Abstract
Background
The current second-line treatment of advanced gastric or gastroesophageal junction adenocarcinoma remains unsatisfactory. Anti-PD-1 monoclonal antibody combined with anti-angiogenic therapy shows anti-tumor activity and synergistic effect. We aimed to assess the efficacy and safety of the combination therapy of camrelizumab, apatinib, and S-1 in patients with gastric or gastroesophageal junction adenocarcinoma.
Methods
In this open-label, single-arm, phase 2 trial, in each 21-day cycle, eligible patients received 200 mg intravenous camrelizumab in the first day, 500 mg oral apatinib once daily continuously, and specific dose oral S-1 in the first 14 days until the trial was discontinued disease progression, development of intolerable toxicity, or withdrawal of consent. The primary endpoint was objective response rate. The secondary endpoints were disease control rate, progression-free survival and overall survival, and safety. This study was registered at ClinicalTrials.gov, NCT04345783.
Results
Between May 2019 and August 2020, we enrolled a total of 24 patients in this trial. At the data cutoff (December 1, 2020), the median follow-up duration was 8.13 months. Seven of 24 (29.2%, 95%CI 14.9–49.2%) patients reached objective response. The median-progression-free survival was 6.5 months (95%CI 6.01–6.99) and the median overall survival was not reached. Grade 3 or 4 adverse events occurred in 6 (25.0%) patients, including elevated transaminase, thrombocytopenia, fatigue, proteinuria, and intestinal obstruction. No serious treatment-related adverse events or treatment-related deaths occurred.
Conclusions
In this trial, the combination of camrelizumab, apatinib, and S-1 showed promising anti-tumor activity and manageable toxicity as a second-line therapy in patients with advanced gastric or gastroesophageal junction adenocarcinoma, regardless of PD-L1 expression.
Clinical trial registration
NCT04345783.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
According to the 2018 Global Cancer Analysis [1], gastric cancer (GC) is the fifth most common cancer and the third leading cause of cancer death. And patients with advanced gastric or gastroesophageal junction adenocarcinoma exhibited poor prognoses [2,3,4]. At present, the most common treatment options include platinum combined with fluorouracil as the first-line treatment [5], and taxanes combined with ramucirumab as the second-line treatment [6].
Immune checkpoint inhibitor (ICI) therapy, which blocks programmed cell death protein 1 (PD-1) signaling and activating cytotoxic T lymphocytes (CTL) [7], has shown significant anti-tumor activity in several solid tumors and hematological tumors [8, 9]. With cancer immunotherapy emerging and studies on anti-PD-1 therapy as first-line therapy have been carrying out, anti-PD-1 monoclonal antibodies (nivolumab) had been approved by the US Food and Drug Administration (FDA) in first-line treatment for patients with advanced gastric cancer (Her-2 negative and PD-L1 CPS ≥ 5) [10]. However, compared with the traditional chemotherapy, the current immune checkpoint inhibitor therapies are not satisfactory [11, 12]. Given the anti-tumor activity of anti-PD-1 therapy and its synergistic effect with targeted therapy and chemotherapy, researches on anti-PD-1-containing second-line treatments are warranted, and new predictive biomarkers for ICIs are urgently needed. Camrelizumab (SHR-1210), a high-affinity, full-humanized programmed death protein 1 monoclonal antibody [13], in monotherapy or in combination with chemotherapy shows anti-cancer activity and tolerable toxicity in advanced gastric and gastroesophageal junction adenocarcinoma [14, 15].
Tumor angiogenesis, mediated by vascular endothelial growth factor receptor (VEGFR) signaling pathway, plays an important role in tumor proliferation and progression. At present, many studies [8, 16] have found that anti-angiogenesis therapy can modulate immunosuppression and enhance the anti-tumor activity of anti-PD-1 therapy. Apatinib, a small-molecule VEGFR tyrosine kinase inhibitor, effectively inhibits tumor angiogenesis and modulates immunosuppression [17, 18]. In addition, apatinib can effectively reduce camrelizumab-induced reactive cutaneous capillary endothelial proliferation (RCCEP) [19]. Therefore, anti-PD-1 therapy in combination with anti-angiogenesis therapy has a good prospect in advance gastric cancer therapy [20, 21].
S-1 is a fluorouracil-derived combination anti-cancer agent consisting of tegafur (FT), gimeracil (CDHP), and oteracil (OXO) [22]. Numerous studies demonstrated that S-1 was suitable for adjuvant, first-line, and second-line chemotherapy for gastric cancer [23, 24].
