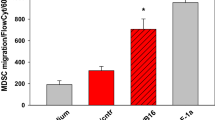
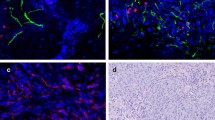
Abstract
Schwann cells are the principal glial cells of the peripheral nervous system which maintain neuronal homeostasis. Schwann cells support peripheral nerve functions and play a critical role in many pathological processes including injury-induced nerve repair, neurodegenerative diseases, infections, neuropathic pain and cancer. Schwann cells are implicated in a wide range of diseases due, in part, to their ability to interact and modulate immune cells. We discuss the accumulating examples of how Schwann cell regulation of the immune system initiates and facilitates the progression of various diseases. Furthermore, we highlight how Schwann cells may orchestrate an immunosuppressive tumor microenvironment by polarizing and modulating the activity of the dendritic cells.



Similar content being viewed by others
Abbreviations
- ADORA1:
-
Adenosine A1 receptor
- CCL:
-
C–C motif chemokine ligand
- CIDP:
-
Chronic inflammatory demyelinating polyneuropathy
- CXCL:
-
C–X–C motif chemokine ligand
- DC:
-
Dendritic cell
- EAN:
-
Experimental allergic neuritis
- ECM:
-
Extracellular matrix
- Gas6:
-
Growth arrest-specific protein 6
- GBS:
-
Guillain–Barre syndrome
- LFA:
-
Lymphocyte function-associated antigen
- LIF:
-
Leukemia inhibitory factor
- LPC:
-
Lysophosphatidylcholine
- MBP:
-
Myelin basic protein
- MCP-1:
-
Monocyte chemoattractant protein-1
- MDSC:
-
Myeloid-derived suppressor cell
- MIP:
-
Macrophage inflammatory protein
- Nox1:
-
NADPH oxidase 1
- P0:
-
Myelin protein zero
- P2RX:
-
P2X purinoceptor
- PLA2 :
-
Phospholipase A2
- PMP-22:
-
Peripheral myelin protein 22
- PNI:
-
Perineural invasion
- PNS:
-
Peripheral nervous system
- RAGE:
-
Receptor for advanced glycation end products
- SAPP:
-
Spontaneous autoimmune peripheral polyneuropathy
- SC:
-
Schwann cell
- TµE:
-
Tumor microenvironment
- TRPA1:
-
Transient receptor potential ankyrin 1
References
Jessen KR, Mirsky R (2016) The repair Schwann cell and its function in regenerating nerves. J Physiol 594:3521–3531. https://doi.org/10.1113/JP270874
Ydens E, Lornet G, Smits V, Goethals S, Timmerman V, Janssens S (2013) The neuroinflammatory role of Schwann cells in disease. Neurobiol Dis 55:95–103. https://doi.org/10.1016/j.nbd.2013.03.005
Bunimovich YL, Keskinov AA, Shurin GV, Shurin MR (2017) Schwann cells: a new player in the tumor microenvironment. Cancer Immunol Immunother 66:959–968. https://doi.org/10.1007/s00262-016-1929-z
Demir IE, Tieftrunk E, Schorn S, Saricaoglu OC, Pfitzinger PL, Teller S et al (2016) Activated Schwann cells in pancreatic cancer are linked to analgesia via suppression of spinal astroglia and microglia. Gut 65:1001–1014. https://doi.org/10.1136/gutjnl-2015-309784
Tzekova N, Heinen A, Kury P (2014) Molecules involved in the crosstalk between immune- and peripheral nerve Schwann cells. J Clin Immunol 34(Suppl 1):S86–104. https://doi.org/10.1007/s10875-014-0015-6
Deborde S, Wong RJ (2017) How Schwann cells facilitate cancer progression in nerves. Cell Mol Life Sci 74:4405–4420. https://doi.org/10.1007/s00018-017-2578-x
Martyn GV, Shurin GV, Keskinov AA, Bunimovich YL, Shurin MR (2019) Schwann cells shape the neuro-immune environs and control cancer progression. Cancer Immunol Immunother. https://doi.org/10.1007/s00262-018-02296-3
DeFrancesco-Lisowitz A, Lindborg JA, Niemi JP, Zigmond RE (2015) The neuroimmunology of degeneration and regeneration in the peripheral nervous system. Neuroscience 302:174–203. https://doi.org/10.1016/j.neuroscience.2014.09.027
