Abstract
Esophageal cancer is a highly malignant disease that despite surgery and adjuvant therapies has an extremely poor outcome. Dendritic cell (DC) immunotherapy as a novel promising strategy could be an alternative for treating this malignancy. Effective DC-mediated immune responses can be achieved by raising cytotoxic T lymphocyte (CTL) response against multiple antigens through loading DCs with total tumor RNA. However, the efficacy of this strategy first needs to be evaluated in a pre-clinical setting. The aim of the study was to set up an ex vivo autologous human readout assay for assessing the effects of DC-mediated cytotoxic responses, using total tumor RNA as an antigen load. Biopsy specimens of seven esophageal cancer patients were used to establish primary cultures of normal and cancer cells and to obtain autologous RNA for loading DCs. Mature DCs loaded with either normal or tumor RNA were obtained and subsequently used to raise various lymphocytes populations. Apoptosis levels of the autologous cultures were measured before and after incubating the cultures with the different lymphocytes populations. The mean apoptosis levels in the tumor cell cultures, induced by lymphocytes instructed by DCs loaded with tumor RNA, significantly increased with 15.6% ±2.9 SEM (range 3.4–24.5%, t-test, P < 0.05). Incubation of the normal cultures with the lymphocytes populations showed a mean non-significant increase in apoptosis of 0.4% ±3.4 SEM (range −13.9 to 9.8%, t-test, P = 0.7). Here, we introduce a practical, patient-specific autologous readout assay for pre-clinical testing of DC-mediated cytotoxic responses. Additionally, we demonstrated that the use of autologous tumor RNA as a strategy for raising cytotoxic responses against multiple tumor antigens could be effective for treating esophageal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are two major types of esophageal cancer: esophageal squamous cell carcinoma and esophageal adenocarcinoma. For decades, the incidence of esophageal squamous cell carcinoma has been unchanged and is approximately 1 per 100,000 cases per year. Of major concern is the steadily increasing incidence of esophageal adenocarcinoma, which has become an important health problem [36, 39]. While esophageal squamous cell carcinoma is associated with poor socio-economic status, smoking habits and alcohol intake [19], esophageal adenocarcinoma has a strong association with Barrett’s esophagus [43]. Barrett’s esophagus is a metaplastic premalignant transformation of the esophageal epithelium associated with gastro-esophageal reflux disease (GERD) [11, 15, 47]. Although the two types of esophageal cancer have different pathophysiology, the clinical outcomes of both are poor. Even after surgical resection, the overall 5 years survival rate of these patients is less than 15%, and adjuvant treatments such as chemo- and radiotherapy have only little effect on patient outcome [7, 17, 40, 42].
Dendritic cell (DC) therapy, as a promising strategy to treat cancer, has been intensively investigated in the last few years. Dendritic cells (DCs) are specialized antigen-presenting cells involved in innate and adaptive immune responses [4, 24]. Functional DCs can be generated from human peripheral blood monocytes and be further matured into DCs that in turn can be used as vaccines for treating malignancies [2, 6, 46, 48]. To generate a cytotoxic T-cell (CTL) response against tumor cells, specific tumor antigens have to be presented to T-lymphocytes by immuno-activatory DCs. Therefore, the immunogenicity of the tumor associated antigens that are used for loading the DCs is crucial. Different antigens have been used and tested for their immunopotency. These include synthetic peptides [16, 35, 45], tumor lysates [27] and cDNA or RNA encoding for specific tumor-associated antigens as well as total tumor mRNA [20, 29, 33, 49, 51]. The introduction of autologous total tumor RNA as an antigen source for loading DCs has several advantages. Such a strategy will not restrict DC vaccination therapy to patients with certain HLA haplotypes. Moreover, it is most suitable to treat cancers with heterogeneous phenotypes, such as esophageal cancers and other solid malignancies that have variable expression of diverse tumor antigens. It is reasonable to assume that normal RNA that will be co-transferred with the total tumor RNA into the DCs may not result in immune responses, since there is tolerance towards self-proteins through depletion of self-specific T cells. Nevertheless, when using total RNA, it is of importance to evaluate whether this strategy would induce a break in the tolerance against self-antigens. A valid pre-clinical method, which would enable us to test the efficacy of immunoactivatory DCs loaded with total tumor RNA to induce T-cell reactivity in an autologous system, could be of use to monitor direct adverse effects as well as to predict potential clinical responses.
The aim of our study was to create an effective autologous ex vivo readout system to evaluate cytotoxic responses induced by DCs based on total tumor RNA as an antigen load. To this aim, primary cultures were established from biopsies of normal and tumor tissues taken during endoscopy from esophageal cancer patients. DCs were generated from peripheral blood monocytes of the patients and loaded with either autologous normal or total tumor RNA, and subsequently studied for their immuno-stimulatory capacity. Hereupon, the DCs were used to stimulate the patient’s lymphocytes to obtain several lymphocytes populations that were subsequently tested for their cytolytic responses against the autologous primary cell cultures.
In this study we introduce a patient-tailored approach using an ex vivo cell culture readout system for evaluating autologous DC-induced cytotoxic responses. We were able to generate fully mature DCs loaded with total tumor RNA that in the autologous ex vivo cultures were able to elicit cytotoxic responses specifically against the autologous cancer cells, while no significant direct adverse effects were seen against the normal cells.
Materials and methods
Patient’s material
The study was approved by the Academic Medical Center (Amsterdam, The Netherlands) Hospital’s medical ethical committee. After informed consent and written permission, 12 patients who met the inclusion criteria (see supplementary data) were included. Before the endoscopic procedure, 64 ml of blood was drawn and collected in heparinized vials for extraction of peripheral blood mononuclear cells (PBMCs). Patients underwent endoscopic procedures for classifying, staging and grading of the esophageal cancer. During this procedure, 12 extra biopsies of each patient were taken to be used for culturing purposes and for RNA isolation; biopsies were obtained from both normal squamous epithelium taken at least 3 cm above the mass and from the malignancy. Matching biopsies from the same spots were taken for histopathological diagnosis.