Camrelizumab, apatinib, and S-1 had, respectively, shown clinically meaningful anti-tumor activity and manageable toxicities for patients with advanced gastric or gastroesophageal junction adenocarcinoma [15, 25]. On this basis, we postulated the combination of camrelizumab and apatinib and S-1 might improve the clinical outcomes and we carried out this phase 2, prospective trial to explore the efficacy and safety of this combinatorial regimen in patients with gastric or gastroesophageal junction adenocarcinoma refractory to first-line treatments.
Method
Patient characteristics
This study is a phase 2, single-arm, prospective study of camrelizumab and apatinib and S-1 at the Beijing Friendship Hospital affiliated to Capital Medical University. The eligible criteria included age of 18 to 80 years old; histologically confirmed metastatic or recurrent gastric or gastroesophageal junction adenocarcinoma, with only one previous treatment regimen (disease progressed during the first-line regimen or within 6 months after last adjuvant chemotherapy); Her-2 negative; an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 and a life expectancy of at least 3 months; measurable lesions following the Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1)[26]; an adequate bone marrow hematopoietic (neutrophil count ≥ 1.5*109/L, platelet count ≥ 75 × 109/L, and hemoglobin ≥ 90 g/L), hepatic (total bilirubin ≤ 25.7umol/L, aspartate aminotransferase and alanine aminotransferase ≤ 100 U/L [for patients with liver metastases ≤ 200 U/L]) and renal function (blood creatinine ≤ 177umol/L and urinary protein ≤ 2 + , urine protein to creatinine ratio < 3·5, or 24 h urine protein ≤ 3500 mg). Major exclusion criteria included: previous treatment with anti-PD-1 or PD-L1 monoclonal antibodies; previous treatment with VEGFR-TKIs or S-1; active or history of autoimmune disease; history of organ transplantation; serious cardiovascular and cerebrovascular diseases; neurological and mental illness; or other serious comorbidities.
Ethical committee clearance
The trial was approved by the ethics board of the Beijing Friendship Hospital affiliated to Capital Medical University and was done in accordance with the Declaration of Helsinki. All patients provided written informed consent. This trial was registered with ClinicalTrials.gov, number NCT04345783.
Procedures
In each 21-day cycle, eligible patients received 200 mg intravenous camrelizumab in the first day, 500 mg oral apatinib once daily continuously and specific dose oral S-1 in the first 14 days (40 mg twice daily if the patient’s body surface area < 1.25 m2; 50 mg twice daily if 1.25–1.5 m2; and s60 mg twice daily if the patient’s body surface area > 1.5 m2) until the trial was discontinued.
The trial was discontinued when the tumor progressed, or intolerable adverse events occurred, or patient withdraw of consent, or necessary discontinuation taken by investigators.
Tumor response was evaluated at baseline and every 6 weeks, by two investigators using enhanced CT or MRI according to the RECIST, v 1.1. And the best response would be confirmed by repeating imaging studies in 4 weeks. The detail criteria were shown in RECIST criteria v1.1. The physical examination and laboratory tests were monitored every 3 weeks, including: hematological examinations, serum chemistry, ECG, urine test, thyroid function, etc. Adverse events were graded and documented according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE, version 4.03) during the course of treatment.
The management of adverse events mainly included supportive cure, dose adjustment, and interruption of dosage. We managed the camrelizumab-related adverse events according to the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Management of Immunotherapy-Related Toxicities [27]. The apatinib and S-1 dose could be reduced if patients had intolerable grade 2 or grade 3 treatment-related adverse events, which were judged by the investigators. The dose reduction and interruption were permitted for no more than two times and dose re-escalation was not permitted in subsequent cycles. If patients had grade 4 AEs, the treatment would be discontinued.
Baseline molecular characteristics, including PD-L1 combined positive score (CPS), tumor mutational burden (TMB) status, and mismatch repair (MMR)/microsatellite instability (MSI), were assessed using formalin-fixed paraffin-embedded tissue specimens from archival tumor tissue samples. Combined positive score (CPS), defined as the number of PD-L1-positive tumor cells, lymphocytes, and macrophages as a proportion of the total number of tumor cells, was assessed by investigators using the PD-L1 immuno-histochemistry 22C3 pharmDx. TMB and MMR status were measured from the extracted DNA from archival tumor samples using the next-generation sequencing (illumina sequencing) method that targets 642 genes corresponding to 1·78 Mb of sequencing data. The TMB-high cutoff line is defined as the top 25% (12 mut/MB) of all subjects from single center. All patients’ molecular and genetic information was measured in GloriousMed Clinical Laboratory Co.
