Abstract
Background
Lenvatinib combined with pembrolizumab showed a promising result in an early phase study for hepatocellular carcinoma (HCC). The efficacy and safety of lenvatinib plus pembrolizumab for patients with unresectable HCC (uHCC) beyond the first-line setting were unclear.
Methods
Seventy-one consecutive patients who received lenvatinib plus pembrolizumab for uHCC were prospectively enrolled. Effect of lenvatinib combinations on Albumin-Bilirubin (ALBI) score and factors associated with progression-free survival (PFS) and overall survival (OS) were analyzed.
Results
Of the 71 cases, 58 (81.7%) were in BCLC C. There were 44 (62%) for the first-line systemic treatment, and 27 (38%) had experienced targeted therapy or nivolumab treatment. The objective response rate and disease control rate (DCR) were 34.1% and 84.1% for the first-line setting, and 18.5% and 70.4% for systemic therapy-experienced cases (Response Evaluation Criteria in Solid Tumors version 1.1, RECIST v1.1), respectively. The mean ALBI score was stable during the treatment course. After a median of 9.3 months of follow-up, the median PFS was 9.3 months versus 4.4 months, and the median OS was not estimable yet versus 12 months for Child–Pugh A versus B patients, respectively. Prior nivolumab failure was the only significant factor associated with poorer PFS (HR = 3.253, p = 0.004). Child–Pugh class B (HR = 2.646, p = 0.039) and prior nivolumab failure (HR = 3.340, p = 0.014) were independent factors for poorer OS in multivariate analysis.
Conclusions
A high DCR was observed by lenvatinib/pembrolizumab combination without adverse effect on ALBI score for systemic therapy-naïve and -experienced uHCC. Suboptimal response to prior nivolumab-failed patients requires further exploration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver and ranks as the fourth leading cause of cancer-related mortality in the world [1]. Early stage HCC can be curatively treated by surgery, transplantation, or ablation [2]. Locoregional treatment by transarterial chemoembolization (TACE) is the standard of care for intermediate-stage HCC, but the complete response rate is low [3, 4]. For patients with advanced HCC, systemic treatment by sorafenib can only provide a limited response [5,6,7].
In recent years, several antiangiogenic multikinase inhibitors (MKI) or antibody and immunotherapy by immune checkpoint inhibitors (ICIs) have become available for advanced HCC [5, 6, 8,9,10,11,12,13]. Nivolumab and pembrolizumab are both ICIs that block programmed death 1 (PD-1) and have shown response rates of 17–20% in phase I/II studies [12, 13]. The phase III study of pembrolizumab in a second-line setting failed to reach a prespecified p-value, but clinically meaningful outcomes were observed [14].
Vascular endothelial growth factor (VEGF) inhibitor could enhance antitumor immunity restored by PD-1/PD-L1 antibody by antagonizing the VEGF-mediated immunosuppressive effect within the tumor microenvironment [15, 16]. Promising results with survival benefits have been confirmed for atezolizumab plus bevacizumab in a phase III trial for HCC [17]. Currently, there are multiple phase III clinical trials being conducted to examine combination therapies of ICI plus MKI or ICI plus ICI. These combinations include lenvatinib plus pembrolizumab, atezolizumab plus cabozantinib, durvalumab plus tremelimumab, and nivolumab plus ipilimumab in a first-line setting [10, 18,19,20,21]. Among this list, lenvatinib plus pembrolizumab has shown promising data for the response rate and survival in the phase 1b study [18].
Previous clinical trials excluded patients with high-risk tumors (tumor volume ≥ 50% of the liver volume, main portal vein invasion, or biliary tract invasion) and decompensated liver function [8, 18]. Thus, it remains unclear whether such patients could respond to lenvatinib plus pembrolizumab, and safety profiles should be further delineated. Therefore, this study reports the treatment responses, risks of treatment-related adverse effects (TRAEs), albumin-bilirubin (ALBI) score changes [22], and survival in response to lenvatinib plus pembrolizumab in the first-line setting or in cases where prior systemic treatment failed in patients with unresectable HCC. Furthermore, we also identify predictors associated with survival.
Materials and methods
Patients
This study prospectively enrolled consecutive 71 patients with unresectable HCC who received lenvatinib plus pembrolizumab from July 2019 to February 2021 at Taipei Veterans General Hospital. Both lenvatinib and pembrolizumab had been approved for HCC by the Taiwan Food and Drug Administration (TFDA) before this study began. The key inclusion criteria included age > 20 years; diagnosis of HCC confirmed by pathologic or radiographic features according to the American Association for the Study of Liver Diseases (AASLD) criteria; and HCC status of Barcelona Clinic Liver Cancer (BCLC) stage B or C not amenable or refractory to TACE. High-risk tumors were allowed in this study, including tumors with size ≥ 50% of the liver size, main portal vein invasion (Vp4), or biliary tract invasion.