Primary cell cultures of esophageal normal squamous epithelium and esophageal cancer epithelium
For establishing the autologous ex vivo test model, biopsies from normal esophageal epithelium and from esophageal cancer were used to establish primary cell cultures. Histological examination of the matching biopsies of the tumors showed that on estimate the biopsy specimens contained at least 50% (range 50–80%) of tumor cells. The culture medium MCDB 153 (Sigma) was modified by adding 5% fetal bovine serum, 0.4 (μg/ml hydrocortisone, (Sigma), 20 ng/ml epidermal growth factor (GIBCO, Grand Island, NY), 10−10 mol/l cholera toxin (Sigma), 140 μg/mL bovine pituitary extract (Sigma), 20 μg/ml adenine (Sigma), 100 U/ml penicillin (GIBCO), 100 μg/ml streptomycin (GIBCO), 0.25 μg/ml amphotericin B (GIBCO), 5 μg/ml insulin-transferrin (GIBCO) and 4 mmol/l glutamine. The explant method was used as described before [38]. Briefly, biopsies from normal and tumor esophageal mucosa were collected aseptically into MCDB153 modified medium during routine endoscopy of patient with esophageal cancer. Specimens were processed within half an hour of procurement as follows: biopsy specimens were minced into fragments of 1–2 mm3 in size. The pieces of tissue were placed in a 24-well plate and anchored by a sterile glass microscope slide before adding the growth medium. MCDB 153 modified medium, 1 ml/well, was added and the cultures were placed at 37°C and with 5% CO2. Fresh medium was replaced every 3 days for 3 weeks until the measurement of autologous cytotoxic responses.
RNA isolation
Biopsies from normal and cancer tissues were collected in Trizol reagent (Life Technologies Inc., Invitrogen, Breda, The Netherlands) and processed according to the manufacturer’s instructions. Briefly, tissues were lyzed by adding 200 (μl Trizol. After phenol/chloroform extraction, RNA was precipitated with isopropanol, washed with 70% ethanol and air dried. The RNA was then dissolved in RNase-free H2O and stored at −80°C until required. Using the Nanodrop® apparatus (type ND-1000, Wilmington, USA), 1 μl of total RNA was used to quantitate the RNA by spectrophotometry.
Isolation of lymphocytes and monocytes from peripheral blood of the patient
Lymphocytes and monocytes from patients were isolated from 64 ml of peripheral blood collected in heparinized vials, using the Ficoll–Percoll gradient separation method [12]. A first separation of peripheral blood mononuclear cells (PBMCs) was done using Ficoll–Hypaque solution (Amersham, Pharmacia, Piscataway, NJ, USA). A further separation of monocytes from lymphocytes was done using a Percoll (Amersham Biosciences Europe Freiburg, Germany) density gradient separation, as described previously [8]. Briefly, PBMCs were washed in Roswell Park Memorial Institute (RPMI) 1640 medium (BioWhittaker-Cambrex Bioscience, Walkersville, MD, USA) at 1,500 rpm for 5 min twice. In the meantime, 19.8 ml of Percoll was mixed with 2.2 ml of 10× phosphate buffered saline (PBS) to obtain a standard isotonic percoll solution (SIP), and then with Iscove’s Modified Dulbecco’s Medium (IMDM) (BioWhittaker), to obtain three solutions at different concentrations (60, 47.5 and 34% SIP). PBMCs were then re-suspended in 2.5 ml of 60% SIP, then 5 ml of 47.5% SIP and 2 ml of 34% SIP were added and a centrifugation at 3,100 rpm for 45 min was performed. After centrifugation, the upper layer (monocytes) was collected as well as the lower layer (lymphocytes). Once separated, monocytes and lymphocytes were washed twice in IMDM, and either used immediately or cryo-preserved in a dimethylsulfoxide (DMSO)/fetal calf serum (FCS) (2:8) solution (Merck, Darmstadt, Germany; GIBCO BRL, Grand Island, NY, USA, respectively).
Electroporation of monocytes with normal and tumor autologous total RNA
Monocytes characterized as CD14+, CD83−, CD86−, CD209− were used for electroporation with autologous total normal or tumor RNA following the procedure recently described by Milano et al. [54]. To monitor for the electroporation efficacy, in vitro transcribed (IVT) GFP-RNA was used. The procedure was as follows: monocytes freshly isolated from blood were washed twice in IMDM and electroporated using the Amaxa cell line Nucleofector Kit V (Amaxa GmbH, Cologne, Germany): 0.5 up to 1 × 106 cells were mixed with Cell Line Nucleofector solution V, and 5 μg/ml of RNA was added to the cuvette to be electroporated using the Nucleofector program U16 of the Amaxa Nucleofector device. After electroporation, 1 million/ml monocytes were cultured in 24-well plate using IMDM with 10% FCS and 2% penicillin/streptomycin (GIBCO) and the cells were placed at 37°C and with 5% CO2 for 1 h. Then the cells were washed twice with IMDM and 1000 U/ml of IL-4 and 800 U/ml of GM-CSF were added to initiate the maturation process. After 6 days at the stage of immature DCs, the cytokines IL-1β, TNF-α and LPS (10, 25, 0.02 ng/ml respectively) were added to further enhance the maturation process. The cells were analyzed for the expression of maturation markers at day 6 and 8 by FACS.
