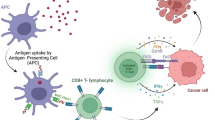
Abstract
Dendritic cell (DC) immunotherapy has shown significant promise in animal studies as a potential treatment for cancer. Its application in the clinic depends on the results of human trials. Here, we review the published clinical trials of cancer immunotherapy using exogenously antigen-exposed DCs. We begin with a short review of general properties and considerations in the design of such vaccines. We then review trials by disease type. Despite great efforts on the part of individual investigative groups, most trials to date have not yielded data from which firm conclusions can be drawn. The reasons for this include nonstandard DC preparation and vaccination protocols, use of different antigen preparations, variable means of immune assessment, and nonrigorous criteria for defining clinical response. While extensive animal studies have been conducted using DCs, optimal parameters in humans remain to be established. Unanswered questions include optimal cell dose, use of mature versus immature DCs for vaccination, optimal antigen preparation, optimal route, and optimal means of assessing immune response. It is critical that these questions be answered, as DC therapy is labor- and resource-intensive. Cooperation is needed on the part of the many investigators in the field to address these issues. If such cooperation is not forthcoming, the critical studies that will be required to make DC therapy a clinically and commercially viable enterprise will not take place, and this therapy, so promising in preclinical studies, will not be able to compete with the many other new approaches to cancer therapy presently in development. Trials published in print through June 2003 are included. We exclude single case reports, except where relevant, and trials with so many variables as to prevent interpretation about DC therapy effects.
Similar content being viewed by others
Abbreviations
- CEA:
-
carcinoembryonic antigen
- CTL:
-
cytotoxic T lymphocyte
- DC:
-
dendritic cell
- DTH:
-
delayed-type hypersensitivity
- EpCAM:
-
epithelial cell adhesion molecule
- FCS:
-
fetal calf serum
- Flt3L:
-
Flt3 ligand
- G-CSF:
-
granulocyte colony stimulating factor
- GM-CSF:
-
granulocyte-macrophage colony stimulating factor
- HBsAg:
-
hepatitis B surface antigen
- HLA:
-
human leukocyte antigen
- HPV:
-
human papillomavirus
- IFN:
-
interferon
- Ig:
-
immunoglobulin
- IL:
-
interleukin
- KLH:
-
keyhole limpet hemocyanin
- MHC:
-
major histocompatibility complex
- MUC1:
-
mucin gene antigen
- PAP:
-
prostatic acid phosphatase
- PBMC:
-
peripheral blood mononuclear cell
- PEG:
-
polyethylene glycol
- PSA:
-
prostate-specific antigen
- PSMA:
-
prostate-specific membrane antigen
- PTH:
-
parathyroid hormone
- RT-PCR:
-
reverse transcription polymerase chain reaction
- TNF-α:
-
tumor necrosis factor α
References
Andersen MH, Keikavoussi P, Brocker EB, Schuler-Thurner B, Jonassen M, Sondergaard I, Straten PT, Becker JC, Kampgen E (2001) Induction of CTL responses in melanoma patients by dendritic cell vaccination: cessation of CTL responses is associated with disease progression. Int J Cancer 94:820
Andreesen R, Scheibenbogen C, Brugger W, Krause S, Meerpohl H-G, Leser H-G, Engler H, Lohr GW (1990) Adoptive transfer of tumor cytotoxic macrophages generated in vitro from circulating blood monocytes: a new approach to cancer immunotherapy. Cancer Res 50:7450
