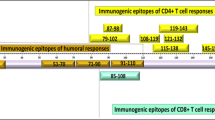
Abstract
About 85% of patients with colorectal cancer (CRC) have the non-microsatellite instability-high (non-MSI-H) subtype, and many cannot benefit from immune checkpoint blockade. A potential reason for this is that most non-MSI-H colorectal cancers are immunologically “cold” due to poor CD8+ T cell infiltration. In the present study, we screened for potential cancer-testis antigens (CTAs) by comparing the bioinformatics of CD8+ T effector memory (Tem) cell infiltration between MSI-H and non-MSI-H CRC. Two ODF2-derived epitope peptides, P433 and P609, displayed immunogenicity and increased the proportion of CD8+ T effector memory (Tem) cells in vitro and in vivo. The adoptive transfer of peptide pool-induced CTLs inhibited tumor growth and enhanced CD8+ T cell infiltration in tumor-bearing NOD/SCID mice. The mechanistic study showed that knockdown of ODF2 in CRC cells promoted interleukin-15 expression, which facilitated CD8+ T cell proliferation. In conclusion, ODF2, a CTA, was negatively correlated with CD8+ T cell infiltration in “cold” non-MSI-H CRC and was selected based on the results of bioinformatics analyses. The corresponding HLA-A2 restricted epitope peptide induced antigen-specific CTLs. Immunotherapy targeting ODF2 could improve CTA infiltration via upregulating IL-15 in non-MSI-H CRC. This tumor antigen screening strategy could be exploited to develop therapeutic vaccines targeting non-MSI-H CRC.







Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin 72(1):7–33. https://doi.org/10.3322/caac.21708
Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, Chen W (2022) Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 135(5):584–590. https://doi.org/10.1097/CM9.0000000000002108
Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A (2020) Colorectal cancer statistics, 2020. CA Cancer J Clin 70 (3):145-164. https://doi.org/10.3322/caac.21601
Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, Church SE, Lafontaine L, Fischer M, Fredriksen T, Sasso M, Bilocq AM, Kirilovsky A, Obenauf AC, Hamieh M, Berger A, Bruneval P, Tuech JJ, Sabourin JC, Le Pessot F, Mauillon J, Rafii A, Laurent-Puig P, Speicher MR, Trajanoski Z, Michel P, Sesboue R, Frebourg T, Pages F, Valge-Archer V, Latouche JB, Galon J (2016) Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity 44(3):698–711. https://doi.org/10.1016/j.immuni.2016.02.025
Shi R, Li Y, Ran L, Dong Y, Zhou X, Tang J, Han L, Wang M, Pang L, Qi Y, Wu Y, Gao Y (2022) Screening and identification of HLA-A2-restricted neoepitopes for immunotherapy of non-microsatellite instability-high colorectal cancer. Sci China Life Sci 65(3):572–587. https://doi.org/10.1007/s11427-021-1944-5
Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill A, Sawyer MB, Hendlisz A, Neyns B, Svrcek M, Moss RA, Ledeine JM, Cao ZA, Kamble S, Kopetz S, Andre T (2018) Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol 36(8):773–779. https://doi.org/10.1200/JCO.2017.76.9901
Ganesh K (2022) Optimizing immunotherapy for colorectal cancer. Nat Rev Gastroenterol Hepatol 19(2):93–94. https://doi.org/10.1038/s41575-021-00569-4
Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin JA, Miller WH Jr, Italiano A, Kao S, Piha-Paul SA, Delord JP, McWilliams RR, Fabrizio DA, Aurora-Garg D, Xu L, Jin F, Norwood K, Bang YJ (2020) Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 21(10):1353–1365. https://doi.org/10.1016/S1470-2045(20)30445-9
Yt L, ZJ S, (2021) Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics 11(11):5365–5386. https://doi.org/10.7150/thno.58390
Saxena M, van der Burg SH, Melief CJM, Bhardwaj N (2021) Therapeutic cancer vaccines. Nat Rev Cancer 21(6):360–378. https://doi.org/10.1038/s41568-021-00346-0