The exclusion criteria were patients who had concurrent cancer other than HCC, hepatic encephalopathy, severe comorbidities (end-stage renal disease, acute coronary syndrome, cerebrovascular accident, severe heart failure, severe arrhythmia, and severe trauma), Child–Pugh class C, and underlying autoimmune diseases. The dose of lenvatinib complied with the standard body weight (BW)-based recommendation (12 mg for BW ≧ 60 kg, 8 mg for BW < 60 kg, orally once daily). Pembrolizumab was administered intravenously at 200 mg or 2–3 mg/kg every 3 weeks [23,24,25].
This study was approved by the Institutional Review Board of Taipei Veterans General Hospital (IRB number: 2020–06-033BC, 2021–07-040BC). The study complied with the standards of the Declaration of Helsinki and current ethical guidelines. All patients signed informed consent forms.
Assessment and follow-up
For this prospective study, all patients had a uniform protocol for monitoring and assessment. Tumor assessment imaging (computed tomography, magnetic resonance imaging, or both) was performed before starting the treatment and repeated every 9 weeks. Serum alpha-fetoprotein (AFP) was measured every 3 weeks until image-confirmed progression occurred. Early AFP response (> 10%) for patients with baseline AFP ≥ 10 ng/mL was defined as a reduction of AFP > 10% within 4 weeks of the treatment [25]. Any AFP response for patients with baseline AFP ≥ 10 ng/mL was defined as any degree of AFP reduction within 4 weeks of the treatment. The response was assessed according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) [26] and hepatocellular carcinoma-specific modified RECIST (mRECIST) [27]. TRAEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0).
End points
The primary end point was the tumor response, including the objective response rate (ORR) and disease control rate (DCR) evaluated by RECIST v1.1 and mRECIST. The secondary end points were progression-free survival (PFS), overall survival (OS), time to response (TTR), time to progression (TTP, defined as time from starting treatment to the first progressive disease (PD)), duration of response (DOR, defined as time from the first partial response (PR) or complete response (CR) to PD or death), and incidence of TRAEs.
Clinical features, biochemistry tests, serological markers and PD-L1 expression analysis
The following clinical features and biochemical data were collected for analysis: age, sex, BCLC stage, Child–Pugh score, serum AFP, alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine, albumin, total bilirubin levels, platelet count, and prothrombin time (international normalized ratio (INR)). Serum biochemistry tests were done using a systemic multi-autoanalyzer (Technicon SMAC, Technicon Instruments Corp., Tarrytown, NY). Serum AFP was measured using radio-immunoassay kits (Abbott Laboratories, North Chicago, IL and Serono Diagnostic SA, Coinsin/VD, Switzerland, respectively).
PD-L1expression was measured by an immunohistochemistry pharmDx assay (Agilent Technologies, Santa Clara, CA, USA) with anti-PD-L1 22C3 antibody on archival or newly obtained biopsy HCC tissues [25]. Expression levels are reported as the tumor proportion score (TPS) and combined positive score (CPS) [28]. TPS or CPS ≧ 1% was defined as a positive result.
Statistical analysis
Categorical data were compared using Fisher’s exact test or Pearson’s chi-squared analysis. ORRs were calculated with the 95% confidence interval (CI) using the Clopper-Pearson method. For patients with confirmed PR or CR, DOR and TTR were analyzed. The changes in ALBI score were estimated by a linear mixed model, which can be used to analyze an outcome measured repeatedly from the same individuals. The Kaplan–Meier method with the log-rank test was applied for OS, PFS, TTP, and DOR.
Variables that achieved statistical significance (p < 0.05) or were close to significance (p < 0.10) in the univariate analysis were examined in a multivariate analysis. For all analyses, p < 0.05 was considered statistically significant. All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS 28.0 for Windows, SPSS Inc, Chicago, IL) and GraphPad Prism version 9.1 (GraphPad Software, La Jolla, CA, USA).
Results
Patient characteristics
The baseline characteristics of the 71 HCC patients are listed in Table 1. The median age was 63 years, and 87.3% of the patients were male. Most patients were within Child–Pugh class A (n = 51, 71.8%), ECOG performance status of 0/1 (n = 62, 87.3%), and BCLC C (n = 58, 81.7%). The etiology of HCC was predominantly hepatitis B virus (HBV) (63.4%).