Gaudet AD, Popovich PG, Ramer MS (2011) Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation 8:110. https://doi.org/10.1186/1742-2094-8-110
Lee H, Jo EK, Choi SY, Oh SB, Park K, Kim JS et al (2006) Necrotic neuronal cells induce inflammatory Schwann cell activation via TLR2 and TLR3: implication in Wallerian degeneration. Biochem Biophys Res Commun 350:742–747. https://doi.org/10.1016/j.bbrc.2006.09.108
Ozaki A, Nagai A, Lee YB, Myong NH, Kim SU (2008) Expression of cytokines and cytokine receptors in human Schwann cells. NeuroReport 19:31–35. https://doi.org/10.1097/WNR.0b013e3282f27e60
Bolin LM, Verity AN, Silver JE, Shooter EM, Abrams JS (1995) Interleukin-6 production by Schwann cells and induction in sciatic nerve injury. J Neurochem 64:850–858
Stratton JA, Holmes A, Rosin NL, Sinha S, Vohra M, Burma NE et al (2018) macrophages regulate Schwann cell maturation after nerve injury. Cell Rep. 24(2561–2572):e2566. https://doi.org/10.1016/j.celrep.2018.08.004
Reichert F, Saada A, Rotshenker S (1994) Peripheral nerve injury induces Schwann cells to express two macrophage phenotypes: phagocytosis and the galactose-specific lectin MAC-2. J Neurosci 14:3231–3245
Saada A, Reichert F, Rotshenker S (1996) Granulocyte macrophage colony stimulating factor produced in lesioned peripheral nerves induces the up-regulation of cell surface expression of MAC-2 by macrophages and Schwann cells. J Cell Biol 133:159–167. https://doi.org/10.1083/jcb.133.1.159
Armati PJ, Pollard JD, Gatenby P (1990) Rat and human Schwann cells in vitro can synthesize and express MHC molecules. Muscle Nerve 13:106–116. https://doi.org/10.1002/mus.880130204
Samuel NM, Mirsky R, Grange JM, Jessen KR (1987) Expression of major histocompatibility complex class I and class II antigens in human Schwann cell cultures and effects of infection with Mycobacterium leprae. Clin Exp Immunol 68:500–509
Kingston AE, Bergsteinsdottir K, Jessen KR, Van der Meide PH, Colston MJ, Mirsky R (1989) Schwann cells co-cultured with stimulated T cells and antigen express major histocompatibility complex (MHC) class II determinants without interferon-gamma pretreatment: synergistic effects of interferon-gamma and tumor necrosis factor on MHC class II induction. Eur J Immunol 19:177–183. https://doi.org/10.1002/eji.1830190128
Bergsteinsdottir K, Kingston A, Jessen KR (1992) Rat Schwann cells can be induced to express major histocompatibility complex class II molecules in vivo. J Neurocytol 21:382–390
Tofaris GK, Patterson PH, Jessen KR, Mirsky R (2002) Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J Neurosci 22:6696–6703. https://doi.org/10.1523/JNEUROSCI.22-15-06696.2002
Stratton JA, Shah PT (2016) Macrophage polarization in nerve injury: do Schwann cells play a role? Neural Regen Res. 11:53–57. https://doi.org/10.4103/1673-5374.175042
Taskinen HS, Roytta M (2000) Increased expression of chemokines (MCP-1, MIP-1alpha, RANTES) after peripheral nerve transection. J Peripher Nerv Syst 5:75–81
Cattin AL, Burden JJ, Van Emmenis L, Mackenzie FE, Hoving JJ, Garcia Calavia N et al (2015) Macrophage-induced blood vessels guide schwann cell-mediated regeneration of peripheral nerves. Cell 162:1127–1139. https://doi.org/10.1016/j.cell.2015.07.021
Horie H, Kadoya T, Hikawa N, Sango K, Inoue H, Takeshita K et al (2004) Oxidized galectin-1 stimulates macrophages to promote axonal regeneration in peripheral nerves after axotomy. J Neurosci 24:1873–1880. https://doi.org/10.1523/JNEUROSCI.4483-03.2004