Detection of markers and MHC class I and II in monocytes and DCs
Monocytes were harvested by pipetting, washed and re-suspended in FACS buffer (5 g BSA, 0.1 g NaN3, 100 mM EDTA in 1 l PBS) at a concentration of 1 million cells/ml, then incubated with various fluorochrome-conjugated antibodies on ice for 30 min in the dark, then washed again and analyzed using the FACSCALIBUR apparatus (Becton-Dickinson, Franklin Lakes, NJ, USA) and BD CellQuest Pro software. PE or FITC-conjugated antibodies, specific for CD14, CD83, CD86, CD209, CCR7, HLA-A,B,C and HLA-DR (BD, San Jose, CA, USA) were used as appropriate, at a concentration of 1:25. Sample stained for FITC- and PE-conjugated IgG2a/IgG1 isotype controls (BD Biosciences) were included in the staining procedure. For all cases, markers and MHC class I and II expression were measured during maturation from monocytes to immature and mature DCs. Measurements were performed of both electroporated and not electroporated (control) cells.
Stimulation of autologous lymphocytes with electroporated DCs
After 8 days from the isolation of monocytes, the resulting mature DCs were twice co-incubated for 7 days with autologous lymphocytes at a proportion of 1:4 (5 × 105 DCs and 2 × 106 lymphocytes) in a 24-well plate in IMDM medium with 5% FCS and 2% penicillin–streptomycin (GIBCO). Before and after the first and second stimulation (day 1, day 7 and day 14 of the co-culture), aliquots of lymphocytes were taken to measure changes in the CD4/8 ratio by flow cytometry and the supernatants were collected to measure the production of inflammatory cytokines. Finally, per patient, two populations of lymphocytes were obtained: lymphocytes stimulated by DCs electroporated with tumor RNA and a second population of lymphocytes stimulated by DCs electroporated with normal RNA.
Measuring of the cytotoxic responses on the ex vivo cell cultures
After stimulation with the normal and tumor RNA electroporated DCs, the two different populations of lymphocytes mentioned above were washed in IMDM and added to the autologous cultured normal and tumor epithelial cells at a target/effector ratio of 2 × 104/4 × 105cells for 5 h. For each patient, autologous tumor and normal epithelial cell cultures were incubated with lymphocytes either stimulated by DCs loaded with autologous normal RNA or with autologous tumor RNA (see supplementary picture). The epithelial normal and tumor cells were washed with cold PBS and detached by adding 0.5 ml trypsin (GIBCO, Auckland, NZ) for 5 min at 37°C; cells were then collected in 1 ml MCDB 153 modified medium, spun down and re-suspended in Annexin V buffer (2.38 g HEPES, 8.8 g NaCl, 0.38 g KCl, 0.2 g CaCl2, 0.20 g MgCl2) at a concentration of 1 million cells/ml. Apoptosis of the normal and cancer cells was measured by using the following antibodies: anti-human Annexin-V APC conjugated (ICQ, Groningen, The Netherlands); Via-probe 7AAD (necrosis marker; R&D System); anti-human EpCam FITC conjugated (epithelial specific marker; Miltenyi Biotech, Auburn, CA); anti-human CD3 PE conjugated (T-cells marker; R&D System). Data were acquired using BD Cell Quest Pro Software. Apoptotic epithelial cells were gated as double positive for AnnexinV and EpCam, and negative for CD3 and 7AAD.
Cytometric bead array (CBA) multiplex assays
During stimulation of lymphocytes with normal and tumor RNA electroporated DCs, the supernatants were collected from the samples of the patients at day 7 (after the first stimulation) and at day 14 (after the second stimulation) and analyzed for cytokine contents using cytometric bead array (CBA). Experiments were performed using the CBA inflammation kit (BD) following the manufacturer’s instructions, and the standard procedure was followed as previously described [10]. The acquired data were analyzed using the BD calibration and analysis software.
Statistical analysis
Statistical analyses were performed using the software Graph Pad Prism®. Statistical tests were applied to seven independent experiments. Differences among the values were determined using both Student’s paired t-test and one-way ANOVA. The significance was determined as P < 0.05.
Results
Patients and primary cultures
A total of 12 patients were enrolled in the study. Informed consent was obtained from each participant. Because of difficulties in establishing primary cultures, such as a high rate of apoptosis due to the initial state of certain tissues and bacterial contamination, a final number of seven male esophageal cancer patients with a mean age of 68 (range 61–78) were analyzed for autologous CTL responses. Histopathology of the biopsies of the cancers was determined by an expert pathologist and revealed esophageal adenocarcinoma in five patients and squamous cell carcinoma in two patients. Patient data are summarized in Table 1.
Immuno-phenotyping of mature DCs
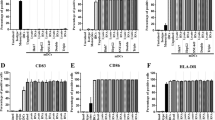
The immuno-phenotype of DCs was analyzed by FACS. The percentages of gated positive cells for the markers CD14, CD83, CD86, CCR7 and CD209 were as follows: at day 1, at the monocyte stage, on average 95% of the gated cells were positive for CD14, whereas all the other markers were expressed in less than 20% of cells. At the immature stage, CD83, CD86, CD209 and CCR7 expression was seen on average in 50–60%, while CD14 expression decreased to 20%. After further 2 days of maturation, CD83, CD86, CD209 and CCR7 expression increased to at least 90%, while CD14 decreased on average to less than 20% of the gated cells (Fig. 1A). Expression of these markers was comparable between electroporated and not electroporated (control) cells, indicating that the process of electroporation did not influence the maturation process.
