.4049/jimmunol.2100522
Li S, Simoni Y, Becht E et al (2020) Human tumor-infiltrating MAIT cells display hallmarks of bacterial antigen recognition in colorectal cancer. Cell Rep Med 1:100039. https://doi.org/10.1016/j.xcrm.2020.100039
Parrot T, Healy K, Boulouis C, Sobkowiak MJ, Leeansyah E, Aleman S, Bertoletti A, Sällberg Chen M, Sandberg JK (2021) Expansion of donor-unrestricted MAIT cells with enhanced cytolytic function suitable for TCR redirection. JCI Insight 6:e140074. https://doi.org/10.1172/jci.insight.140074
Acknowledgements
This work was funded by the Canadian Cancer Society (CCS) (Innovation Grant 706396 to S.M. Mansour Haeryfar) with support from the Spring Yard Cleanup for Cancer, and by the London Regional Cancer Program (Keith Samitt Translational Research Catalyst Grant to Saman Maleki Vareki) with support from London Health Sciences Foundation. We are grateful to cancer patients and their families as well as healthy blood donors who participated in this study.
Funding
This work was funded by the Canadian Cancer Society (CCS) (Innovation Grant 706396 to S.M. Mansour Haeryfar) with support from the Spring Yard Cleanup for Cancer, and by the London Regional Cancer Program (Keith Samitt Translational Research Catalyst Grant to Saman Maleki Vareki) with support from London Health Sciences Foundation.
Author information
Authors and Affiliations
Contributions
MN designed and performed experiments, analyzed and interpreted data, and wrote the initial manuscript draft. CLS and MM recruited and obtained samples from healthy controls and participated in study design. SNP recruited fecal material donors, prepared fecal material for transplantation, administered FMT capsules and managed patient data. RF processed patient blood samples and managed patient data. JPB participated in study design. MSS participated in study design and oversaw the process of fecal material preparation and administration. RF recruited patients and participated in study design. SMV obtained funding, provided patient samples, participated in study design, analyzed data and edited the manuscript. SMMH obtained funding, conceived the idea, designed experiments, analyzed and interpreted data, and extensively edited the manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest. S.M. Mansour Haeryfar currently serves on the Editorial Board of Cancer Immunology, Immunotherapy.
Ethics approval
Human specimens were collected and used as per study protocols 5545 and 114962 approved by the Western University Research Ethics Board for Health Sciences Research Involving Human Subjects.
Consent to participate
Informed consent was obtained from all patients and healthy blood donors who participated in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ninkov, M., Schmerk, C.L., Moradizadeh, M. et al. Improved MAIT cell functions following fecal microbiota transplantation for metastatic renal cell carcinoma. Cancer Immunol Immunother 72, 1247–1260 (2023). https://doi.org/10.1007/s00262-022-03329-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03329-8