Kwa MS, van Schaik IN, De Jonge RR, Brand A, Kalaydjieva L, van Belzen N et al (2003) Autoimmunoreactivity to Schwann cells in patients with inflammatory neuropathies. Brain 126:361–375. https://doi.org/10.1093/brain/awg030
Aranami T, Yamamura T (2013) Pathogenesis of chronic inflammatory demyelinating polyneuropathy. Nihon Rinsho 71:850–854
Armati PJ, Mathey EK (2014) Clinical implications of Schwann cell biology. J Peripher Nerv Syst. 19:14–23. https://doi.org/10.1111/jns5.12057
Armati PJ, Pollard JD (1996) Immunology of the Schwann cell. Baillieres Clin Neurol 5:47–64
Csurhes PA, Sullivan AA, Green K, Pender MP, McCombe PA (2005) T cell reactivity to P0, P2, PMP-22, and myelin basic protein in patients with Guillain–Barre syndrome and chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol Neurosurg Psychiatry 76:1431–1439. https://doi.org/10.1136/jnnp.2004.052282
Sanvito L, Makowska A, Mahdi-Rogers M, Hadden RD, Peakman M, Gregson N et al (2009) Humoral and cellular immune responses to myelin protein peptides in chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol Neurosurg Psychiatry 80:333–338. https://doi.org/10.1136/jnnp.2008.159798
Mathey EK, Park SB, Hughes RA, Pollard JD, Armati PJ, Barnett MH et al (2015) Chronic inflammatory demyelinating polyradiculoneuropathy: from pathology to phenotype. J Neurol Neurosurg Psychiatry 86:973–985. https://doi.org/10.1136/jnnp-2014-309697
Jang SY, Yoon BA, Shin YK, Yun SH, Jo YR, Choi YY et al (2017) Schwann cell dedifferentiation-associated demyelination leads to exocytotic myelin clearance in inflammatory segmental demyelination. Glia. 65:1848–1862. https://doi.org/10.1002/glia.23200
Yang M, Peyret C, Shi XQ, Siron N, Jang JH, Wu S et al (2015) Evidence from human and animal studies: pathological roles of CD8(+) T cells in autoimmune peripheral neuropathies. Front Immunol. 6:532. https://doi.org/10.3389/fimmu.2015.00532
Spierings E, de Boer T, Wieles B, Adams LB, Marani E, Ottenhoff TH (2001) Mycobacterium leprae-specific, HLA class II-restricted killing of human Schwann cells by CD4+ Th1 cells: a novel immunopathogenic mechanism of nerve damage in leprosy. J Immunol. 166:5883–5888. https://doi.org/10.4049/jimmunol.166.10.5883
Lilje O (2002) The processing and presentation of endogenous and exogenous antigen by Schwann cells in vitro. Cell Mol Life Sci 59:2191–2198
Wekerle H, Schwab M, Linington C, Meyermann R (1986) Antigen presentation in the peripheral nervous system: Schwann cells present endogenous myelin autoantigens to lymphocytes. Eur J Immunol 16:1551–1557. https://doi.org/10.1002/eji.1830161214
Meyer zu Horste G, Heidenreich H, Mausberg AK, Lehmann HC, ten Asbroek AL, Saavedra JT et al (2010) Mouse Schwann cells activate MHC class I and II restricted T-cell responses, but require external peptide processing for MHC class II presentation. Neurobiol Dis 37:483–490. https://doi.org/10.1016/j.nbd.2009.11.006
Meyer Zu Horste G, Heidenreich H, Lehmann HC, Ferrone S, Hartung HP, Wiendl H et al (2010) Expression of antigen processing and presenting molecules by Schwann cells in inflammatory neuropathies. Glia 58:80–92. https://doi.org/10.1002/glia.20903
Duan RS, Jin T, Yang X, Mix E, Adem A, Zhu J (2007) Apolipoprotein E deficiency enhances the antigen-presenting capacity of Schwann cells. Glia. 55:772–776. https://doi.org/10.1002/glia.20498