Outcomes
The primary endpoint was objective response rate (ORR). According to RECIST v1.1, the objective response rate was defined as the proportion of patients with measurable disease who achieved complete response (CR) or partial response (PR). The secondary endpoints were the disease control rates (DCR), progression-free survival (PFS) and overall survival (OS) and safety. The DCR was defined as the proportion of patients achieving CR or PR or stable disease (SD). Progression-free survival is defined as the duration from the beginning of the treatment to the disease progression, or death from any cause. Overall survival is defined as the duration from the beginning of the treatment to death from any cause. Exploratory endpoints included the correlation between PD-L1, TMB, and gene mutation with efficacy and prognosis.
Statistical analysis
Using the Simon’s optimal two-stage design, with the power of 80% and one-sided α of 5%, the planned sample size was 22 patients. In ATTRACTION-2 [28], the response rate of nivolumab with any PD-L1 status was 11%. Considering the efficacy of anti-PD-1 monoclonal antibody combined with anti-angiogenesis targeted therapy, in our study, with any PD-L1 status, we set the threshold ORR to 10% and the expected ORR to 35%. In the first stage, we enrolled 8 patients. If 1 or more patients reached an objective response, we enrolled 14 or more patients in the second stage. Ultimately, if 5 or more patient of 22 reached an objective response, the primary endpoint was met.
We analyzed data in two populations: the full analysis set and biomarkers analysis group. The full analysis set included all enrolled patients. The biomarkers analysis group was a subgroup of patients with available molecular characteristics. We analyzed efficacy and safety data in the full analysis set. We analyzed the correlation between biomarkers and efficacy in the biomarker analysis set.
We assessed calculated the 95% CIs of ORR and DCR using exact binomial test and calculated the risk ratio and P of subgroup using Fisher exact probability test. We assessed the PFS, OS, 95%CIs, and HR using the Kaplan–Meier Model and log-rank test. Safety date was analyzed in all patients enrolled with at least one dose of the regimen. In addition, we did the post hoc exploratory analysis between subgroups, such as biomarkers (PD-L1 expression, TMB expression), sex, age, ECOG performance status, history of gastrectomy, number of metastases, liver metastases, peritoneal metastases, and so on. All statistical analyses were two-sided and significance was set at P < 0.05. The software used for all statistical analyses was SPSS Statistics (version 25.0).
Results
Patient characteristics
In the first stage of this study, 1 patient had a partial response. Therefore, with a significant treatment response, 16 more patients were enrolled in the second stage. Between May 2019 and December 2020, we enrolled a total of 24 patients with full efficacy and safety data. And 19 patients with molecular characteristics date were included in biomarker analysis set, all of whom were assessed with MSI status, TMB status, PD-L1 CPS, and other gene mutation information. The patients’ baseline characteristics are shown in Table 1.
Efficacy
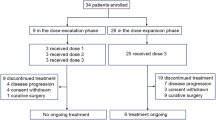
At the time of data cutoff (December 1, 2020), with the median follow-up duration 7.58 months, 19 patients have discontinued this trial, while 5 patients were still on treatment. Nine patients discontinued this protocol treatment due to disease progression, 3 patients due to intolerable adverse events, 2 patients due to consent withdraw, and 2 patient received radical gastrectomy with the patients’ request and assessment of investigators, in addition, 3 patients dropped out of the study after treatment was interrupted by the COVID-19 for more than 1.5 months. The flowchart of the study is shown in Fig. 1.
Of all enrolled patients, 1 (4.2%) patient achieved complete response, 6 (25.0%) patients achieved partial response, 16 (66.7%) patients reached stable disease, and 3 (12.5%) patients were progressive disease. As the primary endpoint, objective response was achieved in 7 of 24 patients (ORR: 29.2%, 95%CI 14.9–49.2%). And disease control was achieved in 23 of 24 patients (DCR: 95.8%, 95%CI 79.8–99.2%) (Table 2). And among the patients with CR or PR, the median duration of response (mDOR) reached 6.86 weeks (95%CI 6.12–7.60) (Fig. 2).
