Abstract
Angiogenesis is a vital process in the progression and metastasis of solids tumors including gastric adenocarcinoma. Tumors induce angiogenesis by secreting proangiogenic molecules such as vascular endothelial growth factor A (VEGF-A), and VEGF-A inhibition has become a common therapeutic strategy for many cancers. Several drugs targeting the VEGF-A pathway have been approved for clinical use in selected solid tumors, and several anti-VEGF-A strategies have been examined for gastric cancer. Phase II studies suggested that bevacizumab, an anti-VEGF antibody, can increase the efficacy of chemotherapy for advanced gastric cancer, but two international phase III trials failed to show an overall survival benefit. Two more recent international phase III trials have examined ramucirumab, an antibody targeting the primary receptor for VEGF-A, as second-line therapy for advanced gastric cancer and found a survival benefit both as single agent therapy and when combined with chemotherapy. Finally, correlative science studies suggest that the VEGF-A pathway may have varying importance in gastric cancer progression depending on ethnicity or race. This article will review the preclinical and clinical studies on the role of the VEGF-A pathway inhibition in gastric cancer.
Similar content being viewed by others
VEGF-A
Angiogenesis is the physiological process through which new blood vessels form from pre-existing blood vessels. In 1971, Dr. Judah Folkman proposed the hypothesis that tumor growth is angiogenesis-dependent [1]. He showed evidence that tumors could not enlarge beyond a few millimeters in size without recruiting new blood vessels and that tumors secreted a diffusible substance that could stimulate endothelial cell proliferation. Subsequently, numerous pro- and antiangiogenic factors have been discovered, with one of the first discovered and most important factors being vascular endothelial growth factor A (VEGF-A) [2]. The primary functions of VEGF-A are to promote blood vessel dilation and permeability and to induce new blood vessel formation. VEGF-A is overexpressed by the vast majority of tumors studied, and circulating levels of VEGF-A are elevated in many patients with cancer [3].
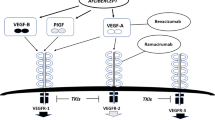
There are five structurally related VEGF ligands: VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor (PlGF) (Fig. 1) [4]. The VEGF ligands exist as homodimers linked by disulfide bonds. Each ligand is expressed as several different variants because of alternative splicing or posttranslational processing, and each variant binds differently to VEGF receptors and coreceptors. VEGFs are produced by many different cell types and can act in an autocrine and paracrine manner on VEGF receptors. There are three primary VEGF receptors, VEGFR-1, VEGFR-2, and VEGFR-3, which are all receptor tyrosine kinases [4]. VEGF-A exerts its effects primarily through VEGFR-1 (a.k.a. Flt-1) and VEGFR-2 (a.k.a. Flk-1, KDR) [5]. VEGFR-1 and VEGFR-2 are expressed primarily on endothelial cells and consist of seven extracellular immunoglobulin-like domains, a transmembrane region, and an intracellular consensus tyrosine kinase domain [6]. VEGFR-1 by itself is generally thought to transmit only weak mitogenic signals but can heterodimerize with VEGFR-2, forming a complex with strong signaling properties [7]. VEGFR-2 seems to mediate the major growth and permeability actions of VEGF-A [8]. Neuropilins 1 and 2 (NRP-1, NRP-2) act as coreceptors and enhance VEGF-A binding to VEGFR-2 [9]. VEGFR-3 (a.k.a. Flt-4) is activated by binding of VEGF-C and VEGF-D. VEGFR-3 and its ligands play important roles in the regulation of tumor lymphangiogenesis [10]. VEGF-C produced by tumors can bind to VEGFR-3 and induce lymphatic leakage, enlargement, and sprouting, which can facilitate metastasis to lymph nodes [11].
VEGF-A Inhibition in Solid Tumors
Inhibition of VEGF-A signaling can effectively suppress tumor angiogenesis in numerous animal models [5]. The VEGF-A pathway has been the target of numerous drugs that are in clinical development or are currently approved by the United States Food and Drug Administration (FDA) (Table 1). Bevacizumab is a recombinant, humanized version of a murine anti-VEGF-A monoclonal antibody [12]. By binding circulating VEGF-A, bevacizumab prevents VEGF-A from binding its receptors and thus blocks VEGF-A activity. Bevacizumab has been demonstrated to increase time to disease progression in patients with metastatic renal cell cancer [13]. When combined with chemotherapeutic drugs, bevacizumab improved progression-free or overall survival in patients with metastatic colorectal cancer [14], metastatic breast cancer [15], and metastatic non-squamous non-small-cell lung cancer [16]. Bevacizumab is also effective as monotherapy for recurrent glioblastoma multiforme [17]. Small molecule receptor tyrosine kinase inhibitors with anti-VEGF-A activity (e.g. sunitinib, sorafenib, pazopanib, and vandetanib) have been FDA approved for metastatic renal cell carcinoma, primitive neuroectodermal tumor, hepatocellular carcinoma, and medullary thyroid cancer.