Mao XJ, Zhang XM, Zhang HL, Quezada HC, Mix E, Yang X et al (2010) TNF-alpha receptor 1 deficiency reduces antigen-presenting capacity of Schwann cells and ameliorates experimental autoimmune neuritis in mice. Neurosci Lett 470:19–23. https://doi.org/10.1016/j.neulet.2009.12.045
Pollard JD, Baverstock J, McLeod JG (1987) Class II antigen expression and inflammatory cells in the Guillain–Barre syndrome. Ann Neurol 21:337–341. https://doi.org/10.1002/ana.410210404
Mancardi GL, Cadoni A, Zicca A, Schenone A, Tabaton M, De Martini I et al (1988) HLA-DR Schwann cell reactivity in peripheral neuropathies of different origins. Neurology. 38:848–851. https://doi.org/10.1212/wnl.38.6.848
Mitchell GW, Williams GS, Bosch EP, Hart MN (1991) Class II antigen expression in peripheral neuropathies. J Neurol Sci 102:170–176
Van Rhijn I, Van den Berg LH, Bosboom WM, Otten HG, Logtenberg T (2000) Expression of accessory molecules for T-cell activation in peripheral nerve of patients with CIDP and vasculitic neuropathy. Brain 123(Pt 10):2020–2029. https://doi.org/10.1093/brain/123.10.2020
Im JS, Tapinos N, Chae GT, Illarionov PA, Besra GS, DeVries GH et al (2006) Expression of CD1d molecules by human schwann cells and potential interactions with immunoregulatory invariant NK T cells. J Immunol. 177:5226–5235. https://doi.org/10.4049/jimmunol.177.8.5226
Murata K, Dalakas MC (2000) Expression of the co-stimulatory molecule BB-1, the ligands CTLA-4 and CD28 and their mRNAs in chronic inflammatory demyelinating polyneuropathy. Brain 123(Pt 8):1660–1666. https://doi.org/10.1093/brain/123.8.1660
Kiefer R, Dangond F, Mueller M, Toyka KV, Hafler DA, Hartung HP (2000) Enhanced B7 costimulatory molecule expression in inflammatory human sural nerve biopsies. J Neurol Neurosurg Psychiatry 69:362–368. https://doi.org/10.1136/jnnp.69.3.362
Allard DE, Wang Y, Li JJ, Conley B, Xu EW, Sailer D et al (2018) Schwann cell-derived periostin promotes autoimmune peripheral polyneuropathy via macrophage recruitment. J Clin Invest. 128:4727–4741. https://doi.org/10.1172/JCI99308
Neal JW, Gasque P (2016) The role of primary infection of Schwann cells in the aetiology of infective inflammatory neuropathies. J Infect 73:402–418. https://doi.org/10.1016/j.jinf.2016.08.006
Serrano-Coll H, Salazar-Pelaez L, Acevedo-Saenz L, Cardona-Castro N (2018) Mycobacterium leprae-induced nerve damage: direct and indirect mechanisms. Pathog Dis. https://doi.org/10.1093/femspd/fty062
Fonseca AB, Simon MD, Cazzaniga RA, de Moura TR, de Almeida RP, Duthie MS et al (2017) The influence of innate and adaptative immune responses on the differential clinical outcomes of leprosy. Infect Dis Poverty. 6:5. https://doi.org/10.1186/s40249-016-0229-3
Park AJ, Rendini T, Martiniuk F, Levis WR (2016) Leprosy as a model to understand cancer immunosurveillance and T cell anergy. J Leukoc Biol 100:47–54. https://doi.org/10.1189/jlb.5RU1215-537RR
Mattos KA, Oliveira VG, D’Avila H, Rodrigues LS, Pinheiro RO, Sarno EN et al (2011) TLR6-driven lipid droplets in Mycobacterium leprae-infected Schwann cells: immunoinflammatory platforms associated with bacterial persistence. J Immunol. 187:2548–2558. https://doi.org/10.4049/jimmunol.1101344
Mattos KA, Lara FA, Oliveira VG, Rodrigues LS, D’Avila H, Melo RC et al (2011) Modulation of lipid droplets by Mycobacterium leprae in Schwann cells: a putative mechanism for host lipid acquisition and bacterial survival in phagosomes. Cell Microbiol 13:259–273. https://doi.org/10.1111/j.1462-5822.2010.01533.x
Hartlehnert M, Derksen A, Hagenacker T, Kindermann D, Schafers M, Pawlak M et al (2017) Schwann cells promote post-traumatic nerve inflammation and neuropathic pain through MHC class II. Sci Rep. 7:12518. https://doi.org/10.1038/s41598-017-12744-2