There were 44 (62%) patients who were treated in the first-line setting, while the other 27 (38%) patients had experienced prior systemic therapy including MKIs or ICI (sorafenib (n = 15), sorafenib followed by regorafenib (n = 8), lenvatinib (n = 2), nivolumab (n = 9)). There were 28 patients who had high-risk tumors, including tumor volume ≧ 50% of the liver volume (n = 17), main portal vein invasion (n = 15), and biliary tract invasion (n = 2). Compared with patients who received prior systemic therapy, patients in the first-line setting had a higher proportion with ALBI grade 1 and higher N/L ratios.
Tumor response
During a median follow-up duration of 9.3 months (range, 0.7 to 17.8 months), CR was achieved in 1 (1.5%) patient according to RECIST v1.1 and 4 (5.6%) patients according to mRECIST. The ORR was 28.2% (95% CI, 17.5% to 38.9%) according to RECIST v1.1 and 57.7% (95% CI, 46% to 69.5%) according to mRECIST. DCR was 78.9% (95% CI, 69.1% to 88.6%) according to RECIST v1.1 and 80.3% (95% CI, 70.8% to 89.8%) according to mRECIST (Table 2).
For the responders, the median TTR was 2.3 months (range, 1.8 to 8.6) according to RECIST v1.1. The median DOR according to RECIST v1.1 was 9.3 months (95% CI, NE) (Table 2). The ORR for the first-line setting was 34.1%, which is numerically higher than the result of 18.5% for cases that had experienced prior systemic therapy (RECIST v1.1). Compared with the first-line setting, numerically lower ORR and DCR were observed in patients with prior systemic therapy according to both RECIST v1.1 and mRECIST (Table 2). For patients with only MKI experience, the ORR and DCR were 27.8% (vs. 34.1%, p = 0.629) and 83.3% (vs. 84.1%, p = 1.000) compared with those in the first-line setting (RECIST v1.1), respectively.
The ORR for the patients with high-risk tumors was numerically lower than that of patients with low-risk tumors (25% vs. 30.2%, p = 0.632). The DCR was similar between the two groups (85.7% vs. 74.4%, p = 0.254). The ORR was 25% for Child–Pugh B patients, which was numerically lower than the result of 29.4% for Child–Pugh A patients (p = 0.710) (RECIST v1.1) (Supplementary Table 1). Among all 71 patients, the median TTP was 11.2 months (95% CI, 5.1–17.3) according to RECIST v1.1 and 10.9 months (95% CI, 8.4–13.5) according to mRECIST. The TTP was 15.3 months (95% CI, 3.6–27.0) for patients in the first-line setting and 9.4 months (95% CI, 2.8–16.1) for patients with prior systemic therapy (RECIST v1.1).
PFS and OS
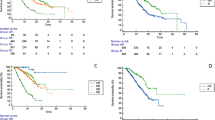
The median PFS was 8.7 months (95% CI, 6.1–11.3 months) according to RECIST1.1 (Fig. 1a), 9.2 months (95% CI, 6.1–12.3 months) for patients in the first-line setting, and 4.9 months (95% CI, 0.3–9.5 months) for cases that experienced prior systemic therapy (RECIST v1.1, p = 0.092, Fig. 1b). The median OS was 16.4 months overall (95%CI, 11.9–21.0 months, Fig. 1c), 16.4 months (95% CI, 12.4–20.5) for patients in the first-line setting, and not estimable for patients with prior systemic therapy (RECIST v1.1, p = 0.682, Fig. 1d).
Progression-free survival (PFS) and overall survival (OS) of entire cohort of HCC patients. Kaplan–Meier estimates of PFS according to RECIST v1.1 a among all 71 patients b stratified by systemic therapy-naïve and -experienced patients; OS c among entire 71 patients and d stratified by systemic therapy-naïve and -experienced patients
Compared to patients with Child–Pugh A, poorer median PFS was noted for Child–Pugh B patients (9.3 months vs. 4.4 months), but the difference was not statistically significant (Fig. 2a). Child–Pugh class also affected OS: patients with Child–Pugh A had significantly better median OS than those with Child–Pugh B (not reached vs. 12 months, p = 0.017) (Fig. 2b). Among the 44 patients in the first-line setting, the median PFS was 10.6 months (95% CI, 5.7–15.5) for patients with Child–Pugh A and 8.0 months (95% CI, 1.9–14.1) for patients with Child–Pugh B (RECIST v1.1, p = 0.177, Fig. 2c). The median OS was not estimable for patients with Child–Pugh A and 8.0 months (95% CI, 0.3–15.7) for patients with Child–Pugh B (RECIST v1.1, p = 0.010, Fig. 2d).
