Abstract
Introduction
The density and distribution of the tumor immune microenvironment associated with brain metastases (BM) from gynecologic malignancies are unknown and have not been previously reported. We sought to describe the clinical features of a cohort of patients with BM from gynecologic malignancies and to characterize the tumor immune microenvironment from available archival surgical specimens.
Methods
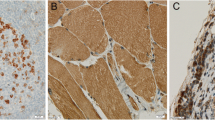
We performed a retrospective review of electronic medical records from 2002 to 2018 for patients with BM from gynecologic malignancies. Data on patient characteristics, treatment regimens, and clinical outcomes were procured. CD4, CD8, CD45RO, CD68, CD163, and FOXP3 immunohistochemistry were evaluated from available archival surgical specimens from primary disease site and neurosurgical resection.
Results
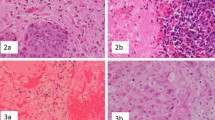
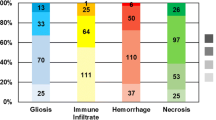
A cohort of 44 patients with BM from gynecologic malignancies was identified, 21 (47.7%) endometrial primaries and 23 (52.3%) ovarian primaries. Tumor-infiltrating lymphocytes (TILs) and tumor-associated macrophages (TAMs) were evaluated in 13 primary cases and 15 BM cases. For the 13 primary cases, CD4+ TILs were evident in 76.9% of cases, CD8+ in 92.3%, CD45RO+ in 92.3%, and FOXP3+ in 46.2%, as well as CD68+ TAMs in 100% and CD163+ in 100%. For the 15 BM cases, CD4+ TILs were evident in 60.0% of cases, CD8+ in 93.3%, CD45RO+ in 73.3%, and FOXP3+ in 35.7%, as well as CD68+ TAMs in 86.7% and CD163+ in 100%.
Conclusion
An active tumor immune microenvironment is present with similar distribution in the primary disease site and BM from patients with gynecologic malignancies.





Similar content being viewed by others
Availability of data and material
All data included herein.
References
Deng K, Yang C, Tan Q et al (2018) Sites of distant metastases and overall survival in ovarian cancer: a study of 1481 patients. Gynecol Oncol 150:460–465. https://doi.org/10.1016/j.ygyno.2018.06.022
Mao W, Wei S, Yang H et al (2020) Clinicopathological study of organ metastasis in endometrial cancer. Future Oncol Lond Engl 16:525–540. https://doi.org/10.2217/fon-2020-0017
Anupol N, Ghamande S, Odunsi K et al (2002) Evaluation of prognostic factors and treatment modalities in ovarian cancer patients with brain metastases. Gynecol Oncol 85:487–492
Chura JC, Marushin R, Boyd A et al (2007) Multimodal therapy improves survival in patients with CNS metastasis from uterine cancer: a retrospective analysis and literature review. Gynecol Oncol 107:79–85. https://doi.org/10.1016/j.ygyno.2007.05.027
Uccella S, Morris JM, Multinu F et al (2016) Primary brain metastases of endometrial cancer: a report of 18 cases and review of the literature. Gynecol Oncol 142:70–75. https://doi.org/10.1016/j.ygyno.2016.04.013
Gien LT, Kwon JS, D’Souza DP et al (2004) Brain metastases from endometrial carcinoma: a retrospective study. Gynecol Oncol 93:524–528. https://doi.org/10.1016/j.ygyno.2004.02.006
Divine LM, Kizer NT, Hagemann AR et al (2016) Clinicopathologic characteristics and survival of patients with gynecologic malignancies metastatic to the brain. Gynecol Oncol 142:76–82. https://doi.org/10.1016/j.ygyno.2016.04.030
Yano H, Nagao S, Yamaguchi S (2020) Leptomeningeal metastases arising from gynecological cancers. Int J Clin Oncol 25:391–395. https://doi.org/10.1007/s10147-019-01556-1
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264. https://doi.org/10.1038/nrc3239
Patel SP, Kurzrock R (2015) PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther 14:847–856. https://doi.org/10.1158/1535-7163.MCT-14-0983