Numerous preclinical and clinical studies have demonstrated that anti-VEGF-A therapies can increase the efficacy of chemotherapy and radiation therapy. The mechanisms by which this occurs are unclear. One mechanism put forth by Dr. Rakesh Jain (Massachusetts General Hospital) is vascular normalization [18]. Malignant tumors generally have high levels of VEGF-A leading to dysfunctional vasculature. This tumor vasculature contains tortuous, dilated, and highly permeable vessels, which lead to areas of poor perfusion, hypoxia, and high interstitial pressure. This abnormal vasculature can be transiently normalized by antiangiogenic therapies [18, 19]. Jain and colleagues demonstrated that anti-VEGF-A agents can normalize tumor blood flow and oxygenation and thus improve the delivery of chemotherapy or improve the efficacy of radiation. There are several other mechanisms by which anti-VEGF-A therapies may increase the efficacy of chemotherapy or radiation. Anti-VEGF-A agents may enhance the direct effect of chemotherapy or radiation on dividing endothelial cells in tumor vasculature [20–22]. VEGF-A is recognized to prolong endothelial cell survival, so neutralization of VEGF-A may increase the deleterious effects of chemotherapy and radiation on endothelial cells. Alternatively, VEGF-A inhibition may break the tumor vascular niche where tumor stem cells reside and allow tumor stem cells to be more sensitive to chemotherapy [23, 24]. Tumor stem cells are an especially important target because they are resistant to chemotherapy and radiation, have high metastatic potential, and may be the cells responsible for the regrowth of tumors after anticancer therapy [25].
It is increasingly apparent that solid tumors possess or develop evasive or adaptive mechanisms of resistance to anti-VEGF-A and other antiangiogenic therapies. First, tumors may produce angiogenic factors that overcome the neutralizing antiangiogenic therapy. Second, tumors may become tolerant to hypoxia, subsequently becoming more invasive and resistant to treatment [26–28]. Third, tumor cells can form their own vessel lining or co-op nearby vessels [29]. Fourth, tumor cells can recruit bone marrow-derived cells or activate cancer-associated fibroblast, thus circumventing any damage to their existing vasculature [30]. Fifth, tumors can subvert the blockade of the pathway through alternative angiogenic signaling cascades [30–32].
Gastric Cancer
It is estimated that there are over 1 million cases of gastric cancer worldwide per year and there are over 700,000 deaths each year [33]. Thus, gastric cancer is the fourth most common cancer and the second leading cause of cancer death. Gastric adenocarcinoma accounts for about 95 % of gastric cancer cases. The incidence of gastric adenocarcinoma varies tremendously throughout the world and country by country. The highest incidence countries are in Eastern Asia (e.g., Korea, Japan, and China) [34, 35]. The incidence of gastric cancer in the USA and Western Europe has been steadily declining and is currently only about one-sixth that of Eastern Asia [36]. Despite this, the incidence of proximal gastric cancer in Western countries is rising. Overall, males are affected twice as frequently as females, and the average age of presentation is between 60 and 70 years old.
Gastric adenocarcinoma is often asymptomatic in its early stages and in later stages causes weight loss, epigastric pain or discomfort, gastrointestinal bleeding, vomiting, and anorexia. In Japan and Korea, high awareness and common endoscopic screening for gastric cancer have led the proportion of patients presenting with early gastric cancer (i.e., T1 tumors) to reach about 50 %. Unfortunately, in other countries, gastric cancer is found most frequently in advanced stages.
Early T1 tumors (into the lamina propria or submucosa) have little risk of lymph node or distant metastasis and can be effectively managed by endoscopic resection. For more advanced tumors that have not metastasized, treatment generally includes surgical resection of the tumor and the surrounding lymph nodes. Patients with tumors extending into the muscularis layer of the stomach or beyond or who have lymph node metastases are at significant risk of harboring occult micrometastatic disease and are often treated with adjuvant chemotherapy or a combination of chemotherapy and radiation. For unresectable or metastatic gastric cancers, primary palliative chemotherapy can be used. Overall survival for patients with metastatic disease is 3–5 months with best supportive care, and this is extended to 9–11 months with chemotherapy [37]. A minority of gastric adenocarcinomas overexpress human epidermal growth receptor 2 (HER-2), and the addition of trastuzumab to chemotherapy prolonged survival in these patients from 11 to 14 months in a randomized trial [38].