Progression-free survival (PFS) and overall survival (OS) based on Child–Pugh class and tumor risk. Kaplan–Meier estimates of a PFS according to RECIST v1.1 among all 71 patients; b OS among entire cohort; c PFS according to RECIST v1.1 in the first-line-setting patients; d OS in the first-line-setting patients; e PFS according to RECIST v1.1; f OS stratified by tumor risk
Although the PFS (7.0 vs. 9.0 months, p = 0.596) was not significantly different between patients with high-risk and low-risk tumors (Fig. 2e), poorer OS was observed among patients with high-risk tumors (12 months vs. not reached, p = 0.025) in the Kaplan–Meier survival analysis (Fig. 2f). The PFS and OS of patients based on treatment responses are shown in Fig. 3a, b. Patients who achieved an objective response had significantly better PFS (15.3 vs. 2.1 months, p < 0.001) and OS (Not reached vs. 11.8 months, p = 0.018) than PD patients.
Progression-free survival (PFS) and overall survival (OS) according to the treatment response to lenvatinib plus pembrolizumab. Kaplan–Meier curves of a PFS according to RECIST v1.1; b OS stratified by treatment response. CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease
Early AFP response
For the 53 patients with baseline AFP ≥ 10 ng/ml, 42 (79.2%) had AFP reduction within 4 weeks with a median AFP change to − 43.6%. Interestingly, both patients with and without objective response according to RECIST v1.1 had significant AFP reduction (median: − 48.6% vs. − 36.3%, p = 0.456). There was no significant difference in ORR (33.3% vs. 28.6%, p = 1.000) and DCR (87.2% vs. 71.4%, p = 0.222) between patients with and without early AFP reduction (> 10%) [25].
ALBI score changes and safety profiles
The median number of cycles of pembrolizumab was 7 cycles (range, 1–26 cycles), and the median dose of lenvatinib was 84.7% of the standard dose (interquartile range, 68.7% to 100%). Figure 4a shows the changes in ALBI score among patients who received lenvatinib plus pembrolizumab according to a linear mixed model. The mean ALBI score remained stable during the treatment course (p = 0.099). In addition, the ALBI score in the systemic therapy-naïve subgroup (n = 44) and systemic therapy-experienced subgroup (n = 27) both showed no significant change with respect to the treatment cycles (p = 0.406, Fig. 4b; p = 0.102 Fig. 4c).
TRAEs occurred in 97.2% (69/71) of patients (Supplementary Table 2), including 27 (96.4%) of the systemic therapy-naïve patients and 42 (97.7%) of the systemic therapy-experienced cases. The most common any-grade TRAE was fatigue (56.3%), followed by hypertension (49.3%), palmer-plantar syndrome (46.5%), decreased appetite (40.8%), and hypothyroidism (38%). The most common AEs with grade ≧ 3 were hepatitis (7%), hypertension (4.2%), decreased appetite (4.2%), and skin psoriasis flare (4.2%). Variceal bleeding was noted in 4 patients. Dose reduction of lenvatinib occurred in 20 (28.2%) cases.
AEs led to interruption of pembrolizumab treatment in 9 (12.7%) cases, and 29 (40.8%) cases used steroids due to an impression of immune-related adverse events. Of the 29 cases that used steroid treatment, 10 cases received IV methylprednisolone (0.5–1 mg/kg/day) for grade 3/4 immune-related adverse events and grade 2 pneumonitis. The other 19 patients received a low-dose (< 10 mg/day) oral form of prednisolone for skin rash, pruritus, and grade 1/2 hepatitis. Discontinuation of the combination treatment because of TRAEs was noted in 3 (4.2%) patients (1 case of colitis and 2 cases of pneumonitis). In general, patients with prior systemic therapy did not have significantly higher risk of any grade of TRAE than patients in the first-line setting except for a higher chance of pruritus (44.4% vs. 15.9%) in patients with prior systemic therapy. The occurrence rate of grade 3/4 TRAEs was 35.7% in patients with high-risk tumors (18.6% in patients with low-risk tumors, p = 0.105) and 35% in patients with Child–Pugh B (21.6% in patients with Child–Pugh A, p = 0.242) (Supplementary Table 3).