At the data cutoff time, 16 (66.7%) patients were still alive and 8 (33.3%) patients had died. As the secondary endpoint, the median-progression-free survival (mPFS) was 6.5 months (95%CI 6.01–6.99), the median overall survival (mOS) was not reached (95%CI 10.3–NA), the 6-month overall survival with 75.0%, and the 12-month overall survival with 41.6% (Fig. 3).
Biomarkers
In the exploratory analysis, among the 19 patients with complete genetic characteristics, 9 (47.4%) patients were PD-L1 CPS ≥ 1, 10 (52.6%) patients were PD-L1 CPS < 1. 1 (5.3%) patient reached complete response, 5 (26.3%) patients reached partial response, and 12 (63.2%) patients reached stable disease. The overall objective response was 31.58% (95%CI 15.37–53.99%) (Table 2). 4 (44.4%) of 9 patients with PD-L1 CPS ≥ 1 achieved objective response, and 2 (20%) of 10 patients with PD-L1 CPS < 1 achieved objective response (risk ratio 2.2, 95%CI 0.53–9.53, P = 0.350). Moreover, 3 (60%) of 5 patients with a TMB-high and 3 (21.4%) of 14 patients with a TMB-low reached objective response. There were 2 patients with microsatellite instability high, one reached CR and the other reached PR. The details of genetic variations are shown in Fig. 4.
At the data cutoff time, in the biomarker analysis set, the median PFS were 6.20 (95%CI 4.94–7.46) in patient with PD-L1 CPS ≥ 1 and 6.53 m (95%CI 6.47–6.60) in patients with PD-L1 CPS < 1 (HR: 1.94, 95%CI 0.43 to 8.79, P = 0.391), and no statistically significant difference was showed. The median PFS was not reached (95% CI 4·0 to not reached) with a TMB-high and 6.50 months (95% CI 5.78—7.23) with a TMB-low (HR: 0.451, 95%CI 0.090 to 2.263) (Fig. 5). In the post hoc analysis, several gene mutations with a central distribution tendency were screened out, including GNAS, KMT2B, NF1, NOTCH2/3, PTPRS, APC, TP53BP1, TP53 co-mutation with DNA Damage Response and Repair (DDR) pathway genes, which seem to indicate better efficacy and survival prognosis. We assigned patients with any these mutations to the subgroup A, and patients without to the subgroup B. The objective response rate of subgroup A was 45.5% (95% CI 18.1–75.4%), and that of subgroup B was 12.5% (95% CI 0.7–53.3%) (risk ratio 3.6, 95%CI 0.52–25.4, P = 0.177). The median PFS was not reached in subgroup A, and was 5.6 m (95% CI 3.4–7.8) in subgroup B (Fig. 5).
Kaplan–Meier curves for overall survival and progression-free survival of subgroup analysis. a The progression-free survival for PD-L1 status subgroups. b The overall survival for PD-L1 status subgroups. c. The progression-free survival for TMB status subgroups. d The overall survival for TMB status subgroups. e. The progression-free survival for gene mutations subgroups. f The overall survival for gene mutations subgroups
Safety
All 24 patients were included in the safety analysis. The overall incidence of any-grade adverse events was 100%. Most adverse events were graded 1 or 2. The most common AEs were anemia in 18 (75%) patients, hypoalbuminemia in 14 patients (58.3%), leukopenia in 11 patients (45.8%), thrombocytopenia in 11 patients (45.8%), aminotransferase increased in 10 patients (41.7%). Six patients (25.0%) had grade 3–4 adverse events, including aminotransferase AST increased, thrombocytopenia, fatigue, proteinuria, and intestinal obstruction.
There were 4 (16.7%) patients with dose reduction of S-1 for hematological adverse events, and 7 (29.2%) patients dose reduction or interruption of apatinib for anorexia, fatigue, abdominal pain, and diarrhea, and 3 patients (12.5%) patients dose interruption of camrelizumab for increased AST. Eventually, there were 3 patients discontinued from this trial for trAEs. No treatment-related deaths occurred. Details of adverse events are shown in Table 3.
Discussion
With an objective response rate reaching 29.2%, this prospective trial met the primary endpoint and shows significant efficacy and manageable adverse events of camrelizumab combined with apatinib and S-1 in patient with advanced GC or EGJC refractory to first-line treatment.