Bonaventura P, Shekarian T, Alcazer V, Valladeau-Guilemond J, Valsesia-Wittmann S, Amigorena S, Caux C, Depil S (2019) Cold tumors: a therapeutic challenge for immunotherapy. Front Immunol 10:168. https://doi.org/10.3389/fimmu.2019.00168
Liu W, Tang H, Li L, Wang X, Yu Z, Li J (2021) Peptide-based therapeutic cancer vaccine: Current trends in clinical application. Cell Prolif 54(5):e13025. https://doi.org/10.1111/cpr.13025
Taniguchi H, Iwasa S, Yamazaki K, Yoshino T, Kiryu C, Naka Y, Liew EL, Sakata Y (2017) Phase 1 study of OCV-C02, a peptide vaccine consisting of two peptide epitopes for refractory metastatic colorectal cancer. Cancer Sci 108(5):1013–1021. https://doi.org/10.1111/cas.13227
Kibe S, Yutani S, Motoyama S, Nomura T, Tanaka N, Kawahara A, Yamaguchi T, Matsueda S, Komatsu N, Miura M, Hinai Y, Hattori S, Yamada A, Kage M, Itoh K, Akagi Y, Sasada T (2014) Phase II study of personalized peptide vaccination for previously treated advanced colorectal cancer. Cancer Immunol Res 2(12):1154–1162. https://doi.org/10.1158/2326-6066.CIR-14-0035
Hubbard JM, Cremolini C, Graham RP, Moretto R, Mitchell JL, Wessling J, Toke ER, Csiszovszki Z, Lőrincz O, Molnar L, Somogyi E, Megyesi M, Pantya K, Tóth J, Páles P, Miklós I, Falcone A (2020) Evaluation of safety, immunogenicity, and preliminary efficacy of PolyPEPI1018 off-the-shelf vaccine with fluoropyrimidine/bevacizumab maintenance therapy in metastatic colorectal cancer (mCRC) patients. J Clin Oncol 38(15_suppl):4048–4048. https://doi.org/10.1200/JCO.2020.38.15_suppl.4048
Bezu L, Kepp O, Cerrato G, Pol J, Fucikova J, Spisek R, Zitvogel L, Kroemer G, Galluzzi L (2018) Trial watch: peptide-based vaccines in anticancer therapy. Oncoimmunology 7(12):e1511506. https://doi.org/10.1080/2162402X.2018.1511506
Fridman WH, Pages F, Sautes-Fridman C, Galon J (2012) The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12(4):298–306. https://doi.org/10.1038/nrc3245
Maimela NR, Liu S, Zhang Y (2019) Fates of CD8+ T cells in tumor microenvironment. Comput Struct Biotechnol J 17:1–13. https://doi.org/10.1016/j.csbj.2018.11.004
Wang Y, Song X, Zheng Y, Liu Z, Li Y, Qian X, Pang X, Zhang Y, Yin Y (2018) Cancer/testis antigen MAGEA3 interacts with STAT1 and remodels the tumor microenvironment. Int J Med Sci 15(14):1702–1712. https://doi.org/10.7150/ijms.27643
Chen F, Zou Z, Du J, Su S, Shao J, Meng F, Yang J, Xu Q, Ding N, Yang Y, Liu Q, Wang Q, Sun Z, Zhou S, Du S, Wei J, Liu B (2019) Neoantigen identification strategies enable personalized immunotherapy in refractory solid tumors. J Clin Investig 129(5):2056–2070. https://doi.org/10.1172/JCI99538
Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, Benner C, Chanda SK (2019) Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun 10(1):1523. https://doi.org/10.1038/s41467-019-09234-6
Charoentong P, Finotello F, Angelova M, Mayer C, Efremova M, Rieder D, Hackl H, Trajanoski Z (2017) Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep 18(1):248–262. https://doi.org/10.1016/j.celrep.2016.12.019
Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q, Li B, Liu XS (2020) TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res 48(W1):W509–W514. https://doi.org/10.1093/nar/gkaa407
Shi RR, Liu J, Zou Z, Qi YM, Zhai MX, Zhai WJ, Gao YF (2013) The immunogenicity of a novel cytotoxic T lymphocyte epitope from tumor antigen PL2L60 could be enhanced by 4-chlorophenylalanine substitution at position 1. Cancer Immunol Immunother 62(11):1723–1732. https://doi.org/10.1007/s00262-013-1478-7
Wu Y, Zhai W, Sun M, Zou Z, Zhou X, Li G, Yan Z, Qi Y, Gao Y (2017) A novel recombinant multi-epitope vaccine could induce specific cytotoxic T lymphocyte response in vitro and in vivo. Protein Pept Lett 24(6):573–580. https://doi.org/10.2174/0929866524666170419152700