Factors associated with PFS and OS
In the univariate analysis, ECOG status ≥ 1, INR > 1.2, and prior nivolumab experience were factors associated with PFS according to RECIST v1.1. In the multivariate analysis, previous experience with nivolumab (HR = 3.253, 95% CI: 1.473 to 7.183, p = 0.004) was the only independent risk factor for PFS (Supplementary Table 4 and Fig. 5a). Factors associated with OS in the univariate analysis were ECOG status ≧ 1, bile duct involvement, Child–Pugh class B, and prior systemic therapy using nivolumab. In the multivariate analysis, Child–Pugh class B (HR = 2.646, 95% CI: 1.053 to 6.651) and previous nivolumab experience (HR = 3.340, 95% CI:1.277 to 8.734) were significant prognostic factors for poorer OS (Table 3 and Fig. 5b). Among patients naïve to nivolumab, the PFS and OS of those with MKI experience were 9.4 months (vs. 9.2 months, p = 0.772) and not reached (vs. 16.4 months, p = 0.261), respectively, in comparison to those in the first-line setting (Supplementary Fig. 1).
Role of PD-L1 expression in tumor response and survival
There were 48 patients with available liver-tumor tissues for the determination of PD-L1 expression (25 archival specimens and 23 freshly derived specimens). In general, the ORR was higher in TPS-positive cases (58.3% vs. 25%, p = 0.073) and CPS-positive cases (50% vs. 23.3%, p = 0.058) based on RECIST v1.1 (Supplementary Table 5). The median PFS for TPS-positive and negative patients were 15.3 months and 8.7 months (p = 0.208, Fig. 6a), while those for CPS-positive and negative patients were 15.3 months and 8.7 months (p = 0.103, Fig. 6b), respectively. The median OS for TPS-positive and negative patients were not reached and 16.4 months (TPS, p = 0.095, Fig. 6c), while those for CPS-positive and negative patients were not reached and 14.5 months (CPS, p = 0.037, Fig. 6d), respectively.
Association of PD-L1 with progression-free survival (PFS) and overall survival (OS). Kaplan–Meier curves of PFS according to RECIST v1.1 stratified by a TPS ≥ 1% or < 1%; b CPS ≥ 1% or < 1%; OS stratified by c TPS ≥ 1% or < 1%; d CPS ≥ 1% or < 1%. CPS, combined positive score; TPS, tumor proportion score
Discussion
To the best of our knowledge, there has been no study comparing lenvatinib plus pembrolizumab in the first-line setting and systemic-experienced uHCC and in Child–Pugh B patients. In our study, we found that the treatment-related adverse events from lenvatinib plus pembrolizumab were similar between systemic therapy-naïve and -experienced uHCC patients, but the PFS and OS are compromised in patients with prior systemic therapy, particularly in patients with prior nivolumab treatment failure. Our findings provide important information on lenvatinib plus pembrolizumab for uHCC patients with high-risk tumors, Child–Pugh class B, and prior systemic treatment exposure. For the responders, the response was also enduring.
The ORR and DCR of lenvatinib plus pembrolizumab were generally better than those of MKI or ICI monotherapy [8, 13, 14]. But for patients with prior systemic therapy, the ORR of lenvatinib plus pembrolizumab (18.5% according to RECIST v1.1) was similar to that of lenvatinib (18.8% in REFLECT study) or pembrolizumab monotherapy (18.3% in KEYNOTE-240 study) [8, 14]. This finding indicated that a certain degree of MKI and ICI resistance might exist after prior systemic therapy. Nevertheless, the DCR of lenvatinib plus pembrolizumab was still high across systemic-naïve and -experienced cases.
There has been no applicable baseline factor to predict the response to ICI immunotherapy for HCC in previous studies [25]. In this study, prior nivolumab failure was the only factor significantly associated with PFS. This finding implies that prior anti-PD-1 treatment may have a detrimental effect on repeated anti-PD-1 immunotherapy by blocking the same immune checkpoint.
Our previous real-world cohort study has demonstrated that liver reserve (Child–Pugh class A) determined overall survival on ICI therapy [25]. In this study, Child–Pugh class B and previous nivolumab experience were risk factors for OS. Previous studies suggest that MKI could enhance the response to ICI immunotherapy [29], but the current study showed that prior MKI treatment did not have a positive effect on the response to lenvatinib plus pembrolizumab. Atezolizumab plus bevacizumab is still being developed as a first-line treatment for advanced HCC, so the outcomes of this treatment followed by lenvatinib/pembrolizumab still require further exploration.