Cellular differentiation markers in monocytes, immature DCs and mature DCs. a FACS profiles of the cellular differentiation markers CD14, CD86, CD83, CD209 and CCR-7; after electroporation in mature DCs CD14 expression is seen in less than 20% of the cells, while the maturation markers are found in at least 90% of the gated cells. b Electroporation efficacy assessed by FACS analysis shows high levels of GFP expression of DCs loaded with IVT GFP-RNA after 72 h and at day 8 of maturation. Bright gray lines represent control cells; dark gray areas represent positive cells
Electroporation efficacy as measured by GFP expression
The electroporation efficacy as monitored by electroporation of IVT GFP-RNA showed GFP expression in 50–60% of the gated cells in all cases (Fig. 1B). This expression was found 24 h after electroporation and was maintained during the maturation process of the DCs until day 8.
MHC class I and II expression
MHC class I was seen on average in 90% of the gated cells in the monocyte stage. At day 6 (immature DC state), 90% of cells maintained expression, and at day 8 (mature DC state), MHC class I increased to 95% (Fig. 2).
MHC class I and II expression before and after transfection of the DCs. FACS analysis of MHC class I and II (HLA-A, B, C and HLA-DR expression) in monocytes before and after electroporation at the immature stage (day 6) and mature stage (day 8) and in not electroporated mature DCs. Before electroporation, the MHC class I and II expression is seen on average in 90% of the gated cells. The expression levels of both MHC class I and II molecules is maintained through the maturation process and it is still high (on average 90%) in mature DCs. Bright gray lines represent control cells; dark gray areas represent positive cells
MHC class II was seen on average in 90% in the monocyte stage. At day 6 (immature DC state), the expression level was retained, and at day 8 (mature DC state), MHC class II expression increased to 96% of the gated cells (Fig. 2). No significant differences were detected between the expression levels of electroporated and not electroporated (control) cells.
CD4/8 ratios in lymphocytes
Before stimulation, the CD4/8 ratio was 2.5 ± 0.1 SD (Fig. 3). After one week of stimulation by DCs electroporated with normal RNA, lymphocytes showed a ratio shift to 2.2 ± 0.9 SD and to 2.1 ± 1.1 after the second week of stimulation. Thus, there was no significant difference in the CD4/8 ratio in the lymphocytes stimulated before and after stimulation by DCs loaded with normal RNA (two tailed paired t-test, P = 0.8). In contrast, after one and two weeks of stimulating the lymphocytes by DCs electroporated with tumor RNA, the ratio shifted to 2.1 ± 1.2 SD, and to 0.7 ± 0.3 SD, respectively, which was a significantly different shift (two tailed paired t-test, **P = 0.006).
CD4/8 ratios of stimulated lymphocytes. CD4/8 ratios of lymphocytes as measured by FACS: the CD4/8 ratio of the lymphocytes before stimulation as measured in all cases was 2.5 ± 0.1 SD (a, b). a After the subsequent stimulation of the lymphocytes by DCs electroporated with normal RNA, the CD4/8 ratio shifted to 2.2 ± 0.9 SD; and after the second stimulation to 2.1 ± 1.1 SD (two tailed paired t-test, P = 0.8). b Lymphocytes stimulated by DCs electroporated with tumor RNA showed a CD4/8 ratio of 2.1 ± 1.2 SD after the first week of stimulation and 0.7 ± 0.3 SD after the second stimulation (two tailed paired t-test, **P = 0.006)
IFN-γ production by stimulated lymphocytes
Before stimulation, IFN-γ production of the lymphocytes was virtually negative. After stimulation with not electroporated DCs or DCs electroporated with normal RNA, the IFN-γ level increased to 2,041 ± 650 SEM and 2425 ± 294 SEM pg/ml, respectively. After stimulation of the lymphocytes by DCs electroporated with tumor RNA, the IFN-γ production significantly increased to 5,742 ± 592 SEM pg/ml (ANOVA, Bonferroni multiple comparison post test, ***P < 0.001; Fig. 4).
IFN-γ release by lymphocytes stimulated with normal and tumor RNA-electroporated DCs. IFN-γ release as measured in lymphocytes before and after stimulation with electroporated DCs. Lymphocytes stimulated by DCs electroporated with tumor RNA show a significantly higher IFN-γ release than lymphocytes stimulated with not loaded DCs or lymphocytes stimulated by DCs electroporated with normal RNA. (ANOVA, Bonferroni multiple comparison post test, ***P < 0.001). Data are expressed as means ± SEM of the different experiments
Cytotoxicity responses as measured by apoptosis in the epithelial cell cultures
Apoptosis levels after 5 h of incubation with the cytotoxic populations, as calculated with respect to the basal apoptosis levels of the cultures (Fig. 5a,d), were as follows: After incubation of the autologous normal epithelial cell cultures with lymphocytes stimulated by DCs electroporated with normal RNA and tumor RNA, the mean increase in apoptosis levels were 0.4% ± 3.4 SEM (range −13.9 to 9.8%, two tailed paired t-test, P = 0.7) and −1.3% ±3.2 SEM (range −14 to13%, two tailed paired t-test 0.2 respectively, Figs. 5b, c, 6a). The apoptosis levels in the tumor cell cultures co-incubated with lymphocytes stimulated by DCs electroporated with normal RNA increased with 2.3% ±2.1 SEM (range −4.7 to 12.7%, two tailed paired t-test, P = 0.2; Figs. 5e, 6). The mean level of apoptosis in the tumor cell cultures incubated with lymphocytes instructed by tumor RNA electroporated DCs increased significantly with 15.6% ±2.9 SEM (range 3.4–24.5%, two tailed paired t-test, P < 0.05 Figs. 5f, 6b).