Kiguchi N, Maeda T, Kobayashi Y, Fukazawa Y, Kishioka S (2010) Macrophage inflammatory protein-1alpha mediates the development of neuropathic pain following peripheral nerve injury through interleukin-1beta up-regulation. Pain 149:305–315. https://doi.org/10.1016/j.pain.2010.02.025
Saika F, Kiguchi N, Kobayashi Y, Fukazawa Y, Kishioka S (2012) CC-chemokine ligand 4/macrophage inflammatory protein-1beta participates in the induction of neuropathic pain after peripheral nerve injury. Eur J Pain 16:1271–1280. https://doi.org/10.1002/j.1532-2149.2012.00146.x
Abdo H, Calvo-Enrique L, Lopez JM, Song J, Zhang MD, Usoskin D et al (2019) Specialized cutaneous Schwann cells initiate pain sensation. Science 365:695–699. https://doi.org/10.1126/science.aax6452
Goncalves NP, Vaegter CB, Andersen H, Ostergaard L, Calcutt NA, Jensen TS (2017) Schwann cell interactions with axons and microvessels in diabetic neuropathy. Nat Rev Neurol. 13:135–147. https://doi.org/10.1038/nrneurol.2016.201
Yagihashi S, Matsunaga M (1979) Ultrastructural pathology of peripheral nerves in patients with diabetic neuropathy. Tohoku J Exp Med 129:357–366
Mizisin AP (2014) Mechanisms of diabetic neuropathy: Schwann cells. Handb Clin Neurol. 126:401–428. https://doi.org/10.1016/B978-0-444-53480-4.00029-1
Kalichman MW, Powell HC, Mizisin AP (1998) Reactive, degenerative, and proliferative Schwann cell responses in experimental galactose and human diabetic neuropathy. Acta Neuropathol 95:47–56
Viader A, Sasaki Y, Kim S, Strickland A, Workman CS, Yang K et al (2013) Aberrant Schwann cell lipid metabolism linked to mitochondrial deficits leads to axon degeneration and neuropathy. Neuron 77:886–898. https://doi.org/10.1016/j.neuron.2013.01.012
Sbai O, Devi TS, Melone MA, Feron F, Khrestchatisky M, Singh LP et al (2010) RAGE-TXNIP axis is required for S100B-promoted Schwann cell migration, fibronectin expression and cytokine secretion. J Cell Sci 123:4332–4339. https://doi.org/10.1242/jcs.074674
Vincent AM, Callaghan BC, Smith AL, Feldman EL (2011) Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol. 7:573–583. https://doi.org/10.1038/nrneurol.2011.137
Herder C, Lankisch M, Ziegler D, Rathmann W, Koenig W, Illig T et al (2009) Subclinical inflammation and diabetic polyneuropathy: MONICA/KORA Survey F3 (Augsburg, Germany). Diabetes Care 32:680–682. https://doi.org/10.2337/dc08-2011
Conti G, Scarpini E, Baron P, Livraghi S, Tiriticco M, Bianchi R et al (2002) Macrophage infiltration and death in the nerve during the early phases of experimental diabetic neuropathy: a process concomitant with endoneurial induction of IL-1beta and p75NTR. J Neurol Sci 195:35–40
Tang W, Lv Q, Chen XF, Zou JJ, Liu ZM, Shi YQ (2013) CD8(+) T cell-mediated cytotoxicity toward Schwann cells promotes diabetic peripheral neuropathy. Cell Physiol Biochem 32:827–837. https://doi.org/10.1159/000354485
De Logu F, Nassini R, Materazzi S, Carvalho Goncalves M, Nosi D, Rossi Degl’Innocenti D et al (2017) Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice. Nat Commun. 8:1887. https://doi.org/10.1038/s41467-017-01739-2
Watkins LR, Milligan ED, Maier SF (2001) Glial activation: a driving force for pathological pain. Trends Neurosci 24:450–455
Hald A, Nedergaard S, Hansen RR, Ding M, Heegaard AM (2009) Differential activation of spinal cord glial cells in murine models of neuropathic and cancer pain. Eur J Pain 13:138–145. https://doi.org/10.1016/j.ejpain.2008.03.014