Most clinical trials excluded patients with high-risk tumors [8, 13, 14, 18]. Tumor burden ≧ 50% liver volume, main portal vein invasion, and bile duct involvement were not factors associated with PFS and OS in this study. This indicates that patients with high-risk tumors are still potential candidates for lenvatinib plus pembrolizumab treatment, but only if their liver function is well preserved. In addition, our study confirmed that there was no negative effect on ALBI score during lenvatinib plus pembrolizumab treatment.
Although nearly all patients experienced adverse events, only 3 patients discontinued the treatment due to such events. No unexpected adverse effects were discovered, and the incidence rates of TRAEs were similar in the therapy-naïve and -experienced patients. Only a higher frequency of pruritus was observed in patients with prior systemic therapy. AEs with grade ≧ 3 were rare, including pneumonitis, psoriasis, hypertension, diarrhea, and hepatitis.
We previously proposed a “10–10 rule” based on early AFP response to predict ORR and survival of ICI monotherapy in advanced HCC [25]. But AFP reduction is common when using lenvatinib/pembrolizumab combinations, AFP reduction could happen in patients without objective response, and early AFP reduction did not have a predictive role for lenvatinib/pembrolizumab combinations. The predictive value of PD-L1 expression in response to ICI for HCC has not yet been confirmed [12, 13, 30]. A positive result for PD-L1 expression has been associated with significantly better OS and PFS [31, 32]. In the present study, CPS-positive cases had a trend of higher ORR and better median OS than CPS-negative patients. These findings suggest that PD-L1 expression has a potential role in selecting candidates for lenvatinib plus pembrolizumab treatment for uHCC.
There are several limitations to this study. First, there was no control arm for comparison. However, the ORR and DCR of lenvatinib plus pembrolizumab were unequivocally higher than in previous studies on ICI and MKI monotherapy. Second, the case number was not large, although this has been the largest prospective real-world study on lenvatinib plus pembrolizumab for unresectable HCC so far. Nevertheless, our findings highlight the potential for future expansion of indications for lenvatinib plus pembrolizumab for uHCC patients with high-risk tumors, Child–Pugh B, or previous MKI experience.
In conclusion, the real-world data on lenvatinib plus pembrolizumab showed comparable responses and survival to an early phase clinical trial without unexpected adverse effects, even in patients with high-risk tumors and Child–Pugh B status.
Availability of data and material
The dataset used for this study is available from the corresponding author upon reasonable request.
Abbreviations
- AASLD:
-
American association for the study of liver disease
- AFP:
-
Alpha-fetoprotein
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BCLC:
-
Barcelona-clinic-liver-cancer
- CI:
-
Confidence interval
- CR:
-
Complete response
- CPS:
-
Combined positive score
- CT:
-
Computed tomography
- DCR:
-
Disease control rate
- DOR:
-
Duration of response
- ECOG:
-
Eastern cooperative oncology group
- HBsAg:
-
Hepatitis B surface antigen
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- ICI:
-
Immune check point inhibitor
- INR:
-
International normalized ratio
- MKI:
-
Multikinase inhibitor
- MRI:
-
Magnetic resonance image
- NLR:
-
Neutrophil-to-lymphocyte ratio
- NUCs:
-
Nucleos/tide analogues
- OR:
-
Odds ratio
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PD:
-
Progressive disease
- PD-(L)1:
-
Programmed death (ligand) 1
- PFS:
-
Progression-free survival
- PR:
-
Partial response
- (m)RECIST:
-
Modified response evaluation criteria in solid tumors
- TACE:
-
Transarterial chemoembolization
- TFDA:
-
Taiwan food and drug administration
- TPS:
-
Tumor proportion score
- TTP:
-
Time to progression
- TTR:
-
Time to response
- TRAE:
-
Treatment-related adverse effects
- v1.1:
-
Version 1.1
- VEGF(R):
-
Vascular endothelial growth factor (receptor)
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int j cancer 136(5):E359-386. https://doi.org/10.1002/ijc.29210
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L (2018) EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 69(1):182–236. https://doi.org/10.1016/j.jhep.2018.03.019
Lee IC, Hung YW, Liu CA, Lee RC, Su CW, Huo TI, Li CP, Chao Y, Lin HC, Hou MC, Huang YH (2019) A new ALBI-based model to predict survival after transarterial chemoembolization for BCLC stage B hepatocellular carcinoma. Liver Int 39(9):1704–1712. https://doi.org/10.1111/liv.14194