As previous studies reported, combination of a VEGFR inhibitor and an anti-PD-1 antibody produced strong synergistic anti-tumor activity. Apatinib, a small-molecule VEGFR tyrosine kinase inhibitor, effectively inhibits tumor angiogenesis and modulates immunosuppression. In addition, the previous study on PD-1 blockades showed that the anti-tumor effect of cytotoxic agents remains important in AGC. 5-Fluorouracil is an indispensable part of chemotherapy for AGC, and S-1 is suitable for all stages of the treatment of advanced gastric cancer. These results suggest the feasibility of camrelizumab plus apatinib and S-1. On this basis, we conducted this exploratory study of camrelizumab plus apatinib and S-1 in second-line setting in patients with advanced gastric cancer.
Currently, given first-line treatments for advanced gastric cancer, anti-PD-1 monotherapy has shown a survival benefit for patients. In the ATTRACTION-2 trial [28], nivolumab monotherapy in third-line or later-line setting improved the overall survival of patients advanced gastric cancer by 5.26 months (95% CI 4·60–6·37) irrespective of PD-L1 status, although the ORR was only 11.2% (95%CI 7.7–15.6). Compared with monotherapy, with the significant efficacy, the anti-PD-1 combination therapy has been listed in the latest treatment guidelines [10, 29]. For first-line treatment of advanced gastric cancer, as CheckMate-649 study [10] reported, nivolumab plus chemotherapy showed significant anti-tumor activity in patients with CPS ≥ 5, with ORR reached 60%, median OS reached 13.8 m, and median PFS reached 7.7 m. In the analysis of the Attraction-4 (ONO-4538–37) trial [30], nivolumab plus chemotherapy group had significantly better ORR (57.5% vs 47.8%) and mPFS (10.45 m vs 8.34 m) than the control group. The phase 3 EPOC 1706 trial [31] reported lenvatinib plus pembrolizumab with an obvious anti-tumor activity in patients with advanced gastric cancer as the first-line or second-line treatment, in which the objective response rate reached 69% and the median-progression-free survival was 7.1 months. There are relatively few studies on second-line therapy for advanced gastric cancer. In KEYNOTE-061 trial [11], Pembrolizumab monotherapy did not significantly improve survival compared with paclitaxel as second-line therapy for advanced GC or GEJC with PD-L1 CPS ≥ 1. However, In the Takako Eguchi Nakajima’s study [32], the combination of nivolumab, ramucirumab, and paclitaxel as second-line treatment resulted in an ORR of 37.2% (95%CI, 23.0–53.5%), a median PFS of 5.1 months (95%CI, 4.5–6.5 months), and a median survival of 13.1 months (95%CI, 8.0–16.6 months). In our trial, in a second-line treatment setting, anti-PD-1 therapy in combination with chemotherapy and targeted therapy showed significant efficacy and survival benefit for patients with advanced gastric cancer.
In RAINBOW study [6], with the ORR reaching 28% and the mPFS reaching 4.4 months, ramucirumab plus Paclitaxel showed a significant improvement as second-line treatment for patients with AGC. In our study, the ORR was 29.2% and mPFS was 6.5 months, comparable with the response in Japanese patients receiving paclitaxel plus ramucirumab in the RAINBOW trial. In addition, it is important to take into account the differences of treatment response in Japanese and western patient cohorts. In general, anti-angiogenesis targeted therapy seemed to lead to higher response rates in Asia [33]. Given the best response of different patients to PD-1 blockades varying greatly, cross-trial comparisons require careful interpretation, and researches on anti-PD-1-containing treatments are still warranted.
The biomarker analysis included PD-L1, TMB status, and gene mutations from NGS data (Table 1). In patients with full genetic information, the ORR was 44.4% and 20.0%, respectively, in patients with CPS ≥ 1 and CPS < 1. However, similar to the ATTRACTION-4 trial [30], the Kaplan–Meier survival curves in different PD-L1 expression status showed no significant difference (Fig. 4). As reported in previous studies [34], MMR (MSI), TMB, and DNA damage response and repair are closely related. In many solid tumors, patients with high microsatellite instability were associated with high response rates to immunotherapies. TMB has also been evaluated as a predictor of response to PD-1 blockade, given patients with higher TMB showing a greater likelihood of response [35]. However, TMB cannot serve as biomarkers for Immune checkpoint blockade precisely. In our trials, the mPFS of patients with higher mutational loads was not reached, which was better than 6.5 months in patients with lower TMB (HR:0.3695, 95%CI 0.075 to 1.831). It seems to indicate better treatment outcomes in patients with higher mutational loads.