Ducourneau VR, Dolique T, Hachem-Delaunay S, Miraucourt LS, Amadio A, Blaszczyk L et al (2014) Cancer pain is not necessarily correlated with spinal overexpression of reactive glia markers. Pain 155:275–291. https://doi.org/10.1016/j.pain.2013.10.008
Zhou YQ, Liu Z, Liu HQ, Liu DQ, Chen SP, Ye DW et al (2016) Targeting glia for bone cancer pain. Expert Opin Ther Targets. 20:1365–1374. https://doi.org/10.1080/14728222.2016.1214716
Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ et al (2013) Autonomic nerve development contributes to prostate cancer progression. Science 341:1236361. https://doi.org/10.1126/science.1236361
Zhao CM, Hayakawa Y, Kodama Y, Muthupalani S, Westphalen CB, Andersen GT et al (2014) Denervation suppresses gastric tumorigenesis. Sci Transl Med 6:250ra115. https://doi.org/10.1126/scitranslmed.3009569
Peterson SC, Eberl M, Vagnozzi AN, Belkadi A, Veniaminova NA, Verhaegen ME et al (2015) Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell 16:400–412. https://doi.org/10.1016/j.stem.2015.02.006
Saloman JL, Albers KM, Li D, Hartman DJ, Crawford HC, Muha EA et al (2016) Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc Natl Acad Sci USA 113:3078–3083. https://doi.org/10.1073/pnas.1512603113
Pawlowski A, Weddell G (1967) The lability of cutaneous neural elements. Br J Dermatol 79:14–19
Dubeykovskaya Z, Si Y, Chen X, Worthley DL, Renz BW, Urbanska AM et al (2016) Neural innervation stimulates splenic TFF2 to arrest myeloid cell expansion and cancer. Nat Commun. 7:10517. https://doi.org/10.1038/ncomms10517
Kaminishi M, Shimizu N, Shimoyama S, Yamaguchi H, Tsuji E, Aoki F et al (1997) Denervation promotes the development of cancer-related lesions in the gastric remnant. J Clin Gastroenterol 25(Suppl 1):S129–S134
Seifert P, Spitznas M (2002) Axons in human choroidal melanoma suggest the participation of nerves in the control of these tumors. Am J Ophthalmol 133:711–713
Ayala GE, Dai H, Powell M, Li R, Ding Y, Wheeler TM et al (2008) Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res 14:7593–7603. https://doi.org/10.1158/1078-0432.CCR-08-1164
Albo D, Akay CL, Marshall CL, Wilks JA, Verstovsek G, Liu H et al (2011) Neurogenesis in colorectal cancer is a marker of aggressive tumor behavior and poor outcomes. Cancer 117:4834–4845. https://doi.org/10.1002/cncr.26117
Tomita T (2012) Localization of nerve fibers in colonic polyps, adenomas, and adenocarcinomas by immunocytochemical staining for PGP 9.5. Dig Dis Sci 57:364–370. https://doi.org/10.1007/s10620-011-1876-7
Terada T, Matsunaga Y (2001) S-100-positive nerve fibers in hepatocellular carcinoma and intrahepatic cholangiocarcinoma: an immunohistochemical study. Pathol Int 51:89–93
Zhou M, Patel A, Rubin MA (2001) Prevalence and location of peripheral nerve found on prostate needle biopsy. Am J Clin Pathol 115:39–43. https://doi.org/10.1309/2APJ-YKBD-97EH-67GW
Shurin GV, Kruglov O, Ding F, Lin Y, Hao X, Keskinov AA et al (2019) Melanoma-induced reprogramming of Schwann cell signaling aids tumor growth. Cancer Res 79:2736–2747. https://doi.org/10.1158/0008-5472.CAN-18-3872
Demir IE, Boldis A, Pfitzinger PL, Teller S, Brunner E, Klose N et al (2014) Investigation of Schwann cells at neoplastic cell sites before the onset of cancer invasion. J Natl Cancer Inst. https://doi.org/10.1093/jnci/dju184
Deborde S, Omelchenko T, Lyubchik A, Zhou Y, He S, McNamara WF et al (2016) Schwann cells induce cancer cell dispersion and invasion. J Clin Invest. 126:1538–1554. https://doi.org/10.1172/JCI82658