VEGF-A Inhibition in Gastric Cancer
Laboratory Studies
There have been several studies demonstrating the efficacy of anti-VEGF-A therapies in animal models of gastric cancer. There are several methods of disrupting the VEGF-A pathway including blocking VEGF-A secretion from tumors cells, neutralizing the VEGF-A ligand, blocking VEGF-A binding to VEGF receptors, and blocking downstream signaling of the VEGF receptors. Sun et al. [39] used lentivirus-mediated siRNA to knock down VEGF-A expression in SGC7901 gastric cancer cells leading to reduced growth of tumor xenografts. As noted previously, bevacizumab binds human VEGF-A and prevents its binding to VEGF receptors. In one study, intraperitoneal administration of bevacizumab inhibited peritoneal metastasis and reduced malignant ascites when MKN-45P human gastric cancer cells were injected into the peritoneal cavity of mice [40]. DC101 is a monoclonal antibody targeting mouse VEGFR-2. Jung et al. [41] injected TMK-1 gastric cancer cells orthotopically into mice and found that DC101 treatment decreased tumor vascularity and increased endothelial cell apoptosis. Numerous small molecule inhibitors of VEGF receptors have been developed, and some have been approved for use in solids tumors other than gastric cancer (Table 1). In a study from Japan, oral administration of AZD2171 (a.k.a. cediranib), which potently blocks VEGFR-1, 2, and 3, significantly reduced the growth of KATO-III and OCUM2M human gastric cancer xenografts [42].
Because of the resistance mechanisms described above, inhibition of the VEGF-A pathway may not work well as a single agent for gastric cancer. Thus, VEGF-A inhibition has been combined with other targeted biological agents as well as with chemotherapy in several preclinical studies. For example, the combination of DC101 and the anti-EGFR antibody C225 inhibited gastric tumor growth in a TMK-1 xenograft gastric cancer mouse model better than either therapy alone [41]. VEGF-Trap, an engineered soluble decoy VEGF receptor, and trastuzumab, an anti-HER2 antibody, had additive inhibitory effects on the tumor growth and angiogenesis of gastric cancer xenografts [43]. Combination of bevacizumab and insulin-like growth factor-1 receptor blockade was also highly effective against gastric cancer xenografts [44]. A heterodimeric Fc-based bispecific antibody simultaneously targeting VEGFR2 and Met exhibited potent antitumor efficacy in gastric cancer xenograft models [45]. Finally, in another xenograft study, combining cisplatin with sunitinib was found to enhance the antitumor effect in gastric tumors grown in mice [46].
Prognostic Significance of VEGF-A in Gastric Cancer Patients
Several studies have examined circulating levels of VEGF-A in patients with gastric cancer and correlated levels with patient and tumor characteristics, outcomes, and response to therapy [47–54]. Table 2 summarizes ten studies of circulating VEGF-A levels in gastric cancer patients. Seven studies found a correlation between circulating VEGF-A levels in plasma or serum and overall survival, and two studies found a correlation between VEGF-A levels and stage of disease. Only one study found no correlation between plasma VEGF-A levels and overall survival. One study evaluated serum VEGF-A levels in the perioperative period in gastric cancer patients and found that serum VEGF-A levels decreased after surgery and that preoperative serum VEGF-A levels were an independent prognostic factor for overall survival [54]. Plasma VEGF-A levels have also been found to be increased in gastric cancer patients with tumors having venous invasion and lymph node metastasis compared to tumors without venous invasion or lymph node metastasis [55]. In summary, the vast majority of studies examining circulating levels of VEGF-A in gastric cancer patients have demonstrated a correlation between VEGF-A levels and overall survival or stage of disease.
Numerous studies have examined the expression of VEGF-A and its primary receptors, VEGFR-1 and VEGFR-2, in tumors and correlated expression of these proteins with patient and tumor characteristics, outcomes, and response to therapy. Table 3 summarizes the studies of VEGF-A expression in tumors and survival in gastric cancer patients. The 24 primary studies are from a variety of countries, use varying cutoffs for positive or negative expression of VEGF-A, and use varying endpoints. However, in summary, 17 primary studies and all three meta-analyses found a correlation between VEGF-A expression and survival. One meta-analysis of 30 studies (n = 2,166) evaluated the correlation between VEGF-A expression detected by immunohistochemistry and survival in gastric cancer patients. The rates of patients with VEGF-A overexpression ranged from 26.7 to 89.9 %. VEGF-A overexpression was found to correlate with poorer prognosis in this meta-analysis [56]. Because the meta-analysis included mainly East Asian studies (23 out of 30 studies), the results could not be generalized to Western countries. By subgroup analysis according to region, the hazard ratio of Asian patients (n = 1,674) was slightly lower than that of non-Asian patients (n = 492) (1.39 versus 1.74).