Hung YW, Lee IC, Chi CT, Lee RC, Liu CA, Chiu NC, Hwang HE, Chao Y, Hou MC, Huang YH (2021) Redefining tumor burden in patients with intermediate-stage hepatocellular carcinoma: the seven-eleven criteria. Liv Cancer. https://doi.org/10.1159/000517393
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F, de Oliveira AC, Santoro A, Raoul J-L, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz J-F, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359(4):378–390. https://doi.org/10.1056/NEJMoa0708857
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a Phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10(1):25–34. https://doi.org/10.1016/s1470-2045(08)70285-7
Lee PC, Chen YT, Chao Y, Huo TI, Li CP, Su CW, Lee MH, Hou MC, Lee FY, Lin HC, Huang YH (2018) Validation of the albumin-bilirubin grade-based integrated model as a predictor for sorafenib-failed hepatocellular carcinoma. Liv int off j Int Assoc Stud Liv 38(2):321–330. https://doi.org/10.1111/liv.13527
Kudo M, Finn RS, Qin S, Han K-H, Ikeda K, Piscaglia F, Baron A, Park J-W, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng A-L (2018) Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. The Lancet 391(10126):1163–1173. https://doi.org/10.1016/S0140-6736(18)30207-1
Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G (2017) Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London England) 389(10064):56–66. https://doi.org/10.1016/s0140-6736(16)32453-9
Zhu AX, Kang Y-K, Yen C-J, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau K-M, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart J-B, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M (2019) Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 20(2):282–296. https://doi.org/10.1016/S1470-2045(18)30937-9
Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF, Bolondi L, Klümpen HJ, Chan SL, Zagonel V, Pressiani T, Ryu MH, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK (2018) Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. New Engl j med 379(1):54–63. https://doi.org/10.1056/NEJMoa1717002
El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim T-Y, Choo S-P, Trojan J, Welling TH, Meyer T, Kang Y-K, Yeo W, Chopra A, AndersondelaCruz JC, Lang L, Neely J, Tang H, Dastani HB, Melero I (2017) Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389(10088):2492–2502. https://doi.org/10.1016/s0140-6736(17)31046-2
Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng A-L, Kudo M, Alistar A, Asselah J, Blanc J-F, Borbath I, Cannon T, Chung K, Cohn A, Cosgrove DP, Damjanov N, Gupta M, Karino Y, Karwal M, Kaubisch A, Kelley R, Van Laethem J-L, Larson T, Lee J, Li D, Manhas A, Manji GA, Numata K, Parsons B, Paulson AS, Pinto C, Ramirez R, Ratnam S, Rizell M, Rosmorduc O, Sada Y, Sasaki Y, Stal PI, Strasser S, Trojan J, Vaccaro G, Van Vlierberghe H, Weiss A, Weiss K-H, Yamashita T (2018) Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 19(7):940–952. https://doi.org/10.1016/s1470-2045(18)30351-6
Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL (2020) Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, Phase III trial. J clin oncol off j Am Soc Clin Oncol 38(3):193–202. https://doi.org/10.1200/jco.19.01307
Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK (2018) Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 15(5):325–340. https://doi.org/10.1038/nrclinonc.2018.29
Kalbasi A, Ribas A (2020) Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol 20(1):25–39. https://doi.org/10.1038/s41577-019-0218-4
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL, Investigators IM (2020) Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 382(20):1894–1905. https://doi.org/10.1056/NEJMoa1915745
Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Kubota T, Dutcus CE, Saito K, Siegel AB, Dubrovsky L, Mody K, Llovet JM (2020) Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol 38(26):2960–2970. https://doi.org/10.1200/JCO.20.00808
Kelley RK, Cheng A-L, Braiteh FS, Park J-W, Benzaghou F, Milwee S, Borgman A, El-Khoueiry AB, Kayali ZK, Zhu AX, Rimassa L (2019) Phase 3 (COSMIC-312) study of cabozantinib (C) in combination with atezolizumab (A) versus sorafenib (S) in patients (pts) with advanced hepatocellular carcinoma (aHCC) who have not received prev. J Clin Oncol. 37(15_suppl):TSP4157–TSP4157. https://doi.org/10.1200/JCO.2019.37.15_suppl.TPS4157