Fridman WH, Pagès F, Sautès-Fridman C, Galon J (2012) The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12:298–306. https://doi.org/10.1038/nrc3245
Taube JM, Klein A, Brahmer JR et al (2014) Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 20:5064–5074. https://doi.org/10.1158/1078-0432.CCR-13-3271
Lorger M, Andreou T, Fife C, James F (2019) Immune checkpoint blockade—how does it work in brain metastases? Front Mol Neurosci. https://doi.org/10.3389/fnmol.2019.00282
Berghoff AS, Preusser M (2015) The inflammatory microenvironment in brain metastases: potential treatment target? Chin Clin Oncol 4:21. https://doi.org/10.3978/j.issn.2304-3865.2015.06.03
Berghoff AS, Ricken G, Widhalm G et al (2015) Tumour-infiltrating lymphocytes and expression of programmed death ligand 1 (PD-L1) in melanoma brain metastases. Histopathology 66:289–299. https://doi.org/10.1111/his.12537
Herrera-Rios D, Mughal SS, Teuber-Hanselmann S et al (2020) Macrophages/microglia represent the major source of indolamine 2,3-dioxygenase expression in melanoma metastases of the brain. Front Immunol 11:120. https://doi.org/10.3389/fimmu.2020.00120
Kluger HM, Zito CR, Barr ML et al (2015) Characterization of PD-L1 expression and associated T-cell infiltrates in metastatic melanoma samples from variable anatomic sites. Clin Cancer Res Off J Am Assoc Cancer Res 21:3052–3060. https://doi.org/10.1158/1078-0432.CCR-14-3073
Taggart D, Andreou T, Scott KJ et al (2018) Anti-PD-1/anti-CTLA-4 efficacy in melanoma brain metastases depends on extracranial disease and augmentation of CD8+ T cell trafficking. Proc Natl Acad Sci U S A 115:E1540–E1549. https://doi.org/10.1073/pnas.1714089115
Batur S, Dulger O, Durak S et al (2019) Concordance of PD-L1 expression and CD8+ TIL intensity between NSCLC and synchronous brain metastases. Bosn J Basic Med Sci. https://doi.org/10.17305/bjbms.2019.4474
Kim R, Keam B, Kim S et al (2019) Differences in tumor microenvironments between primary lung tumors and brain metastases in lung cancer patients: therapeutic implications for immune checkpoint inhibitors. BMC Cancer 19:19. https://doi.org/10.1186/s12885-018-5214-8
Mansfield AS, Aubry MC, Moser JC et al (2016) Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann Oncol Off J Eur Soc Med Oncol 27:1953–1958. https://doi.org/10.1093/annonc/mdw289
Pocha K, Mock A, Rapp C et al (2020) Surfactant expression defines an inflamed subtype of lung adenocarcinoma brain metastases that correlates with prolonged survival. Clin Cancer Res Off J Am Assoc Cancer Res. https://doi.org/10.1158/1078-0432.CCR-19-2184
Téglási V, Pipek O, Lózsa R et al (2019) PD-L1 expression of lung cancer cells, unlike infiltrating immune cells, is stable and unaffected by therapy during brain metastasis. Clin Lung Cancer 20:363-369.e2. https://doi.org/10.1016/j.cllc.2019.05.008
Téglási V, Reiniger L, Fábián K et al (2017) Evaluating the significance of density, localization, and PD-1/PD-L1 immunopositivity of mononuclear cells in the clinical course of lung adenocarcinoma patients with brain metastasis. Neuro-Oncol 19:1058–1067. https://doi.org/10.1093/neuonc/now309
Zhou J, Gong Z, Jia Q et al (2018) Programmed death ligand 1 expression and CD8+ tumor-infiltrating lymphocyte density differences between paired primary and brain metastatic lesions in non-small cell lung cancer. Biochem Biophys Res Commun 498:751–757. https://doi.org/10.1016/j.bbrc.2018.03.053
Duchnowska R, Pęksa R, Radecka B et al (2016) Immune response in breast cancer brain metastases and their microenvironment: the role of the PD-1/PD-L axis. Breast Cancer Res BCR 18:43. https://doi.org/10.1186/s13058-016-0702-8