Clinical Trials on ANTI-VEGF Treatment for Gastric Cancer
Table 4 summarizes the phase II or III clinical trials conducted to evaluate therapies targeting the VEGF-A pathway in gastric cancer.
Bevacizumab
Several phase II studies suggest that the addition of bevacizumab improves the efficacy of chemotherapy in patients with advanced gastric cancer. In 2006, Shah et al. reported a multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. They found a response rate of 65 %, a median time to progression of 8.3 months, and a median overall survival of 12.3 months [57]. Another phase II study of oxaliplatin, docetaxel, and bevacizumab in locally advanced and metastatic gastric and gastroesophageal junction cancers was not as promising, with a response rate of 42 %, progression-free survival or 6.6 months, and overall survival of 11.1 months [58]. Positive results were seen in another phase II study of modified docetaxel, cisplatin, and fluorouracil with bevacizumab in patients with metastatic gastroesophageal adenocarcinoma. Response rate in this study reached 67 % with a median progression-free survival of 12 months and a median overall survival of 16.8 months [59].
The encouraging results seen in phase II studies of chemotherapy and bevacizumab led to the AVAGAST trial, which was an international randomized phase III trial comparing capecitabine and cisplatin chemotherapy with placebo versus capecitabine and cisplatin with bevacizumab in the first-line treatment of advanced gastric cancer [60]. The addition of bevacizumab to chemotherapy was associated with significant increases in progression-free survival (6.7 vs. 5.3 months, p = 0.0037) and overall response rate (46.0 vs. 37.4 %, p = 0.0315). However, the primary endpoint was overall survival, and the median overall survival was 12.1 months (95 % CI, 11.1–13.8 months) in the bevacizumab group and 10.1 months (95 % CI, 9.0–11.3 months) in the placebo group (p = 0.1002). Thus, overall this was a negative study. The percentage of patients with grade 3 or greater toxicity was not significantly different between the two groups (77 % in the placebo group and 76 % in the bevacizumab group). Treatment-related deaths occurred in 2 % of patients in the bevacizumab group and 3 % of patients in the placebo group.
Further analysis of the AVAGAST trial suggested that certain subgroups may have benefited from the addition of bevacizumab to chemotherapy. Patients enrolled in North America and Latin America appeared to have a survival benefit with the addition of bevacizumab (median, 11.5 versus 6.8 months for placebo-chemotherapy; HR, 0.63; 95 % CI, 0.43–0.94), whereas patients enrolled in Asia (90 % from Japan and Korea) appeared to have no benefit (HR, 0.97; 95 % CI, 0.75–1.25). European patients had intermediate results (HR, 0.85; 95 % CI, 0.63–1.14). Further subgroups that may have benefited from the addition of bevacizumab included patients with locally advanced disease compared to metastatic disease and patient with nonmeasurable disease compared to those with measurable disease.
In a subsequent study, Shah et al. examined blood and tumor samples from patients in the AVAGAST trial for biomarkers including plasma levels of VEGF-A and protein expression of NRP-1, VEGFR-1, and VEGFR-2. They found that in non-Asian patients, high baseline plasma VEGF-A levels and low baseline neuropilin-1 levels showed a trend toward improved overall survival [53]. These results coupled with the the finding from subgroup analysis that the addition of bevacizumab to chemotherapy may have some benefit in American patients suggest that the VEGF-A pathway may have varying importance in gastric cancer depending on race. We recently measured levels of serum VEGF-A in 181 Caucasian patients and 115 Asian patients treated at two institutions prior to potentially curative surgical resection for gastric cancer (data submitted for publication). Caucasians had a median VEGF-A level that was 95 % higher than that of Asians as well as a much higher standard deviation (88.1 ± 6,206 vs. 45.2 ± 76.3 pg/ml, p < 0.001). In Caucasian patients, preoperative VEGF-A levels were inversely correlated with overall survival, while in Asian patients, there was no difference in overall survival based on the VEGF-A level.
Biomarkers have been investigated in other phase III clinical trials of bevacizumab for other solid tumors [61]. In summary, these studies show that circulating levels of short vascular endothelial growth factor VEGF-A isoforms, expression of VEGFR-1 in tumors or plasma, and genetic variants in VEGF-A or its receptors are potential biomarker candidates. However, none of these potential biomarkers have been validated or implemented into clinical practice.