Apoptosis levels (Annexin V expression) measured by FACS. FACS analysis of 7AAD and AnnexinV (apoptosis) expression in the normal and tumor primary cell cultures of patient one. Basal level of apoptosis in normal primary cultures (a); apoptosis in normal primary cultures after incubation with lymphocytes stimulated with DCs loaded with either normal RNA (b) or tumor RNA (c); Basal level of apoptosis in primary tumor cultures (d); apoptosis in primary tumor cultures, after incubation with lymphocytes stimulated by DCs loaded with normal RNA (e) and tumor RNA (f). Notice that a high level of apoptosis was induced in the tumor cultures by lymphocytes stimulated with tumor RNA-loaded DCs (f), while there is no increase in apoptosis in case the tumor cells were incubated with lymphocytes stimulated by DCs loaded with normal RNA (e). Microphotographs of the normal epithelial cultures and tumor epithelial cultures are included in the figures and were obtained using an Eclipse TS 100 phase contrast microscope (NIKON)
Summary of the CTL-induced apoptosis levels in the primary ex vivo cultures. Basal level of apoptosis in the normal and the tumor epithelial cells was taken as zero. a On average, the level of apoptosis in the normal epithelial cultures of the patients increased with 0.4% ±3.4 SEM (range −13.9 to 9.8%) and −1.3% ±3.2 SEM (range −14 to 13.4%) when incubated with lymphocytes instructed by DCs loaded with normal and tumor RNA, respectively (two tailed t-test, P = 0.7 and 0.2). b On average the apoptosis levels in the tumor cell cultures incubated with lymphocytes instructed by DCs loaded with tumor RNA increased with 15.6% ±2.9 SEM (range 3.4–24.5%, ** P = 0.002), while with 2.3% ±2.1 SEM (range −4.7 to 12.7%), when incubated with normal RNA-electroporated DCs (P = 0.2)
Discussion
Clinical trials using antigen-pulsed DCs have been conducted in patients with various types of cancer, including myeloid leukemia, glioblastoma, metastatic melanoma, pancreas, colorectal cancer and many others [1, 9, 18, 26, 28, 34]. To our knowledge, no reports have been yet published regarding DC therapy on esophageal cancer. Genotypically and phenotypically, esophageal cancers are highly heterogeneous [23], which make it difficult to choose a single effective antigenic target for treatment of these types of malignancies. Using total tumor RNA as an antigen load for DCs overcomes the need to identify specific tumor antigens, but as well provides the opportunity to evoke more effective anti-cancer cytotoxic T-cell responses against tumors without knowing the exact nature of the targeted antigens [21]. RNA-based DC immunotherapy with the use of total tumor RNA provides the potential to generate a polyclonal immune response to multiple known and unknown tumor antigens without the limitation to specific HLA types. However, when using total tumor RNA for loading DCs, there is a major concern for inducing direct adverse effects through breaking tolerance to self-antigens resulting in autoimmune responses. Therefore, a pre-clinical ex vivo evaluation in an autologous setup to estimate potential adverse effects of this strategy could be useful to predict inauspicious effects in a clinical setting.
In our study, we analyzed the feasibility of an autologous readout system to evaluate cytotoxicity responses against esophageal cancer cells. Furthermore, in this study, the concern of breaking tolerance to self-antigens, when immunizing with total tumor RNA, was addressed by monitoring lysis activity against the patient’s normal epithelial cells in the ex vivo readout system. In general, we found that DCs electroporated with tumor RNA elicited cytotoxic lymphocytes, which were able to recognize and induce significantly high levels of apoptosis in the autologous cultured cancer cells (P < 0.05, Fig. 6), while these lymphocytes did not induce a cytotoxic reaction against the autologous normal epithelial cells (P = 0.2, Fig. 6). In addition, in the control experiments with lymphocytes stimulated by DCs loaded with normal RNA, in general no significant increase in apoptosis levels in the autologous normal epithelial cells was detected. This observation corresponds with a previous study, in which DCs electroporated with renal tumor RNA did not show a cytotoxic response against benign renal parenchyma [50]. It needs to be pointed out, however, that the increase in the level of apoptosis in the normal cultures ranged from −13.9 to 12%. Thus, in certain cases this could mean that the DCs can mediate adverse effects on normal tissues. In one particular case, for instance, the DCs loaded with tumor RNA induced a cytotoxic lymphocytes mediated increase in apoptosis level of 12% in the normal cells and, although this was significantly lower when compared to the apoptosis level of 22% as induced in the tumor cells (P < 0.05, Fig. 6), it would be doubtful whether these DCs would be suitable as a vaccine for patient therapy.
Despite the strong potency of DCs to present tumor antigens, the efficacy of therapeutic DC vaccination against cancer is questioned. So far, only a limited rate of objective tumor regressions has been observed in clinical studies [3, 5, 9, 14, 25, 27, 44]. One reason for the discrepancy between the outcomes as seen in the pre-clinical feasibility assays with respect to the true clinical responses could be that most pre-clinical in vitro tumor models that evaluate direct cytotoxicity of DCs have been performed on either cancer cell lines or mice models [22, 30, 31, 41, 52]. Because of an absent or different immune system, tumor cell lines and animal models in general exhibit remarkable anticancer responses, and these models are not suitable for predicting direct adverse immune responses in humans. Indirectly, the potency of DCs is measured through their ability to induce pro-inflammatory cytokines such as IL-12, for their migratory ability, their potency to induce allogenic T-cell responses and tumor antigen-specific CD8+ T cells [5, 13, 32, 37]. Therefore, another potential application of the test model is its application for predicting clinical anti-tumor responses. In our seven cases, cytotoxic lymphocytes-induced apoptosis was on average 15% in the autologous tumor cultures, but the range of lysis was as low as 4% and as high as 24%. It is possible that these ex vivo responses would correlate with clinical responses, and as such this setup might be used as a prognostic clinical tool. Unquestionably, this should be first evaluated in patient trials, during which the late adverse responses can be monitored as well and long-term safety of total tumor RNA-based DCs can be studied. Of interest is a similar recent study, which demonstrated that DC-induced preclinical ex vivo CTLs’ responses on autologous tumor cell cultures of melanoma patients do correlate with clinical tumor responses [53].