Azuma T, Horie S, Tomita K, Takahashi T, Tanaka Y, Kashiwase K, Nieda M, Takeuchi T, Ohta N, Shibata Y, Hirai H, Kitamura T (2002) Dendritic cell immunotherapy for patients with metastatic renal cell carcinoma: University of Tokyo experience. Int J Urol 9:340
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392:245
Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, Rolland A, Taquet S, Coquery S, Wittkowski KM, Bhardwaj N, Pineiro L, Steinman R, Fay J (2001) Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res 61:6451
Bostanci A, Vogel G (2002) German inquiry finds flaws, not fraud. Science 298:1531
Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L, Brugger W (2000) Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood 96:3102
Burch PA, Breen JK, Buckner JC, Gastineau DA, Kaur JA, Laus RL, Padley DJ, Peshwa MV, Pitot HC, Richardson RL, Smits BJ, Sopapan P, Strang G, Valone FH, Vuk-Pavlovic S (2000) Priming tissue-specific cellular immunity in a phase I trial of autologous dendritic cells for prostate cancer. Clin Cancer Res 6:2175
Bystryn JC (2002) Vaccines for melanoma. Dermatol Clin 20:717
Carroll WL, Thielemans K, Dilley J, Levy R (1986) Mouse x human heterohybridomas as fusion partners with human B cell tumors. J Immunol Methods. 155:61
Chang AE, Redman BG, Whitfield JR, Nickoloff BJ, Braun TM, Lee PP, Geiger JD, Mule JJ (2002). A phase I trial of tumor lysate-pulsed dendritic cells in the treatment of advanced cancer. Clin Cancer Res 8:1021
Cull G, Durrant L, Stainer C, Haynes A, Russell N (1999) Generation of anti-idiotype immune responses following vaccination with idiotype-protein pulsed dendritic cells in myeloma. Br J Haematol 107:648
Davis ID, Jefford M, Parente P, Cebon J (2003) Rational approaches to human cancer immunotherapy. J Leukoc Biol 73:3
Ferlazzo G, Klein J, Paliard X, Wei WZ, Galy A (2000) Dendritic cells generated from CD34+ progenitor cells with flt3 ligand, GM-CSF, IL-4, and TNF-alpha are functional antigen-presenting cells resembling mature monocyte-derived dendritic cells. J Immunother 23:48
Fong L, Hou Y, Rivas A, Benike C, Yuen A, Fisher GA, Davis MM, Engleman EG (2001a) Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc Natl Acad Sci U S A 98:8809
Fong L, Brockstedt D, Benike C, Breen JK, Strang G, Ruegg CL, Engleman EG (2001b) Dendritic cell-based xenoantigen vaccination for prostate cancer immunotherapy. J Immunol 167:7150
Fong L, Brockstedt D, Benike C, Wu L, Engleman EG (2001c) Dendritic cells injected via different routes induce immunity in cancer patients. J Immunol 166:4254
Geiger JD, Hutchinson RJ, Hohenkirk LF, McKenna EA, Yanik GA, Levine JE, Chang AE, Braun TM, Mule JJ (2001) Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res 61:8513
Heiser A, Coleman D, Dannull J, Yancey D, Maurice MA, Lallas CD, Dahm P, Niedzwiecki D, Gilboa E, Vieweg J (2002) Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J Clin Invest 109:409
Hernando JJ, Park TW, Kubler K, Offergeld R, Schlebusch H, Bauknecht T (2002) Vaccination with autologous tumor antigen-pulsed dendritic cells in advanced gynaecological malignancies: clinical and immunological evaluation of a phase I trial. Cancer Immunol Immunother 51:45
Holtl L, Rieser C, Papesh C, Ramoner R, Bartsch G, Thurner M (1998) CD83+ blood dendritic cells as a vaccine for immunotherapy of metastatic renal-cell cancer. Lancet 352:1358