Another phase III trial in China called the AVATAR trial examined the efficacy of capecitabine and cisplatin with or without bevacizumab in patients with locally advanced or metastatic gastric or gastroesophageal junction cancer [62]. This study found that the addition of bevacizumab to capecitabine and cisplatin did not significantly improve progression-free or overall survival. Median progression-free survival was 6.3 months with bevacizumab versus 6.0 months with placebo (HR 0.89, 95 % CI 0.66–1.21), and median overall survival was 10.5 months with bevacizumab versus 11.4 with placebo (HR 1.11, 95 % CI 0.79–1.56; p = 0.5567). Thus, the results of the AVATAR study were consistent with the results seen in the Asian patients in the AVAGAST study. Grade 3–5 adverse events and serious adverse events were 60 and 19 % for the bevacizumab group and 68 vs. 21 % for placebo group.
There is one ongoing phase III trial examining the role of bevacizumab in the adjuvant setting for resectable gastric or gastroesophageal junction cancer. The MAGIC-B trial in Europe is randomizing patients to perioperative epirubicin, cisplatin, and capecitabine (ECX) chemotherapy with or without bevacizumab [63]. Safety results from 200 randomized patients showed that the rate of complications in the two arms was similar. Gastrointestinal perforation (three in the ECX group and one in the ECX plus bevacizumab group) and cardiac events (one in the ECX group and four in the ECX plus bevacizumab group) were uncommon [64].
Receptor Tyrosine Kinase Inhibitors
A number of studies have examined the combination of small molecule inhibitors of VEGF-A receptors alone or in combination with chemotherapy for advanced gastric cancer. For example, Bang et al. reported on a phase II study of sunitinib alone as second-line treatment for advanced gastric cancer. The response rate was only 2.6 %, progression-free survival was 2.3 months, and overall survival was 6.8 months. Thus, single-agent sunitinib was determined to be of insufficient clinical value as second-line treatment for advanced gastric cancer [65]. The Eastern Cooperative Oncology Group (ECOG) performed a phase II study of sorafenib in combination with docetaxel and cisplatin in the first-line treatment of metastatic or advanced gastric and gastroesophageal junction adenocarcinoma. This study showed an overall response rate of 41 %, progression-free survival of 5.8 months, and overall survival of 13.6 months [66]. There have been no phase III trials demonstrating a survival benefit for the addition of VEGF-A receptor tyrosine kinase inhibitors to chemotherapy for advanced gastric cancer.
VEGFR Antibodies: Ramucirumab
There have been two recent positive studies examining the VEGFR-2 antibody ramucirumab for advanced gastric cancer. Ramucirumab is a fully human monoclonal antibody that neutralizes VEGFR-2. A phase I study with patients with advanced solid tumors including gastric cancer showed ramucirumab may have a favorable therapeutic index in treating malignancies amenable to VEGFR-2 inhibition [67]. This led to the REGARD study, which was an international phase III trial that randomized patients with advanced gastric or gastroesophageal junction adenocarcinoma with disease progression after first-line chemotherapy to ramucirumab or placebo in a 2:1 ratio [68]. Median overall survival was 5.2 months in the ramucirumab group and 3.8 months in the placebo group (p = 0.047). Thus, ramucirumab validated VEGFR-2 as a therapeutic target for advanced gastric cancer.
To determine whether ramucirumab could improve the efficacy of chemotherapy, the RAINBOW trial examined patients with similar inclusion criteria as the REGARD trial, namely patients with advanced gastric or gastroesophageal junction adenocarcinoma who had failed first-line chemotherapy [69]. This trial randomized 665 patients to paclitaxel plus ramucirumab or paclitaxel plus placebo, and median overall survival was significantly improved from 7.4 to 9.6 months with the addition of ramucirumab (p = 0.017). Ramucirumab led to a higher incidence of grade 3 or greater neutropenia (40.7 vs. 18.8 % with paclitaxel alone), leukopenia (17.4 vs. 6.7 % with paclitaxel alone), hypertension (14.7 vs. 2.7 % with paclitaxel alone), and fatigue (11.9 vs. 5.5 % with paclitaxel alone).