The main difficulty in our setup is the loss of cultures due to bacterial overgrowth and cell death. Because of these reasons, 5 of 12 cultures were lost. This is not surprising, since esophageal cancers are highly contaminated and may contain necrotic areas. The majority of cultures were lost in the initiation phase of the study. Optimizing the culturing media, targeting biopsies from non-necrotic tumor areas and minimizing the delay between collection of the tissues and setting up of the cultures highly diminished the number of losses.
In this study, loading of the DCs was performed through direct electroporation of the monocytes with total RNA. This method that has been recently described was found to be particularly efficient with a high yield of fully mature immunopotent DCs [54]. Furthermore, the method used did not interfere with the expression of the most important maturation markers such as CD83, CD86, CD80 and CD209 and the expression of MHC class I, which is of pivotal importance for a proper CD8+ T cell priming, and MHC class II, which is of central importance for inducing CD4+ T helper response (Fig. 2).
Another important finding in the present study is that the immunopotency of the DCs loaded with tumor RNA was significantly higher compared to those loaded with normal RNA. This was demonstrated by a significantly more profound shift of the CD4/8 ratio towards CD8+ T cells (Fig. 3), and the threefold increase in the level of IFN-γ (Fig. 4). The more significant shift towards a CD8+ T cells subpopulation induced by tumor RNA-loaded DCs on autologous lymphocytes, as compared with the shift induced by normal RNA-loaded DCs, indicates that lymphocytes stimulated by tumor RNA-loaded DCs were primed towards a cytotoxic population, whereas lymphocytes stimulated by normal RNA-loaded DCs maintained tolerance for self-antigens and as a consequence the shift towards CD8+ T cells was not significant. This hypothesis is further sustained by the additional shift towards CD8+ T cells after the second stimulation of lymphocytes that was only seen in case DCs were electroporated with tumor RNA. This suggests that lymphocytes that were primed in the first stimulation further expanded after the additional stimulation with tumor antigen presenting DCs.
In summary, here we present a human autologous ex vivo readout system that appears to be an effective and useful readout for testing the efficacy of DC-induced cytotoxic responses against esophageal cancer cells, and to monitor the direct adverse effects on autologous normal tissues of esophageal cancer patients. In addition, the minimal lytic effects on the normal cell cultures encourages the use of total tumor RNA-based DC vaccines. This important ex vivo validation study sets the stage to proceed with the establishment of this novel model for pre-clinical testing of CTLs and antigen-presenting cells and to further explore the strategy of total tumor RNA-based DC therapy for treatment of esophageal cancer.
Abbreviations
- DCs:
-
Dendritic cells
- APCs:
-
Antigen presenting cells
- PBMCs:
-
Peripheral blood mononuclear cells
- CTLs:
-
Cytotoxic T-lymphocytes
- CCR7:
-
Chemokine receptor 7
- IL-12, IL-10:
-
Interleukine12, interleukine10
References
Anichini A, Molla A, Mortarini R, Tragni G, Bersani I, Di Nicola M, Gianni AM, Pilotti S, Dunbar R, Cerundolo V, Parmiani G (1999) An expanded peripheral T cell population to a cytotoxic T lymphocyte (CTL)-defined, melanocyte-specific antigen in metastatic melanoma patients impacts on generation of peptide-specific CTLs but does not overcome tumor escape from immune surveillance in metastatic lesions. J Exp Med 190:651–667
Ardavin C, Amigorena S, Reis e Sousa C (2004) Dendritic cells: immunobiology and cancer immunotherapy. Immunity 20:17–23
Babatz J, Rollig C, Lobel B, Folprecht G, Haack M, Gunther H, Kohne CH, Ehninger G, Schmitz M, Bornhauser M (2006) Induction of cellular immune responses against carcinoembryonic antigen in patients with metastatic tumors after vaccination with altered peptide ligand-loaded dendritic cells. Cancer Immunol Immunother 55:268–276
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392:245–252
Banchereau J, Palucka AK (2005) Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol 5:296–306
Berger TG, Feuerstein B, Strasser E, Hirsch U, Schreiner D, Schuler G, Schuler-Thurner B (2002) Large-scale generation of mature monocyte-derived dendritic cells for clinical application in cell factories. J Immunol Methods 268:131–140
Bosset JF, Gignoux M, Triboulet JP, Tiret E, Mantion G, Elias D, Lozach P, Ollier JC, Pavy JJ, Mercier M, Sahmoud T (1997) Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med 337:161–167
Braat H, van den Brande J, van Tol E, Hommes D, Peppelenbosch M, van Deventer S (2004) Lactobacillus rhamnosus induces peripheral hyporesponsiveness in stimulated CD4+ T cells via modulation of dendritic cell function. Am J Clin Nutr 80:1618–1625