Wu Y, Zhai W, Zhou X, Wang Z, Lin Y, Ran L, Qi Y, Gao Y (2018) HLA-A2-restricted epitopes identified from MTA1 could elicit antigen-specific cytotoxic T lymphocyte response. J Immunol Res 2018:2942679. https://doi.org/10.1155/2018/2942679
Sun W, Shi J, Wu J, Zhang J, Chen H, Li Y, Liu S, Wu Y, Tian Z, Cao X, Li N (2018) A modified HLA-A*0201-restricted CTL epitope from human oncoprotein (hPEBP4) induces more efficient antitumor responses. Cell Mol Immunol 15(8):768–781. https://doi.org/10.1038/cmi.2017.155
Thomas R, Shaath H, Naik A, Toor SM, Elkord E, Decock J (2020) Identification of two HLA-A*0201 immunogenic epitopes of lactate dehydrogenase C (LDHC): potential novel targets for cancer immunotherapy. Cancer Immunol Immunother 69(3):449–463. https://doi.org/10.1007/s00262-020-02480-4
Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, Eaton DL, Grogan JL (2014) The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell 26(6):923–937. https://doi.org/10.1016/j.ccell.2014.10.018
van der Gracht ET, Schoonderwoerd MJ, van Duikeren S, Yilmaz AN, Behr FM, Colston JM, Lee LN, Yagita H, van Gisbergen KP, Hawinkels LJ, Koning F, Klenerman P, Arens R (2020) Adenoviral vaccines promote protective tissue-resident memory T cell populations against cancer. J Immunother. https://doi.org/10.1136/jitc-2020-001133
Ito H, Ando T, Arioka Y, Saito K, Seishima M (2015) Inhibition of indoleamine 2,3-dioxygenase activity enhances the anti-tumour effects of a toll-like receptor 7 agonist in an established cancer model. Immunology 144(4):621–630. https://doi.org/10.1111/imm.12413
Uhlen M, Zhang C, Lee S, Sjostedt E, Fagerberg L, Bidkhori G, Benfeitas R, Arif M, Liu Z, Edfors F, Sanli K, von Feilitzen K, Oksvold P, Lundberg E, Hober S, Nilsson P, Mattsson J, Schwenk JM, Brunnstrom H, Glimelius B, Sjoblom T, Edqvist PH, Djureinovic D, Micke P, Lindskog C, Mardinoglu A, Ponten F (2017) A pathology atlas of the human cancer transcriptome. Science. https://doi.org/10.1126/science.aan2507
Ghafouri-Fard S, Abbasi A, Moslehi H, Faramarzi N, Taba Taba Vakili S, Mobasheri MB, Modarressi MH (2010) Elevated expression levels of testis-specific genes TEX101 and SPATA19 in basal cell carcinoma and their correlation with clinical and pathological features. Br J Dermatol 162(4):772–779. https://doi.org/10.1111/j.1365-2133.2009.09568.x
Ghafouri-Fard S, Ousati Ashtiani Z, Sabah Golian B, Hasheminasab SM, Modarressi MH (2010) Expression of two testis-specific genes, SPATA19 and LEMD1, in prostate cancer. Arch Med Res 41(3):195–200. https://doi.org/10.1016/j.arcmed.2010.04.003
Li B, Li X, Mao R, Liu M, Fu L, Shi L, Zhao S, Fu M (2021) Overexpression of ODF1 in gastrointestinal tract neuroendocrine neoplasms: a novel potential immunohistochemical biomarker for well-differentiated neuroendocrine tumors. Endocr Pathol 32(2):301–308. https://doi.org/10.1007/s12022-020-09649-8
Ali M, Foldvari Z, Giannakopoulou E, Boschen ML, Stronen E, Yang W, Toebes M, Schubert B, Kohlbacher O, Schumacher TN, Olweus J (2019) Induction of neoantigen-reactive T cells from healthy donors. Nat Protoc 14(6):1926–1943. https://doi.org/10.1038/s41596-019-0170-6
Yu Z, Liu W, He Y, Sun M, Yu J, Jiao X, Han Q, Tang H, Zhang B, Xian Y, Qi J, Gong J, Xin W, Shi G, Shan F, Zhang R, Li J, Wei M (2021) HLA-A2.1-restricted ECM1-derived epitope LA through DC cross-activation priming CD8(+) T and NK cells: a novel therapeutic tumour vaccine. J Hematol Oncol 14(1):71. https://doi.org/10.1186/s13045-021-01081-7
Azimi N, Brown K, Bamford RN, Tagaya Y, Siebenlist U, Waldmann TA (1998) Human T cell lymphotropic virus type I Tax protein trans-activates interleukin 15 gene transcription through an NF-kappaB site. Proc Natl Acad Sci USA 95(5):2452–2457. https://doi.org/10.1073/pnas.95.5.2452