Thus, as the second-line therapy in advanced gastric cancer, VEGFR-2 inhibition with ramucirumab alone compared to best supportive care or ramucirumab plus chemotherapy compared to chemotherapy alone can increase overall survival. It is unclear why ramucirumab has been found to be effective for advanced gastric cancer while bevacizumab has not. Perhaps there is some advantage to blocking the primary VEGF-A receptor, VEGFR-2, as opposed to blocking the ligand, VEGF-A, but such an advantage has not been supported in animal models and has not been found in other solid tumors. There are growing data on the differences in the biology of gastric cancer in Asians and non-Asians [70], and perhaps the VEGF-A pathway plays a more prominent role in non-Asians. Nearly half of the patients in the AVAGAST study and all the patients in the AVATAR study were Asian. In contrast, only 7 % of the patients in the REGARD trial were Asian. Thus, it is possible that the varying importance of the VEGF-A pathway in Asians and non-Asians explains the dichotomous results of these studies.
Conclusion
The VEGF-A pathway is one of the most important pathways in promoting tumor angiogenesis and is now a validated target in advanced gastric cancer, at least in the second-line setting. While the VEGF-A pathway is important in gastric cancer angiogenesis, there is enough redundancy in the proangiogenic pathways that blocking VEGF-A alone has only a modest effect in slowing the growth of advanced gastric cancer and a somewhat greater effect when combined with chemotherapy. As noted previously, blocking VEGFR-2 with ramucirumab in advanced gastric cancer patients who have progressed on first-line chemotherapy leads to an overall survival benefit compared to best supportive care of 1.4 months, and adding ramucirumab to chemotherapy improves overall survival compared to chemotherapy alone by 2.2 months. Large phase III clinical trials have not demonstrated a benefit in adding bevacizumab to chemotherapy in the first-line treatment of advanced gastric cancer, but there may be a benefit in non-Asian compared to Asian patients, and this possibility requires further exploration. As we look into the future of VEGF-A inhibition in patients with gastric cancer, we will need to determine which patients will benefit from targeting the VEGF-A pathway, what mechanisms lead to the increase in efficacy of chemotherapy following the addition of VEGF-A inhibition, and what intrinsic and acquired escape pathways are used.
References
Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6.
Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803.
Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–80.
Rapisarda A, Melillo G. Role of the VEGF/VEGFR axis in cancer biology and therapy. Adv Cancer Res. 2012;114:237–67.
Ferrara N. The role of vascular endothelial growth factor in angiogenesis. New York: Marcel Dekker, Inc.; 2001.
Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–71.
Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–83.
Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–8.
Ellis LM. The role of neuropilins in cancer. Mol Cancer Ther. 2006;5:1099–107.
Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–60.
Achen MG, Stacker SA. Tumor lymphangiogenesis and metastatic spread-new players begin to emerge. Int J Cancer. 2006;119:1755–60.
Presta LG, Chen H, O’Connor SJ, Chisholm V, Meng YG, Krummen L, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–9.
Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–34.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42.
Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–76.
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50.
Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–5.
Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987–9.
Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62.
Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O’Reilly MS, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–86.
Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–24.
Pietras K, Hanahan D. A multitargeted, metronomic, and maximum-tolerated dose “chemo-switch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J Clin Oncol. 2005;23:939–52.
Folkins C, Man S, Xu P, Shaked Y, Hicklin DJ, Kerbel RS. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67:3560–4.
Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82.
Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68.
Yu JL, Rak JW, Coomber BL, Hicklin DJ, Kerbel RS. Effect of p53 status on tumor response to antiangiogenic therapy. Science. 2002;295:1526–8.
Suzuki H, et al. Paracrine upregulation of VEGF receptor mRNA in endothelial cells by hypoxia-exposed hep G2 cells. Am J Physiol. 1999;276(1 Pt 1):G92–7.
Suzuki. H, Mori M, Kawaguchi C, Adachi M, Miura S, Ishii H. Serum vascular endothelial growth factor in the course of transcatheter arterial embolization of hepatocellular carcinoma. Int J Oncol. 1999;14:1087–90.
Kaur B, Tan C, Brat DJ, Post DE, Van Meir EG. Genetic and hypoxic regulation of angiogenesis in gliomas. J Neurooncol. 2004;70:229–43.
Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603.
Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309.
Relf M, LeJeune S, Scott PA, Fox S, Smith K, Leek R, et al. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997;57:963–9.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Lee J, Demissie K, Lu SE, Rhoads GG. Cancer incidence among Korean-American immigrants in the United States and native Koreans in South Korea. Cancer Control. 2007;14:78–85.
Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol. 2014;20:4483–90.
de Martel C, Forman D, Plummer M. Gastric cancer: epidemiology and risk factors. Gastroenterol Clin North Am. 2013;42:219–40.
Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–9.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97.