Cimino-Mathews A, Ye X, Meeker A et al (2013) Metastatic triple-negative breast cancers at first relapse have fewer tumor-infiltrating lymphocytes than their matched primary breast tumors: a pilot study. Hum Pathol 44:2055–2063. https://doi.org/10.1016/j.humpath.2013.03.010
Lee M, Heo S-H, Song IH et al (2019) Presence of tertiary lymphoid structures determines the level of tumor-infiltrating lymphocytes in primary breast cancer and metastasis. Mod Pathol 32:70–80. https://doi.org/10.1038/s41379-018-0113-8
Liu Y, Komohara Y, Domenick N et al (2012) Expression of antigen processing and presenting molecules in brain metastasis of breast cancer. Cancer Immunol Immunother CII 61:789–801. https://doi.org/10.1007/s00262-011-1137-9
Ogiya R, Niikura N, Kumaki N et al (2017) Comparison of immune microenvironments between primary tumors and brain metastases in patients with breast cancer. Oncotarget 8:103671–103681. https://doi.org/10.18632/oncotarget.22110
Sobottka B, Pestalozzi B, Fink D et al (2016) Similar lymphocytic infiltration pattern in primary breast cancer and their corresponding distant metastases. Oncoimmunology 5:e1153208. https://doi.org/10.1080/2162402X.2016.1153208
Zhu L, Narloch JL, Onkar S et al (2019) Metastatic breast cancers have reduced immune cell recruitment but harbor increased macrophages relative to their matched primary tumors. J Immunother Cancer 7:265. https://doi.org/10.1186/s40425-019-0755-1
Berghoff AS, Ricken G, Wilhelm D et al (2016) Tumor infiltrating lymphocytes and PD-L1 expression in brain metastases of small cell lung cancer (SCLC). J Neurooncol 130:19–29. https://doi.org/10.1007/s11060-016-2216-8
Roussille P, Tachon G, Villalva C et al (2018) Pathological and molecular characteristics of colorectal cancer with brain metastases. Cancers. https://doi.org/10.3390/cancers10120504
Dahlin AM, Henriksson ML, Van Guelpen B et al (2011) Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod Pathol 24:671–682. https://doi.org/10.1038/modpathol.2010.234
Pakneshan S, Safarpour D, Tavassoli F, Jabbari B (2014) Brain metastasis from ovarian cancer: a systematic review. J Neurooncol 119:1–6. https://doi.org/10.1007/s11060-014-1447-9
Pothuri B, Chi DS, Reid T et al (2002) Craniotomy for central nervous system metastases in epithelial ovarian carcinoma. Gynecol Oncol 87:133–137
Berghoff AS, Fuchs E, Ricken G et al (2016) Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology 5:e1057388. https://doi.org/10.1080/2162402X.2015.1057388
Adeegbe DO, Nishikawa H (2013) Natural and induced T regulatory cells in cancer. Front Immunol 4:190. https://doi.org/10.3389/fimmu.2013.00190
Webb JR, Milne K, Kroeger DR, Nelson BH (2016) PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol Oncol 141:293–302. https://doi.org/10.1016/j.ygyno.2016.03.008
Hendry S, Salgado R, Gevaert T et al (2017) Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in Melanoma, Gastrointestinal Tract Carcinomas, Non-Small Cell Lung Carcinoma and Mesothelioma, Endometrial and Ovarian Carcinomas, Squamous Cell Carcinoma of the Head and Neck, Genitourinary Carcinomas, and Primary Brain Tumors. Adv Anat Pathol 24:311–335. https://doi.org/10.1097/PAP.0000000000000161
Akbay EA, Koyama S, Carretero J et al (2013) Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 3:1355–1363. https://doi.org/10.1158/2159-8290.CD-13-0310
Brastianos PK, Carter SL, Santagata S et al (2015) Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov 5:1164–1177. https://doi.org/10.1158/2159-8290.CD-15-0369
