Abstract
Purpose
Brain metastases (BM) are a complication of advanced breast cancer (BC). Histology of melanoma BM offers prognostic value; however, understanding the microenvironment of breast cancer brain metastases (BCBM) is less characterized. This study reports on four histological biomarkers, gliosis, immune infiltrate, hemorrhage, necrosis, and their prognostic significance in BCBM.
Methods
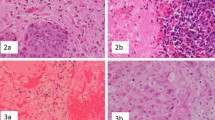
A biobank of 203 human tissues from patients who underwent craniotomy for BCBM was created across four academic institutions. Degree of gliosis, immune infiltrate, hemorrhage, and necrosis were identified and scored via representative H&E stain (0–3+). Overall survival (OS) was estimated using the Kaplan–Meier method. Cox proportional hazards regression evaluated prognostic value of the biomarkers in the context of standard clinical characteristics.
Results
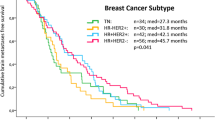
BCBM subtype (available for n = 158) was 36% Her2+, 26% hormone receptor (HR)+/Her2− 38% HR−/Her2− (triple negative, TN). Gliosis was observed in 82% (116/141) of BCBM, with immune infiltrate 44% (90/201), hemorrhage 82% (166/141), and necrosis 87% (176/201). Necrosis was significantly higher in TNBC (p < 0.01). Presence of gliosis, immune infiltrate, and hemorrhage correlated with improved OS (p = 0.03, p = 0.03, p = 0.1), while necrosis correlated with inferior OS (p = 0.01). Improved OS was associated with gliosis in TN (p = 0.02), and immune infiltrate (p = 0.001) and hemorrhage (p = 0.07) in HER2+. In a multivariable model for OS, incorporating these biomarkers with traditional clinical variables improved the model fit (p < 0.001).
Conclusion
Gliosis confers superior prognosis in TNBC BM; immune infiltrate and hemorrhage correlate with superior prognosis in HER2+ BCBM. Understanding the metastatic microenvironment of BCBM refines prognostic considerations and may unveil novel therapeutic strategies.





Similar content being viewed by others
References
Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, Goncalves A (2012) Recent trends in epidemiology of brain metastases: an overview. Anticancer Res 32(11):4655–4662
Sperduto PW, Kased N, Roberge D, Chao ST, Shanley R, Luo X et al (2013) The effect of tumor subtype on the time from primary diagnosis to development of brain metastases and survival in patients with breast cancer. J Neurooncol 112(3):467–472
Hamilton R, Krauze M, Romkes M, Omolo B, Konstantinopoulos P, Reinhart T et al (2013) Pathologic and gene expression features of metastatic melanomas to the brain. Cancer 119(15):2737–2746
Vaz-Luis I, Seah D, Olson EM, Wagle N, Metzger-Filho O, Sohl J et al (2013) Clinicopathological features among patients with advanced human epidermal growth factor-2-positive breast cancer with prolonged clinical benefit to first-line trastuzumab-based therapy: a retrospective cohort study. Clin Breast Cancer 13(4):254–263
Duchnowska R, Peksa R, Radecka B, Mandat T, Trojanowski T, Jarosz B et al (2016) Immune response in breast cancer brain metastases and their microenvironment: the role of the PD-1/PD-L axis. Breast Cancer Res 18(1):43
Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M et al (2016) Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 17(7):976–983
Neman J, Termini J, Wilczynski S, Vaidehi N, Choy C, Kowolik CM et al (2014) Human breast cancer metastases to the brain display GABAergic properties in the neural niche. Proc Natl Acad Sci USA 111(3):984–989
Gril B, Paranjape AN, Woditschka S, Hua E, Dolan EL, Hanson J et al (2018) Reactive astrocytic S1P3 signaling modulates the blood-tumor barrier in brain metastases. Nat Commun 9(1):2705
Lyle LT, Lockman PR, Adkins CE, Mohammad AS, Sechrest E, Hua E et al (2016) Alterations in pericyte subpopulations are associated with elevated blood-tumor barrier permeability in experimental brain metastasis of breast cancer. Clin Cancer Res 22(21):5287–5299
Funding
This study was funded by the NIH/NCI K23 (CKA) and NIH 5K23CA157728-05.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Adam Brufksy is a consultant for Lilly, Amgen, Hexal, Novartis, Roche, Pfizer, Immunomedics, Eisai, and Celgene, and owns stock in Bioarray Technologies. Kimberly Blackwell is employed by Lilly. Jose Pablo Leone receives research funding from Merck. Aki Morikawa receives research funding from Lilly, Genentech, Novartis, Merrimack, and Bayer. Matthew Ewend owns stock in Falcon Therapeutics. Carey Anders is an uncompensated consultant/advisory board member for Novartis, Merrimack, Lily, Nektar, Cascadian, Seattle Genetics, and Genentech, a compensated consultant/advisory board member for Merck, PUMA, and Eisai, receives unrelated research funding from Novartis, Merrimack, PUMA, Lily, Merck, Cascadian, Seattle Genetics, Nektar, Tesaro, and G1-Therapeutics, and receives honoraria from UptoDate and Jones and Bartlett Publishing. The other authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Ethical standard
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent or waiver of consent granted by local IRB was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sambade, M.J., Prince, G., Deal, A.M. et al. Examination and prognostic implications of the unique microenvironment of breast cancer brain metastases. Breast Cancer Res Treat 176, 321–328 (2019). https://doi.org/10.1007/s10549-019-05211-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05211-1