Sun P, Yu H, Zhang WQ, Hu M, Lv R. Lentivirus-mediated siRNA targeting VEGF inhibits gastric cancer growth in vivo. Oncol Rep. 2012;28:1687–92.
Ninomiya S, Inomata M, Tajima M, Ali AT, Ueda Y, Shiraishi N, et al. Effect of bevacizumab, a humanized monoclonal antibody to vascular endothelial growth factor, on peritoneal metastasis of MNK-45P human gastric cancer in mice. J Surg Res. 2009;154:196–202.
Jung YD, Mansfield PF, Akagi M, Takeda A, Liu W, Bucana CD, et al. Effects of combination anti-vascular endothelial growth factor receptor and anti-epidermal growth factor receptor therapies on the growth of gastric cancer in a nude mouse model. Eur J Cancer. 2002;38:1133–40.
Takeda M, Arao T, Yokote H, Komatsu T, Yanagihara K, Sasaki H, et al. AZD2171 shows potent antitumor activity against gastric cancer over-expressing fibroblast growth factor receptor 2/keratinocyte growth factor receptor. Clin Cancer Res. 2007;13:3051–7.
Singh R, Kim WJ, Kim PH, Hong HJ. Combined blockade of HER2 and VEGF exerts greater growth inhibition of HER2-overexpressing gastric cancer xenografts than individual blockade. Exp Mol Med. 2013;45:e52.
Li H, Adachi Y, Yamamoto H, Min Y, Ohashi H, Ii M, et al. Insulin-like growth factor-I receptor blockade reduces tumor angiogenesis and enhances the effects of bevacizumab for a human gastric cancer cell line, MKN45. Cancer. 2011;117:3135–47.
Choi HJ, Kim YJ, Lee S, Kim YS. A heterodimeric Fc-based bispecific antibody simultaneously targeting VEGFR-2 and Met exhibits potent anti-tumor activity. Mol Cancer Ther. 2013;12:2748–59.
Yoon YK, Im SA, Min A, Kim HP, Hur HS, Lee KH, et al. Sunitinib synergizes the antitumor effect of cisplatin via modulation of ERCC1 expression in models of gastric cancer. Cancer Lett. 2012;321:128–36.
Hyodo I, Doi T, Endo H, Hosokawa Y, Nishikawa Y, Tanimizu M, et al. Clinical significance of plasma vascular endothelial growth factor in gastrointestinal cancer. Eur J Cancer. 1998;34:2041–5.
Eroglu A, Demirci S, Ayyildiz A, Kocaoglu H, Akbulut H, Akgul H, et al. Serum concentrations of vascular endothelial growth factor and nitrite as an estimate of in vivo nitric oxide in patients with gastric cancer. Br J Cancer. 1999;80:1630–4.
Yoshikawa T, Tsuburaya A, Kobayashi O, Sairenji M, Motohashi H, Yanoma S, et al. Plasma concentrations of VEGF and bFGF in patients with gastric carcinoma. Cancer Lett. 2000;153:7–12.
Karayiannakis AJ, Syrigos KN, Polychronidis A, Zbar A, Kouraklis G, Simopoulos C, et al. Circulating VEGF levels in the serum of gastric cancer patients: correlation with pathological variables, patient survival, and tumor surgery. Ann Surg. 2002;236:37–42.
Vidal O, Metges JP, Elizalde I, Valentini M, Volant A, Molina R, et al. High preoperative serum vascular endothelial growth factor levels predict poor clinical outcome after curative resection of gastric cancer. Br J Surg. 2009;96:1443–51.
Seo HY, Park JM, Park KH, Kim SJ, Oh SC, Kim BS, et al. Prognostic significance of serum vascular endothelial growth factor per platelet count in unresectable advanced gastric cancer patients. Jpn J Clin Oncol. 2010;40:1147–53.
Van Cutsem E, de Haas S, Kang YK, Ohtsu A, Tebbutt NC, Ming Xu J, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol. 2012;30:2119–27.
Villarejo-Campos P, Padilla-Valverde D, Martin RM, Menendez-Sanchez P, Cubo-Cintas T, Bondia-Navarro JA, et al. Serum VEGF and VEGF-C values before surgery and after postoperative treatment in gastric cancer. Clin Transl Oncol. 2013;15:265–70.
Ohta M, Konno H, Tanaka T, Baba M, Kamiya K, Syouji T, et al. The significance of circulating vascular endothelial growth factor (VEGF) protein in gastric cancer. Cancer Lett. 2003;192:215–25.
Peng L, Zhan P, Zhou Y, Fang W, Zhao P, Zheng Y, et al. Prognostic significance of vascular endothelial growth factor immunohistochemical expression in gastric cancer: a meta-analysis. Mol Biol Rep. 2012;39:9473–84.