Burgdorf SK, Fischer A, Claesson MH, Kirkin AF, Dzhandzhugazyan KN, Rosenberg J (2006) Vaccination with melanoma lysate-pulsed dendritic cells of patients with advanced colorectal carcinoma: report from a phase I study. J Exp Clin Cancer Res 25:201–206
Cook EB, Stahl JL, Lowe L, Chen R, Morgan E, Wilson J, Varro R, Chan A, Graziano FM, Barney NP (2001) Simultaneous measurement of six cytokines in a single sample of human tears using microparticle-based flow cytometry: allergics vs. non-allergics. J Immunol Methods 254:109–118
Cossentino MJ, Wong RK (2003) Barrett’s esophagus and risk of esophageal adenocarcinoma. Semin Gastrointest Dis 14:128–135
Cranmer LD, Trevor KT, Hersh EM (2004) Clinical applications of dendritic cell vaccination in the treatment of cancer. Cancer Immunol Immunother 53:275–306
Dannull J, Nair S, Su Z, Boczkowski D, DeBeck C, Yang B, Gilboa E, Vieweg J (2005) Enhancing the immunostimulatory function of dendritic cells by transfection with mRNA encoding OX40 ligand. Blood 105:3206–3213
Dees EC, McKinnon KP, Kuhns JJ, Chwastiak KA, Sparks S, Myers M, Collins EJ, Frelinger JA, Van Deventer H, Collichio F, Carey LA, Brecher ME, Graham M, Earp HS, Serody JS (2004) Dendritic cells can be rapidly expanded ex vivo and safely administered in patients with metastatic breast cancer. Cancer Immunol Immunother 53:777–785
Demeester SR, Peters JH, Demeester TR (2001) Barrett’s esophagus. Curr Probl Surg 38:558–640
Dhodapkar MV, Krasovsky J, Steinman RM, Bhardwaj N (2000) Mature dendritic cells boost functionally superior CD8(+) T-cell in humans without foreign helper epitopes. J Clin Invest 105:R9–R14
Di Fiore F, Lecleire S, Galais MP, Rigal O, Vie B, David I, Hamidou H, Paillot B, Jacob JH, Michel P (2006) Impact of radiation schedule and chemotherapy duration in definitive chemoradiotherapy regimen for esophageal cancer. Gastroenterol Clin Biol 30:845–851
Fong L, Engleman EG (2000) Dendritic cells in cancer immunotherapy. Annu Rev Immunol 18:245–273
Hashibe M, Boffetta P, Janout V, Zaridze D, Shangina O, Mates D, Szeszenia-Dabrowska N, Bencko V, Brennan P (2007) Esophageal cancer in Central and Eastern Europe: tobacco and alcohol. Int J Cancer 120(7):1518–1522
Heiser A, Maurice MA, Yancey DR, Wu NZ, Dahm P, Pruitt SK, Boczkowski D, Nair SK, Ballo MS, Gilboa E, Vieweg J (2001) Induction of polyclonal prostate cancer-specific CTL using dendritic cells transfected with amplified tumor RNA. J Immunol 166:2953–2960
Kalady MF, Onaitis MW, Padilla KM, Emani S, Tyler DS, Pruitt SK (2002) Enhanced dendritic cell antigen presentation in RNA-based immunotherapy. J Surg Res 105:17–24
Kalady MF, Onaitis MW, Emani S, Abdul-Wahab Z, Pruitt SK, Tyler DS (2004) Dendritic cells pulsed with pancreatic cancer total tumor RNA generate specific antipancreatic cancer T cells. J Gastrointest Surg 8:175–181; discussion 181–182
Krishnadath KK, Wang KK, Taniguchi K, Sebo TJ, Buttar NS, Anderson MA, Lutzke LS, Liu W (2000) Persistent genetic abnormalities in Barrett’s esophagus after photodynamic therapy. Gastroenterology 119:624–630
Lanzavecchia A, Sallusto F (2001) Regulation of T cell immunity by dendritic cells. Cell 106:263–266
Lee WC, Wang HC, Hung CF, Huang PF, Lia CR, Chen MF (2005) Vaccination of advanced hepatocellular carcinoma patients with tumor lysate-pulsed dendritic cells: a clinical trial. J Immunother 28:496–504
Liu G, Ying H, Zeng G, Wheeler CJ, Black KL, Yu JS (2004) HER-2, gp100, and MAGE-1 are expressed in human glioblastoma and recognized by cytotoxic T cells. Cancer Res 64:4980–4986
Maier T, Tun-Kyi A, Tassis A, Jungius KP, Burg G, Dummer R, Nestle FO (2003) Vaccination of patients with cutaneous T-cell lymphoma using intranodal injection of autologous tumor-lysate-pulsed dendritic cells. Blood 102:2338–2344
Morse MA, Nair SK, Boczkowski D, Tyler D, Hurwitz HI, Proia A, Clay TM, Schlom J, Gilboa E, Lyerly HK (2002) The feasibility and safety of immunotherapy with dendritic cells loaded with CEA mRNA following neoadjuvant chemoradiotherapy and resection of pancreatic cancer. Int J Gastrointest Cancer 32:1–6
Muller MR, Grunebach F, Kayser K, Vogel W, Nencioni A, Brugger W, Kanz L, Brossart P (2003) Expression of her-2/neu on acute lymphoblastic leukemias: implications for the development of immunotherapeutic approaches. Clin Cancer Res 9:3448–3453
Muller MR, Grunebach F, Nencioni A, Brossart P (2003) Transfection of dendritic cells with RNA induces CD4- and CD8-mediated T cell immunity against breast carcinomas and reveals the immunodominance of presented T cell epitopes. J Immunol 170:5892–5896
Nair SK, Snyder D, Rouse BT, Gilboa E (1997) Regression of tumors in mice vaccinated with professional antigen-presenting cells pulsed with tumor extracts. Int J Cancer 70:706–715
Nair SK, Boczkowski D, Morse M, Cumming RI, Lyerly HK, Gilboa E (1998) Induction of primary carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes in vitro using human dendritic cells transfected with RNA. Nat Biotechnol 16:364–369