In the exploratory, post hoc analysis, the KM curves with single factor analysis and COX analysis of genetic data failed to find a statistically significant deleterious mutation. Larger studies on the association of genetic mutations and PD-1 blockades are warranted.
In this study, the incidence of adverse events was 100%. However, with symptomatic treatment, and dose reduction, AEs in this study were evaluated as manageable and tolerable. In addition, the incidence of serious adverse events was lower than that of intravenous chemotherapy. In our trial, according to the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Management of Immunotherapy-Related Toxicities, we classified treatment-related adverse events into immune-related adverse events (irAEs) and non-irAEs. Hypothyroidism, hyperthyroidism, reactive cutaneous capillary endothelial proliferation, rash maculopapular, ALT increased and AST increased were grouped to irAEs, diabetes mellitus. In the post hoc analysis, different from a previous study [36], we have not found that patients with irAEs showed a significantly better treatment response. The median PFS in patients with irAEs was 6.5 m and 5.57 m in patients with non-irAEs (HR:0.688, 95%CI 0.1540 to 3.075). It is worth noting that the combination of ICIs and target therapy may affect the assessment of irAEs and the relationship between irAEs and treatment response. In addition, we found patients with ALT/AST increased and rash maculopapular seem to come out with a better progression-free survival. Moreover, different from previous studies on anti-angiogenic therapies [37], patients with hypertension or proteinuria did not show better outcomes. More studies on the correlation between irAEs and immune therapy response are warranted.
There are several limitations in our trial. First, with a non-randomized design and a small sample size from a single institution, sampling error and selection bias could not be ruled out well. Second, follow-up duration was relatively short, and the median overall survival was not reached; nevertheless, our study showed this treatment regimen was effective in improving survival outcomes. Third, no biopsy of the patient's lesion was performed after the first dose to evaluate the efficacy in pathology and molecular biology. And a further study would be needed to investigate the relationship between efficacy and PD-L1 status change during the treatment. Finally, objective response was not assessed by independent central review, which might lead to the overestimation of anti-tumor activity results in this study.
In conclusion, the camrelizumab combined with apatinib and S-1 in this study, for the first time served as a second-line treatment regimen for advanced gastric cancer. Compared with previous studies, with the ORR of 29.2% (95%CI 14.9–49.2%) and median PFS of 6.5 m (95%CI 6.01–6.99), camrelizumab combined with apatinib and S-1 showed significant anti-tumor activity and manageable toxicities in patients with advanced gastric or gastroesophageal junction cancer.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68(1):7–30
Kang JH, Lee SI, Lim DH et al (2012) Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 30(13):1513–1518
Zong L, Abe M, Seto Y et al (2016) The challenge of screening for early gastric cancer in China. Lancet 388(10060):2606
Noh SH, Park SR, Yang HK et al (2014) Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 15(12):1389–1396
Cunningham D, Starling N, Rao S et al (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358(1):36–46
Wilke H, Muro K, Van Cutsem E et al (2014) Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 15(11):1224–1235
Sharma P, Allison JP (2015) Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161(2):205–214
Borghaei H, Paz-Ares L, Horn L et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373(17):1627–1639
Hodi FS, O’Day SJ, McDermott DF et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363(8):711–723
Janjigian YY, Shitara K, Moehler M et al (2021) First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 398(10294):27–40
Shitara K, Ozguroglu M, Bang YJ et al (2018) Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 392(10142):123–133
Shitara K, Van Cutsem E, Bang YJ et al (2020) Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol 6(10):1571–1580
Markham A, Keam SJ (2019) Camrelizumab: first global approval. Drugs 79(12):1355–1361
Huang J, Mo H, Zhang W et al (2019) Promising efficacy of SHR-1210, a novel anti-programmed cell death 1 antibody, in patients with advanced gastric and gastroesophageal junction cancer in China. Cancer 125(5):742–749
Xu J, Zhang Y, Jia R et al (2019) Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin Cancer Res 25(2):515–523