Mariner JM, Lantz V, Waldmann TA, Azimi N (2001) Human T cell lymphotropic virus type I Tax activates IL-15R alpha gene expression through an NF-kappaB site. J Immunol 166(4):2602–2609. https://doi.org/10.4049/jimmunol.166.4.2602
Patidar M, Yadav N, Dalai SK (2016) Interleukin 15: a key cytokine for immunotherapy. Cytokine Growth Factor Rev 31:49–59. https://doi.org/10.1016/j.cytogfr.2016.06.001
Sisto M, Lorusso L, Lisi S (2017) TLR2 signals via NF-kappaB to drive IL-15 production in salivary gland epithelial cells derived from patients with primary Sjogren’s syndrome. Clin Exp Med 17(3):341–350. https://doi.org/10.1007/s10238-016-0429-y
Wang Y, Liu DP, Chen PP, Koeffler HP, Tong XJ, Xie D (2007) Involvement of IFN regulatory factor (IRF)-1 and IRF-2 in the formation and progression of human esophageal cancers. Cancer Res 67(6):2535–2543. https://doi.org/10.1158/0008-5472.CAN-06-3530
Dai Y, Zhao W, Yue L, Dai X, Rong D, Wu F, Gu J, Qian X (2021) Perspectives on immunotherapy of metastatic colorectal cancer. Front Oncol 11:659964. https://doi.org/10.3389/fonc.2021.659964
Golshani G, Zhang Y (2020) Advances in immunotherapy for colorectal cancer: a review. Ther Adv Gastroenterol. https://doi.org/10.1177/1756284820917527
Huyghe N, Baldin P, Van den Eynde M (2020) Immunotherapy with immune checkpoint inhibitors in colorectal cancer: what is the future beyond deficient mismatch-repair tumours? Gastroenterol Rep (Oxf) 8(1):11–24. https://doi.org/10.1093/gastro/goz061
Gomez-Roca C, Yanez E, Im S-A, Alvarez EC, Senellart H, Doherty M, García-Corbacho J, Lopez JS, Basu B, Maurice-Dror C, Gill SS, Ghori R, Kubiak P, Jin F, Norwood KG, Chung HC (2021) LEAP-005: a phase II multicohort study of lenvatinib plus pembrolizumab in patients with previously treated selected solid tumors—results from the colorectal cancer cohort. J Clin Oncol 39(3_suppl):94–94. https://doi.org/10.1200/JCO.2021.39.3_suppl.94
Segal NH, Saro J, Melero I, Ros W, Argiles G, Marabelle A, Rodriguez Ruiz ME, Albanell J, Calvo E, Moreno V, Cleary JM, Eder JP, Karanikas V, Bouseida S, Sandoval F, Sabanes D, Sreckovic S, Hurwitz HI, Paz-Ares L, Tabernero J (2017) Phase I studies of the novel carcinoembryonic antigen T-cell bispecific (CEA-CD3 TCB) antibody as a single agent and in combination with atezolizumab: preliminary efficacy and safety in patients (pts) with metastatic colorectal cancer (mCRC). Ann Oncol 28:v134. https://doi.org/10.1093/annonc/mdx367.036
Liu YT, Sun ZJ (2021) Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics 11(11):5365–5386. https://doi.org/10.7150/thno.58390
Shi W, Qiu Q, Tong Z, Guo W, Zou F, Feng Z, Wang Y, Huang W, Qian H (2020) Synthetic tumor-specific antigenic peptides with a strong affinity to HLA-A2 elicit anti-breast cancer immune response through activating CD8(+) T cells. Eur J Med Chem 189:112051. https://doi.org/10.1016/j.ejmech.2020.112051
McCormack E, Adams KJ, Hassan NJ, Kotian A, Lissin NM, Sami M, Mujic M, Osdal T, Gjertsen BT, Baker D, Powlesland AS, Aleksic M, Vuidepot A, Morteau O, Sutton DH, June CH, Kalos M, Ashfield R, Jakobsen BK (2013) Bi-specific TCR-anti CD3 redirected T-cell targeting of NY-ESO-1- and LAGE-1-positive tumors. Cancer Immunol Immunother 62(4):773–785. https://doi.org/10.1007/s00262-012-1384-4
Oka Y, Tsuboi A, Nakata J, Nishida S, Hosen N, Kumanogoh A, Oji Y, Sugiyama H (2017) Wilms’ tumor gene 1 (WT1) peptide vaccine therapy for hematological malignancies: from CTL epitope identification to recent progress in clinical studies including a cure-oriented strategy. Oncol Res Treat 40(11):682–690. https://doi.org/10.1159/000481353