Holtl L, Rieser C, Papesh C, Ramoner R, Herold M, Klocker H, Radmayr C, Stenzl A, Bartsch G, Thurner M (1999) Cellular and humoral immune responses in patients with metastatic renal cell carcinoma after vaccination with antigen pulsed dendritic cells. J Urol 161:777
Hsu FJ, Levy R (1995) Preferential use of the VH4 immunoglobulin gene family by diffuse large cell lymphoma. Blood 86:3072
Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, Engleman EG, Levy R (1996) Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med 2:52
Itoh T, Ueda Y, Kawashima I, Nukaya I, Fujiwara H, Fuji N, Yamashita T, Yoshimura T, Okugawa K, Iwasaki T, Ideno M, Takesako K, Mitsuhashi M, Orita K, Yamagishi H (2002) Immunotherapy of solid cancer using dendritic cells pulsed with the HLA-A24-restricted peptide of carcinoembryonic antigen. Cancer Immunol Immunother 51:99
Iwashita Y, Tahara K, Goto S, Sasaki A, Kai S, Saike M, Chen C-L, Kawano K, Kitano S (2003) A phase I study of autologous dendritic cell-based immunotherapy for patients with unresectable primary liver cancer. Cancer Immunol Immunother 52:155
Jager E, Jager D, Knuth A (2002) Clinical cancer vaccine trials. Curr Opin Immunol 14:178
Johnson RO, Metter G, Wilson W, Hill G, Krementz E (1976) Phase I evaluation of DTIC (NSC-45388) and other studies in malignant melanoma in the Central Oncology Group. Cancer Treat Rep 60:183
Jonuleit H, Giesecke-Tuettenberg A, Tuting T, Thurner-Schuler B, Stuge TB, Paragnik L, Kandemir A, Lee PP, Schuler G, Knop J, Enk AH (2001) A comparison of two types of dendritic cell as adjuvants for the induction of melanoma-specific T-cell responses in humans following intranodal injection. Int J Cancer 93:243
Kadison AS, Morton DL (2003) Immunotherapy of malignant melanoma. Surg Clin North Am 83:343
Keilholz U, Gore ME (2002) Biochemotherapy for advanced melanoma. Semin Oncol 29:456
Kikuchi T, Akasaki Y, Irie M, Homma S, Ade T, Ohno T (2001) Results of a phase I clinical trial of vaccination of glioma patients with fusions of dendritic and glioma cells. Cancer Immunol Immunother 50:337
Kirk CJ, Mule JJ (2000) Gene-modified dendritic cells for use in tumor vaccines. Hum Gene Ther 11:797
Krause SW, Neumann C, Soruri A, Mayer S, Peters JH, Andreesen R (2002) The treatment of patients with disseminated malignant melanoma by vaccination with autologous cell hybrids of tumor cells and dendritic cells. J Immunother 25:421
Kugler A, Stuhler G, Walden P, Zoller G, Zobywalski A, Brossart P, Trefzer U, Ullrich S, Muller CA, Becker V, Gross AJ, Hemmerlein B, Kanz L, Muller GA, Ringert R-H (2000) Regression of metastatic renal cell carcinoma after vaccination with tumor cell-dendritic cell hybrids. Nat Med 6:332
Lau R, Wang F, Jeffery G, Marty V, Kuniyoshi J, Bade E, Ryback ME, Weber J (2001) Phase I trial of intravenous peptide-pulsed dendritic cells in patients with metastatic melanoma. J Immunother 24:66
Lim SH, Bailey-Wood R (1999) Idiotypic protein-pulsed dendritic cell vaccination in multiple myeloma. Int J Cancer 83:215
Lin C-L, Lo W-F, Lee T-H, Ren Y, Hwang S-L, Cheng Y-F, Chen C-L, Chang Y-S, Lee SP, Rickinson AB, Tam PH (2002) Immunization with Epstein-Barr virus (EBV) peptide-pulsed dendritic cells induces functional CD8+ T-cell immunity and may lead to tumor regression in patients with EBV-positive nasopharyngeal carcinoma. Cancer Res 62:6952