Shah MA, Ramanathan RK, Ilson DH, Levnor A, D’Adamo D, O’Reilly E, et al. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2006;24:5201–6.
El-Rayes BF, Zalupski M, Bekai-Saab T, Heilbrun LK, Hammad N, Patel B, et al. A phase II study of bevacizumab, oxaliplatin, and docetaxel in locally advanced and metastatic gastric and gastroesophageal junction cancers. Ann Oncol. 2010;21:1999–2004.
Shah MA, Jhawer M, Ilson DH, Lefkowitz RA, Robinson E, Capanu M, et al. Phase II study of modified docetaxel, cisplatin, and fluorouracil with bevacizumab in patients with metastatic gastroesophageal adenocarcinoma. J Clin Oncol. 2011;29:868–74.
Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968–76.
Lambrechts D, Lenz HJ, de Haas S, Carmeliet P, Scherer SJ, et al. Markers of response for the antiangiogenic agent bevacizumab. J Clin Oncol. 2013;31:1219–30.
Shen L, Li J, Xu J, Pan H, Dai G, Qin S, et al., Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: randomized, double-blind, phase III study (AVATAR study). Gastric Cancer. 2014 [Epub ahead of print].
Smyth EC, Langley RE, Stenning SP, Stevenson L, Allum WH, Grabsch H, et al. ST03: A randomized trial of perioperative epirubicin, cisplatin plus capecitabine (ECX) with or without bevacizumab (B) in patients (pts) with operable gastric, oesophagogastric junction (OGJ) or lower oesophageal adenocarcinoma. J Clin Oncol 2012;30 (suppl; abstr TPS4143).
Okines AF, Langley RE, Thompson LC, Stenning SP, Stevenson L, Falk S, et al. Bevacizumab with peri-operative epirubicin, cisplatin and capecitabine (ECX) in localised gastro-oesophageal adenocarcinoma: a safety report. Ann Oncol. 2013;24:702–9.
Bang YJ, Kang YK, Kang WK, Boku N, Chung HC, Chen JS, et al. Phase II study of sunitinib as second-line treatment for advanced gastric cancer. Invest New Drugs. 2011;29:1449–58.
Sun W, Powell M, O’Dwyer PJ, Catalano P, Ansari RH, Benson AB 3rd. Phase II study of sorafenib in combination with docetaxel and cisplatin in the treatment of metastatic or advanced gastric and gastroesophageal junction adenocarcinoma: ECOG 5203. J Clin Oncol. 2010;28:2947–51.
Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010;28:780–7.
Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–9.
Wilke H, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S et al. RAINBOW: A global, phase III, randomized, double-blind study of ramucirumab plus paclitaxel versus placebo plus paclitaxel in the treatment of metastatic gastroesophageal junction (GEJ) and gastric adenocarcinoma following disease progression on first-line platinum- and fluoropyrimidine-containing combination therapy rainbow IMCL CP12-0922 (I4T-IE-JVBE). J Clin Oncol. 2014;32 (suppl 3;abstr LBA7).
Strong VE, Song KY, Park CH, Jacks LM, Gonen M, Shah M, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg. 2010;251:640–6.
Enzinger PC, Kwak EL, Szymonifka J, Abrams TA, Regan E, Malinowski P et al. Multicenter randomized phase II trial of cisplatin, irinotecan plus bevacizumab (PCA) versus docetaxel, cisplatin, irinotecan plus bevacizumab (TPCA) in patients (pts) with metastatic esophagogastric cancer (MEGCA). J Clin Oncol. 2012;30 (suppl; abstr 4027).
Shen L, Li J, Xu JM, Pan HM, Dai G, Qin S et al. Efficacy and tolerability of bevacizumab (BEV) plus capecitabine and cisplatin (XP) in Chinese patients (pts) with locally advanced or metastatic gastric/gastroesophageal junction cancer (AGC): Results from the AVATAR study. J Clin Oncol. 2012;30 (suppl 4; abstr 73).
Uronis HE, Bendell JC, Altomare I, Blobe GC, Hsu SD, Morse MA, et al. A phase II study of capecitabine, oxaliplatin, and bevacizumab in the treatment of metastatic esophagogastric adenocarcinomas. Oncologist. 2013;18:271–2.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, D.J., Thomas, N.J., Yoon, C. et al. Vascular Endothelial Growth Factor A Inhibition in Gastric Cancer. Gastric Cancer 18, 33–42 (2015). https://doi.org/10.1007/s10120-014-0397-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-014-0397-4