Lee CC, Soon YY, Lum JHY et al (2020) Frequency of discordance in programmed death-ligand 1 (PD-L1) expression between primary tumors and paired distant metastases in advanced cancers: a systematic review and meta-analysis. Acta Oncol. https://doi.org/10.1080/0284186X.2020.1741678
Ratner E, Bala M, Louie-Gao M et al (2019) Increased risk of brain metastases in ovarian cancer patients with BRCA mutations. Gynecol Oncol 153:568–573. https://doi.org/10.1016/j.ygyno.2019.03.004
Stasenko M, Cybulska P, Feit N et al (2019) Brain metastasis in epithelial ovarian cancer by BRCA1/2 mutation status. Gynecol Oncol 154:144–149. https://doi.org/10.1016/j.ygyno.2019.05.004
Balendran S, Liebmann-Reindl S, Berghoff AS et al (2017) Next-Generation Sequencing-based genomic profiling of brain metastases of primary ovarian cancer identifies high number of BRCA-mutations. J Neurooncol 133:469–476. https://doi.org/10.1007/s11060-017-2459-z
Sekine M, Yoshihara K, Komata D et al (2013) Increased incidence of brain metastases in BRCA1-related ovarian cancers. J Obstet Gynaecol Res 39:292–296. https://doi.org/10.1111/j.1447-0756.2012.01961.x
Colombo I, Garg S, Danesh A et al (2019) Heterogeneous alteration of the ERBB3-MYC axis associated with MEK inhibitor resistance in a KRAS-mutated low-grade serous ovarian cancer patient. Cold Spring Harb Mol Case Stud. https://doi.org/10.1101/mcs.a004341
Bangham M, Goldstein R, Walton H, Ledermann JA (2016) Olaparib treatment for BRCA-mutant ovarian cancer with leptomeningeal disease. Gynecol Oncol Rep 18:22–24. https://doi.org/10.1016/j.gore.2016.10.004
Gray S, Khor XY, Yiannakis D (2019) Niraparib as maintenance therapy in a patient with ovarian cancer and brain metastases. BMJ Case Rep. https://doi.org/10.1136/bcr-2019-230738
Lin NU, Lee EQ, Aoyama H et al (2015) Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 16:e270-278. https://doi.org/10.1016/S1470-2045(15)70057-4
Acknowledgements
We thank Dana-Farber/Harvard Cancer Center in Boston, MA, for the use of the Specialized Histopathology Core, which provided histology and immunohistochemistry service. Dana-Farber/Harvard Cancer Center is supported in part by an NCI Cancer Center Support Grant # NIH 5 P30 CA06516.
Funding
This study was funded by Department of Obstetrics, Gynecology, and Reproductive Science Innovation Award; Icahn School of Medicine at Mount Sinai.
Author information
Authors and Affiliations
Contributions
Corey M. Gill contributed to conceptualization, methodology, formal analysis, investigation, data curation, writing—original draft, writing—review and editing, visualization, and funding acquisition; Megan D’Andrea, Shannon Tomita, Jessa Suhner, Melissa Umphlett, and Konstantin Zakashansky contributed to investigation and writing—review and editing; Stephanie Blank, Nadejda Tsankova, and Raj Shrivastava contributed to resources, writing—review and editing, and supervision; Mary Fowkes contributed to conceptualization, methodology, resources, writing—review and editing, and supervision; Valentin Kolev contributed to conceptualization, methodology, resources, writing—original draft, writing—review and editing, supervision, and funding acquisition.
Corresponding author
Ethics declarations
Ethics approval
Ethics approval was obtained from Icahn School of Medicine at Mount Sinai. Written informed consent was waived.
Conflict of interest
The authors declare that there are no conflicts of interest or competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gill, C.M., D’Andrea, M.R., Tomita, S. et al. Tumor immune microenvironment in brain metastases from gynecologic malignancies. Cancer Immunol Immunother 70, 2951–2960 (2021). https://doi.org/10.1007/s00262-021-02909-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-02909-4