Lindner M, Schirrmacher V (2002) Tumor cell-dendritic cell fusion for cancer immunotherapy: comparison of therapeutic efficiency of polyethylene glycol versus electro-fusion protocols. Eur J Clin Invest 32:207
Lodge PA, Jones LA, Bader RA, Murphy GP, Salgaller ML (2000) Dendritic cell-based immunotherapy of prostate cancer: immune monitoring of a phase II clinical trial. Cancer Res 60:829
Mackensen A, Herbst B, Chen J-L, Kohler G, Noppen C, Herr W, Spagnoli GC, Cerundolo V, Lindemann A (2000) Phase I study in melanoma patients of a vaccine with peptide-pulsed dendritic cells generated in vitro from CD34+ hematopoietic progenitor cells. Int J Cancer 86:385
Mahnke K, Schmitt E, Bonifaz L, Enk AH, Jonuleit H (2002) Immature, but not inactive: the tolerogenic function of immature dendritic cells. Immunol Cell Biol 80:477
Marten A, Flieger D, Renoth S, Weineck S, Albers P, Compes M, Schottker B, Ziske C, Engelhart S, Hanfland P, Krizek L, Faber C, von Ruecker A, Muller S, Sauerbruch T, Schmidt-Wolf IGH (2002) Therapeutic vaccination against metastatic renal cell carcinoma by autologous dendritic cells: preclinical results and outcome of a first clinical phase I/II trial. Cancer Immunol Immunother 51:637
Murphy GP, Tjoa B, Ragde H, Kenny G, Boynton A (1996) Phase I clinical trial: T-cell therapy for prostate cancer using autologous dendritic cells pulsed with HLA-A0201-specific peptides from prostate-specific membrane antigen. Prostate 29:371
Murphy GP, Tjoa BA, Simmons SJ, Jarisch J, Bowes VA, Ragde H, Rogers M, Elgamal A, Kenny GM, Cobb OE, Ireton RC, Troychak MJ, Salgaller ML, Boynton AL (1999) Infusion of dendritic cells pulsed with HLA-A2-specific prostate-specific membrane antigen peptides: a phase II prostate cancer vaccine trial involving patients with hormone-refractory metastatic disease. Prostate 38:73
Nair SK, Morse M, Boczkowski D, Cumming RI, Vasovic L, Gilboa E, Lyerly HK (2002) Induction of tumor-specific cytotoxic T lymphocytes in cancer patients by autologous tumor RNA-transfected dendritic cells. Ann Surg 235:540
Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D (1998) Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med 4:328
Nishiyama T, Tachibana M, Horiguchi Y, Nakamura K, Ikeda Y, Takesako K, Murai M (2001) Immunotherapy of bladder cancer using autologous dendritic cells pulsed with human lymphocyte antigen-A24-specific MAGE-3 peptide. Clin Cancer Res 7:23
Okada H, Pollack IF, Lieberman F, Lunsford LD, Kondziolka D, Schiff D, Attanucci J, Edington H, Chambers W, Kalinski P, Kinzler D, Whiteside T, Elder E, Potter D (2001) Gene therapy of malignant gliomas: a pilot study of vaccinations with irradiated autologous glioma and dendritic cells admixed with IL-4 transduced fibroblasts to elicit an immune response. Hum Gene Ther 12:575
Oosterwijk-Wakka JC, Tiemessen DM, Bleumer I, de Vries IJM, Jongmans W, Adema GJ, Debruyne FMJ, de Mulder PH, Oosterwijk E, Mulders PFA (2002) Vaccination of patients with metastatic renal cell carcinoma with autologous dendritic cells pulsed with autologous tumor antigens in combination with interleukin-2: a phase I study. J Immunother 25:500
O’Rourke MGE, Johnson M, Lanagan C, See J, Yang J, Bell JR, Slater GJ, Kerr BM, Crowe B, Purdie DM, Elliott SL, Ellem KAO, Schmidt CW (2003) Durable complete clinical responses in a phase I/II trial using an autologous melanoma cell/dendritic cell vaccine. Cancer Immunol Immunother 52:387