Shigeta K, Datta M, Hato T et al (2020) Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology 71(4):1247–1261
Roviello G, Ravelli A, Polom K et al (2016) Apatinib: a novel receptor tyrosine kinase inhibitor for the treatment of gastric cancer. Cancer Lett 372(2):187–191
Li J, Qin S, Xu J et al (2013) Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol 31(26):3219–3225
Wang F, Qin S, Sun X et al (2020) Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial. J Hematol Oncol 13(1):47
Park DJ, Thomas NJ, Yoon C et al (2015) Vascular endothelial growth factor a inhibition in gastric cancer. Gastric Cancer 18(1):33–42
Georganaki M, van Hooren L, Dimberg A (2018) Vascular targeting to increase the efficiency of immune checkpoint blockade in cancer. Front Immunol 9:3081
van Groeningen CJ, Peters GJ, Schornagel JH et al (2000) Phase I clinical and pharmacokinetic study of oral S-1 in patients with advanced solid tumors. J Clin Oncol 18(14):2772–2779
Sakuramoto S, Sasako M, Yamaguchi T et al (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357(18):1810–1820
Kim GM, Jeung HC, Rha SY et al (2012) A randomized phase II trial of S-1-oxaliplatin versus capecitabine-oxaliplatin in advanced gastric cancer. Eur J Cancer 48(4):518–526
Feng J, Qin S (2018) The synergistic effects of Apatinib combined with cytotoxic chemotherapeutic agents on gastric cancer cells and in a fluorescence imaging gastric cancer xenograft model. Onco Targets Ther 11:3047–3057
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–47
Thompson JA, Schneider BJ, Brahmer J et al (2019) Management of immunotherapy-related toxicities, version 1.2019. J Natl Compr Canc Netw 17(3):255–89
Kang YK, Boku N, Satoh T et al (2017) Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390(10111):2461–2471
Chung HC, Bang YJ, C SF, et al (2021) First-line pembrolizumab/placebo plus trastuzumab and chemotherapy in HER2-positive advanced gastric cancer: KEYNOTE-811. Fut Oncol (London, England) 17(5):491–501
Boku N, Ryu MH, Kato K et al (2019) Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol 30(2):250–258
Kawazoe A, Fukuoka S, Nakamura Y et al (2020) Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol 21(8):1057–1065
Nakajima TE, Kadowaki S, Minashi K et al (2021) Multicenter phase I/II study of nivolumab combined with paclitaxel plus ramucirumab as second-line treatment in patients with advanced gastric cancer. Clin Cancer Res 27(4):1029–1036
Shitara K, Muro K, Shimada Y et al (2016) Subgroup analyses of the safety and efficacy of ramucirumab in Japanese and Western patients in RAINBOW: a randomized clinical trial in second-line treatment of gastric cancer. Gastric Cancer 19(3):927–938
Luchini C, Bibeau F, Ligtenberg MJL et al (2019) ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol 30(8):1232–1243
Wang F, Wei XL, Wang FH et al (2019) Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann Oncol 30(9):1479–1486
Sato K, Akamatsu H, Murakami E et al (2018) Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer 115:71–74
Lan CY, Wang Y, Xiong Y et al (2018) Apatinib combined with oral etoposide in patients with platinum-resistant or platinum-refractory ovarian cancer (AEROC): a phase 2, single-arm, prospective study. Lancet Oncol 19(9):1239–1246
Acknowledgements
This study was supported by the National Natural Science Foundation of China Youth Science Foundation Project and the Beijing Natural Science Foundation Youth Fund Project. We thank all the patients and their families and all the participating staffs.
Author information
Authors and Affiliations
Contributions
WD and ZZ contributed to conception and design. ZZ, JZ, and WD provided administrative support. WD, ZB, ZZ, JZ, and JY provided study materials or patients. CJ, MZ, and XY collected and assembled the data. CJ, WD, JW, and ZL analyzed and interpreted the data. All authors contributed to manuscript writing. All authors gave final approval of the manuscript. Corresponding authors ZZ and WD have the same contribution to this paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Ethics approval
The trial was approved by the ethics board of the Beijing Friendship Hospital affiliated to Capital Medical University and was done in accordance with the Declaration of Helsinki. All patients provided written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jing, C., Wang, J., Zhu, M. et al. Camrelizumab combined with apatinib and S-1 as second-line treatment for patients with advanced gastric or gastroesophageal junction adenocarcinoma: a phase 2, single-arm, prospective study. Cancer Immunol Immunother 71, 2597–2608 (2022). https://doi.org/10.1007/s00262-022-03174-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03174-9