Hubbard JM, Toke ER, Moretto R, Graham RP, Youssoufian H, Lorincz O, Molnar L, Csiszovszki Z, Mitchell JL, Wessling J, Toth J, Cremolini C (2022) Safety and activity of PolyPEPI1018 combined with maintenance therapy in metastatic colorectal cancer: an open-label, multicenter, phase Ib study. Clin Cancer Res 28(13):2818–2829. https://doi.org/10.1158/1078-0432.CCR-22-0112
Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS, Parkhurst MR, Yang JC, Rosenberg SA (2014) Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 344(6184):641–645. https://doi.org/10.1126/science.1251102
Gjerstorff MF, Andersen MH, Ditzel HJ (2015) Oncogenic cancer/testis antigens: prime candidates for immunotherapy. Oncotarget 6(18):15772–15787. https://doi.org/10.18632/oncotarget.4694
Rosenberg SA, Restifo NP (2015) Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348(6230):62–68. https://doi.org/10.1126/science.aaa4967
Wang B, Wang Y, Sun X, Deng G, Huang W, Wu X, Gu Y, Tian Z, Fan Z, Xu Q, Chen H, Sun Y (2021) CXCR6 is required for antitumor efficacy of intratumoral CD8(+) T cell. J Immunother Cancer. https://doi.org/10.1136/jitc-2021-003100
Kim KH, Kim HK, Kim HD, Kim CG, Lee H, Han JW, Choi SJ, Jeong S, Jeon M, Kim H, Koh J, Ku BM, Park SH, Ahn MJ, Shin EC (2021) PD-1 blockade-unresponsive human tumor-infiltrating CD8(+) T cells are marked by loss of CD28 expression and rescued by IL-15. Cell Mol Immunol 18(2):385–397. https://doi.org/10.1038/s41423-020-0427-6
Beatson R, Tajadura-Ortega V, Achkova D, Picco G, Tsourouktsoglou TD, Klausing S, Hillier M, Maher J, Noll T, Crocker PR, Taylor-Papadimitriou J, Burchell JM (2016) The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9. Nat Immunol 17(11):1273–1281. https://doi.org/10.1038/ni.3552
Klebanoff CA, Gattinoni L, Restifo NP (2012) Sorting through subsets: which T-cell populations mediate highly effective adoptive immunotherapy? J Immunother 35(9):651–660. https://doi.org/10.1097/CJI.0b013e31827806e6
Acknowledgements
We thank the National Natural Science Foundation of China for support. The results shown here are in whole or part based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga.
Funding
This work was supported by the National Science Foundation of China (No. U20A20369, 81822043, U1904147, 82002575), Project of Henan Province (No. 212102310338, LHGJ20190673, 202002077).
Author information
Authors and Affiliations
Contributions
YG and YW conceived and designed the experiments. RS performed most the experiments. LP, MW, YL, CC, and HN performed part of experiments and analyzed. LZ, GY, LQ, and XZ helped with experiment design and provided critical suggestions. XZ, WZ, and YQ helped to revise the manuscript. RS, YW, and YG analyzed and interpreted the results. All authors revised the manuscript, discussed the results, and gave final approval of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for publication
All authors declare that they agree to submit the article for publication.
Ethics approval and consent to participate
This study involving mice was carried out in accordance with the recommendations of the international and national ethical requirements for biomedical research. All studies were conducted in accordance with the standards of the Zhengzhou University Institutional Animal Protection and Ethics Committee with approval number ZZUIRB2021-32.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, R., Zhou, X., Pang, L. et al. Peptide vaccine from cancer-testis antigen ODF2 can potentiate the cytotoxic T lymphocyte infiltration through IL-15 in non-MSI-H colorectal cancer. Cancer Immunol Immunother 72, 985–1001 (2023). https://doi.org/10.1007/s00262-022-03307-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03307-0