Osugi Y, Vuckovic S, Hart DNJ (2002) Myeloid blood CD11c+ dendritic cells and monocyte-derived dendritic cells differ in their ability to stimulate T lymphocytes. Blood 100:2858
Panelli MC, Wunderlich J, Jeffries J, Wang E, Mixon A, Rosenberg SA, Marincola FM (2000) Phase I study in patients with metastatic melanoma of immunization with dendritic cells presenting epitopes derived from the melanoma-associated antigens MART-1 and gp100. J Immunother 23:487
Pecher G, Horing A, Kaiser L, Thiel E (2002) Mucin gene (MUC1) transfected dendritic cells as vaccine: results of a phase I/II clinical trial. Cancer Immunol Immunother 51:669
Perez-Diez A, Marincola FM (2002) Immunotherapy against antigenic tumors: a game with a lot of players. Cell Mol Life Sci 59:230
Rains N, Cannan RJ, Chen W, Stubbs RS (2001) Development of a dendritic cell (DC)-based vaccine for patients with advanced colorectal cancer. Hepatogastroenterology 48:347
Renkvist N, Castelli C, Robbins PF, Parmiani G (2001) A listing of human tumor antigens recognized by T cells. Cancer Immunol Immunother 50:3
Rieser C, Ramoner R, Holtl L, Rogatsch H, Papesh C, Stenzl A, Bartsch G, Thurner M (1999) Mature dendritic cells induce T-helper type-1-dominant immune responses in patients with metastatic renal cell carcinoma. Urol Int 63:151
Rock KL, Fleischacker C, Gamble S (1993) Peptide-priming of cytolytic T cell immunity in vivo using β2-microglobulin as an adjuvant. J Immunol 150:1244
Sadanaga N, Nagashima H, Mashino K, Tahara K, Yamaguchi H, Ohta M, Fujie T, Tanaka F, Inoue H, Takesako K, Akiyoshi T, Mori M (2001) Dendritic cell vaccination with MAGE peptide is a novel therapeutic approach for gastrointestinal carcinomas. Clin Cancer Res 7:2277
Santin AD, Hermonat PL, Ravaggi A, Chiriva-Internati M, Zhan D, Pecorelli S, Parham GP, Cannon MJ (1999) Induction of human papillomavirus-specific CD4+ and CD8+ lymphocytes by E7-pulsed autologous dendritic cells in patients with human papillomavirus type 16- and 18-positive cervical cancer. J Virol 73:5402
Santin AD, Bellone S, Gokden M, Cannon MJ, Parham GP (2002) Vaccination with HPV-18 E7-pulsed dendritic cells in a patient with metastatic cervical cancer. N Engl J Med 346:1752
Schott M, Feldkamp J, Schattenberg D, Seissler J, Scherbaum WA (1999a) Dendritic cell immunotherapy in disseminated parathyroid carcinoma. Lancet 353:1188
Schott M, Seissler J, Feldkamp J, von Schilling C, Scherbaum WA (1999b) Dendritic cell immunotherapy induces antitumor response in parathyroid carcinoma and neuroendocrine pancreas carcinoma. Horm Metab Res 31:662
Schott M, Feldkamp J, Lettmann M, Simon D, Scherbaum WA, Seissler J (2001a) Dendritic cell immunotherapy in a neuroendocrine pancreas carcinoma. Clin Endocrinol 55:271
Schott M, Seissler J, Lettmann M, Fouxon V, Scherbaum WA, Feldkamp J (2001b) Immunotherapy for medullary thryroid carcinoma by dendritic cell vaccination. J Clin Endocrinol Metab 86:4965
Schott M, Feldkamp M, Kobbe G, Scherbaum WA, Seissler J (2002) Calcitonin-specific antitumor immunity in medullary thyroid carcinoma following dendritic cell vaccination. Cancer Immunol Immunother 51:663
Schuler-Thurner B, Dieckmann D, Keikavoussi P, Bender A, Maczek C, Jonuleit H, Roder C, Haendle I, Leisgang W, Dunbar R, Cerundolo V, von den Driesch P, Knop J, Brocker EB, Enk A, Kampgen E, Schuler G (2000) Mage-3 and influenza-matrix peptide-specific cytotoxic T cells are inducible in terminal stage HLA A2.1+ melanoma patients by mature monocyte-derived dendritic cells. J Immunol 165:3492