Nair SK, Morse M, Boczkowski D, Cumming RI, Vasovic L, Gilboa E, Lyerly HK (2002) Induction of tumor-specific cytotoxic T lymphocytes in cancer patients by autologous tumor RNA-transfected dendritic cells. Ann Surg 235:540–549
Nencioni A, Brossart P (2004) Cellular immunotherapy with dendritic cells in cancer: current status. Stem Cells 22:501–513
Nestle FO, Filgueira L, Nickoloff BJ, Burg G (1998) Human dermal dendritic cells process and present soluble protein antigens. J Invest Dermatol 110:762–766
O’Connor JB, Falk GW, Richter JE (1999) The incidence of adenocarcinoma and dysplasia in Barrett’s esophagus: report on the Cleveland Clinic Barrett’s Esophagus Registry. Am J Gastroenterol 94:2037–2042
Osada T, Nagawa H, Takahashi T, Tsuno NH, Kitayama J, Shibata Y (2002) Dendritic cells cultured in anti-CD40 antibody-immobilized plates elicit a highly efficient peptide-specific T-cell response. J Immunother 25:176–184
Palanca-Wessels MC, Barrett MT, Galipeau PC, Rohrer KL, Reid BJ, Rabinovitch PS (1998) Genetic analysis of long-term Barrett’s esophagus epithelial cultures exhibiting cytogenetic and ploidy abnormalities. Gastroenterology 114:295–304
Pera M (2003) Trends in incidence and prevalence of specialized intestinal metaplasia, barrett’s esophagus, and adenocarcinoma of the gastroesophageal junction. World J Surg 27:999–1008; discussion 1006–1008
Peracchia A, Bonavina L, Via A, Incarbone R (1999) Current trends in the surgical treatment of esophageal and cardia adenocarcinoma. J Exp Clin Cancer Res 18:289–294
Pilon-Thomas S, Li W, Briggs JJ, Djeu J, Mule JJ, Riker AI (2006) Immunostimulatory effects of CpG-ODN upon dendritic cell-based immunotherapy in a murine melanoma model. J Immunother 29:381–387
Pulkkinen J, Sipila J, Hujala K, Grenman R (2004) Intraluminal radiotherapy in esophageal cancer. an update. Otolaryngol Pol 58:191–195
Reid BJ, Barrett MT, Galipeau PC, Sanchez CA, Neshat K, Cowan DS, Levine DS (1996) Barrett’s esophagus: ordering the events that lead to cancer. Eur J Cancer Prev 5 Suppl 2:57–65
Roddie H, Klammer M, Thomas C, Thomson R, Atkinson A, Sproul A, Waterfall M, Samuel K, Yin J, Johnson P, Turner M (2006) Phase I/II study of vaccination with dendritic-like leukaemia cells for the immunotherapy of acute myeloid leukaemia. Br J Haematol 133:152–157
Schuler-Thurner B, Schultz ES, Berger TG, Weinlich G, Ebner S, Woerl P, Bender A, Feuerstein B, Fritsch PO, Romani N, Schuler G (2002) Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J Exp Med 195:1279–1288
Schultz ES, Schuler-Thurner B, Stroobant V, Jenne L, Berger TG, Thielemanns K, van der Bruggen P, Schuler G (2004) Functional analysis of tumor-specific Th cell responses detected in melanoma patients after dendritic cell-based immunotherapy. J Immunol 172:1304–1310
Spechler SJ, Zeroogian JM, Antonioli DA, Wang HH, Goyal RK (1994) Prevalence of metaplasia at the gastro-oesophageal junction. Lancet 344:1533–1536
Stift A, Friedl J, Dubsky P, Bachleitner-Hofmann T, Schueller G, Zontsich T, Benkoe T, Radelbauer K, Brostjan C, Jakesz R, Gnant M (2003) Dendritic cell-based vaccination in solid cancer. J Clin Oncol 21:135–142
Strobel I, Berchtold S, Gotze A, Schulze U, Schuler G, Steinkasserer A (2000) Human dendritic cells transfected with either RNA or DNA encoding influenza matrix protein M1 differ in their ability to stimulate cytotoxic T lymphocytes. Gene Ther 7:2028–2035
Su Z, Dannull J, Heiser A, Yancey D, Pruitt S, Madden J, Coleman D, Niedzwiecki D, Gilboa E, Vieweg J (2003) Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res 63:2127–2133
Van Tendeloo VF, Ponsaerts P, Lardon F, Nijs G, Lenjou M, Van Broeckhoven C, Van Bockstaele DR, Berneman ZN (2001) Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood 98:49–56
Warncke M, Dodero A, Dierbach H, Follo M, Veelken H (2006) Murine dendritic cells generated under serum-free conditions have a mature phenotype and efficiently induce primary immune responses. J Immunol Methods 310:1–11
Schmidt CW, Martinez NR, Neller M, Lopez JA, Lanagan CM, O’Connor LE, O’Rourke MGE, Johnson MK, See JL, Slater GJ, Ellem KAO (2006) An immunological insight into effective dendritic cell immunotherapy of metastatic melanoma. Abstract, 9th international conference on dendritic cells, Edinburgh, Scotland, 16–20 September 2006
Milano F, van Baal JW, Rygiel AM, Bergman JJ, van Deventer SJ, Kapsenberg M, Peppelenbosch MP, Krishnadath KK (2007) An improved protocol for generation of immuno-potent DC through direct electroporation Of CD14+ monocytes. J Immunol Methods 321(1–2):94–106
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Milano, F., Rygiel, A.M., Buttar, N. et al. An ex vivo readout for evaluation of dendritic cell-induced autologous cytotoxic T lymphocyte responses against esophageal cancer. Cancer Immunol Immunother 56, 1967–1977 (2007). https://doi.org/10.1007/s00262-007-0341-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-007-0341-0