Abou-Alfa GK, Chan SL, Furuse J, Galle PR, Kelley RK, Qin S, Armstrong J, Darilay A, Vlahovic G, Negro A, Sangro B (2018) A randomized, multicenter phase 3 study of durvalumab (D) and tremelimumab (T) as first-line treatment in patients with unresectable hepatocellular. J Clin Oncol. 36(15_suppl):TPS4144–TPS4144. https://doi.org/10.1200/JCO.2018.36.15_suppl.TPS4144
Yau T, Kang Y-K, Kim T-Y, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou M-M, Matilla A, Tovoli F, Knox JJ, He AR, El-Rayes BF, Acosta-Rivera M, Neely J, Shen Y, Baccan C, Dela Cruz CM, Hsu C (2019) Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC) results from CheckMate 040. J Clin Oncol. 37(15_suppl):4012–4012. https://doi.org/10.1200/JCO.2019.37.15_suppl.4012
Hiraoka A, Kumada T, Tsuji K, Takaguchi K, Itobayashi E, Kariyama K, Ochi H, Tajiri K, Hirooka M, Shimada N, Ishikawa T, Tachi Y, Tada T, Toyoda H, Nouso K, Joko K, Hiasa Y, Michitaka K, Kudo M (2019) Validation of modified ALBI grade for more detailed assessment of hepatic function in hepatocellular carcinoma patients: a multicenter analysis. Liv Cancer 8(2):121–129. https://doi.org/10.1159/000488778
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372(21):2018–2028. https://doi.org/10.1056/NEJMoa1501824
Lala M, Li TR, de Alwis DP, Sinha V, Mayawala K, Yamamoto N, Siu LL, Chartash E, Aboshady H, Jain L (2020) A six-weekly dosing schedule for pembrolizumab in patients with cancer based on evaluation using modelling and simulation. Eur J Cancer 131:68–75. https://doi.org/10.1016/j.ejca.2020.02.016
Lee PC, Chao Y, Chen MH, Lan KH, Lee CJ, Lee IC, Chen SC, Hou MC, Huang YH (2020) Predictors of response and survival in immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. Cancers 12(1):182. https://doi.org/10.3390/cancers12010182
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1 1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liv Dis 30(1):52–60. https://doi.org/10.1055/s-0030-1247132
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366(26):2443–2454. https://doi.org/10.1056/NEJMoa1200690
Cheng AL, Hsu C, Chan SL, Choo SP, Kudo M (2020) Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol 72(2):307–319. https://doi.org/10.1016/j.jhep.2019.09.025
Hui R, Garon EB, Goldman JW, Leighl NB, Hellmann MD, Patnaik A, Gandhi L, Eder JP, Ahn MJ, Horn L, Felip E, Carcereny E, Rangwala R, Lubiniecki GM, Zhang J, Emancipator K, Roach C, Rizvi NA (2017) Pembrolizumab as first-line therapy for patients with PD-L1-positive advanced non-small cell lung cancer: a phase 1 trial. Ann Oncol 28(4):874–881. https://doi.org/10.1093/annonc/mdx008
Sangro B, Melero I, Wadhawan S, Finn RS, Abou-Alfa GK, Cheng A-L, Yau T, Furuse J, Park J-W, Boyd Z, Tang H, Shen Y, Tschaika M, Neely J, El-Khoueiry A (2020) Association of inflammatory biomarkers with clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma. J hepatol 73(6):1460–1469. https://doi.org/10.1016/j.jhep.2020.07.026
Morita M, Nishida N, Sakai K, Aoki T, Chishina H, Takita M, Ida H, Hagiwara S, Minami Y, Ueshima K, Nishio K, Kobayashi Y, Kakimi K, Kudo M (2021) Immunological microenvironment predicts the survival of the patients with hepatocellular carcinoma treated with Anti-PD-1 antibody. Liv Cancer. https://doi.org/10.1159/000516899
Acknowledgements
The authors thank the Clinical Research Core Laboratory of Taipei Veterans General Hospital for providing their facilities to conduct this study.
Funding
The study was supported by grants from Taipei Veteran General Hospital, Taipei, Taiwan (V109C-048, V110C-144, V109A-035).
Author information
Authors and Affiliations
Contributions
CJ Wu: data acquisition, interpretation; statistical analysis; first draft of the manuscript writing and figure preparation. Ya-Wen Hung: data acquisition. Pei-Chang Lee: data acquisition, interpretation; statistical analysis. Chieh-Ju Lee, data acquisition. Chen-Ta Chi, data acquisition. I-Cheng Lee, data acquisition. MC Hou: data quality control. YH Huang: study concept, design, data acquisition and manuscript revision.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of the Taipei Veterans General Hospital (IRB numbers: 2020–06-033BC, 2021–07-040BC). All participants have provided written consent.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, CJ., Lee, PC., Hung, YW. et al. Lenvatinib plus pembrolizumab for systemic therapy-naïve and -experienced unresectable hepatocellular carcinoma. Cancer Immunol Immunother 71, 2631–2643 (2022). https://doi.org/10.1007/s00262-022-03185-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03185-6