Sroka IC, Chopra H, Das L, Gard JM, Nagle RB, Cress AE (2016) Schwann cells increase prostate and pancreatic tumor cell invasion using laminin binding A6 integrin. J Cell Biochem 117:491–499. https://doi.org/10.1002/jcb.25300
Fujii-Nishimura Y, Yamazaki K, Masugi Y, Douguchi J, Kurebayashi Y, Kubota N et al (2018) Mesenchymal-epithelial transition of pancreatic cancer cells at perineural invasion sites is induced by Schwann cells. Pathol Int 68:214–223. https://doi.org/10.1111/pin.12641
Shan C, Wei J, Hou R, Wu B, Yang Z, Wang L et al (2016) Schwann cells promote EMT and the Schwann-like differentiation of salivary adenoid cystic carcinoma cells via the BDNF/TrkB axis. Oncol Rep 35:427–435. https://doi.org/10.3892/or.2015.4366
Zhou Y, Shurin GV, Zhong H, Bunimovich YL, Han B, Shurin MR (2018) Schwann cells augment cell spreading and metastasis of lung cancer. Cancer Res 78:5927–5939. https://doi.org/10.1158/0008-5472.CAN-18-1702
Carroll SL (2012) Molecular mechanisms promoting the pathogenesis of Schwann cell neoplasms. Acta Neuropathol 123:321–348. https://doi.org/10.1007/s00401-011-0928-6
Elmaci I, Altinoz MA, Sari R (2018) Immune pathobiology of Schwannomas: a concise review. J Neurol Surg A Cent Eur Neurosurg. 79:159–162. https://doi.org/10.1055/s-0037-1603949
Shurell E, Singh AS, Crompton JG, Jensen S, Li Y, Dry S et al (2016) Characterizing the immune microenvironment of malignant peripheral nerve sheath tumor by PD-L1 expression and presence of CD8+ tumor infiltrating lymphocytes. Oncotarget. 7:64300–64308. https://doi.org/10.18632/oncotarget.11734
Karmakar S, Reilly KM (2017) The role of the immune system in neurofibromatosis type 1-associated nervous system tumors. CNS Oncol. 6:45–60. https://doi.org/10.2217/cns-2016-0024
Patchett AL, Coorens THH, Darby J, Wilson R, McKay MJ, Kamath KS et al (2019) Two of a kind: transmissible Schwann cell cancers in the endangered Tasmanian devil (Sarcophilus harrisii). Cell Mol Life Sci. https://doi.org/10.1007/s00018-019-03259-2
Liao CP, Booker RC, Brosseau JP, Chen Z, Mo J, Tchegnon E et al (2018) Contributions of inflammation and tumor microenvironment to neurofibroma tumorigenesis. J Clin Invest. 128:2848–2861. https://doi.org/10.1172/JCI99424
Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR, Yang SM (2012) Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol 2012:948098. https://doi.org/10.1155/2012/948098
Funding
The research was supported by the Research Scholar Grant, RSG-19-088-01-CSM (to Y. L. Bunimovich), from the American Cancer Society, and the Hillman Fellows for Innovative Cancer Research Program funded by the Henry L. Hillman Foundation (to Y. L. Bunimovich).
Author information
Authors and Affiliations
Contributions
Designed the study: MRS, YLB. Conducted experiments: GVS, OK, YLB. Wrote the manuscript: SHZ, HK, RK, MRS, YLB.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper is a Focussed Research Review based on a presentation given at the Sixth International Conference on Cancer Immunotherapy and Immunomonitoring (CITIM 2019), held in Tbilisi, Georgia, 29th April–2nd May 2019. It is part of a series of CITIM 2019 papers in Cancer Immunology, Immunotherapy.
Rights and permissions
About this article
Cite this article
Zhang, S.H., Shurin, G.V., Khosravi, H. et al. Immunomodulation by Schwann cells in disease. Cancer Immunol Immunother 69, 245–253 (2020). https://doi.org/10.1007/s00262-019-02424-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-019-02424-7