Schuler-Thurner B, Schultz ES, Berger TG, Weinlich G, Ebner S, Woerl P, Bender A, Feurerstein B, Fritsch PO, Romani N, Schuler G (2002) Rapid induction of tumor-specific type 1 helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J Exp Med 195:1279
Simmons SJ, Tjoa BA, Rogers M, Elgamal A, Kenny GM, Ragde H, Troychak MJ, Boynton AL, Murphy GP (1999) GM-CSF as a systemic adjuvant in a phase II prostate cancer vaccine trials. Prostate 39:291
Small EJ, Fratesi P, Reese DM, Strang G, Laus R, Peshwa MV, Valone FH (2000) Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol 18:3894
Smithers M, O’Connell K, MacFadyen S, Chambers M, Greenwood K, Boyce A, Abdul-Jabbar I, Barker K, Grimmett K, Walpole E, Thomas R (2003) Clinical response after intradermal immature dendritic cell vaccination in metastatic melanoma is associated with immune response to particulate antigen. Cancer Immunol Immunother 52:41
Stift A, Friedl J, Dubsky P, Bachleitner-Hofmann T, Schueller G, Zontsich T, Benkoe T, Radelbauer K, Brostjan C, Jakesz R, Gnant M (2003) Dendritic cell-based vaccination in solid cancer. J Clin Oncol 21:135
Thurner B, Haendle I, Roder C, Dieckmann D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von den Driesch P, Brocker EB, Steinman RM, Enk A, Kampgen E, Schuler G (1999a) Vaccination with Mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cell expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med 190:1669
Thurner B, Roder C, Dieckmann D, Heuer M, Kruse M, Glaser A, Keikavoussi P, Kampgen E, Bender A, Schuler G (1999b) Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J Immunol Methods 223:1
Timmerman JM, Levy R (2000) Linkage of foreign carrier protein to a self-tumor antigen enhances the immunogenicity of a pulsed dendritic cell vaccine. J Immunol 164:4797
Timmerman JM, Czerwinski DK, Davis TA, Hsu FJ, Benike C, Hao ZM, Taidi B, Rajapaksa R, Caspar CB, Okada CY, van Beckhoven A, Liles TM, Engleman EG, Levy R (2002) Idiotype-pulsed dendritic cell vaccination for B-cell lymphoma: clinical and immune responses in 35 patients. Blood 99:1517
Titzer S, Christensen O, Manzke O, Tesch H, Wolf J, Emmerich B, Carsten C, Diehl V, Bohlen H (2000) Vaccination of multiple myeloma patients with idiotype-pulsed dendritic cells: immunological and clinical aspects. Br J Haematol 108:805
Tjoa BA, Erickson SJ, Bowes VA, Ragde H, Kenny GM, Cobb OE, Ireton RC, Troychak MJ, Boynton AL, Murphy GP (1997) Follow-up evaluation of prostate cancer patients infused with autologous dendritic cells pulsed with PSMA peptides. Prostate 32:272
Tjoa BA, Simmons SJ, Bowes VA, Ragde H, Rogers M, Elgamal A, Kenny GM, Cobb OE, Ireton RC, Troychak MJ, Salgaller ML, Boynton AL, Murphy GP (1998) Evaluation of phase I/II clinical trials in prostate cancer with dendritic cells and PSMA peptides. Prostate 36:39
Tjoa BA, Simmons SJ, Elgamal A, Rogers M, Ragde H, Kenny GM, Troychak MJ, Boynton AL, Murphy GP (1999) Follow-up evaluation of a phase II prostate cancer vaccine trials. Prostate 40:125
Toungouz M, Libin M, Bulte F, Faid L, Lehmann F, Duriau D, Laporte M, Gangji D, Bruyns C, Lambermont M, Goldman M, Velu T (2001) Transient expansion of peptide-specific lymphocytes producing IFN-γ after vaccination with dendritic cells pulsed with MAGE peptides in patients with mage-A1/A3-positive tumors. J Leukoc Biol 69:937
Wagner DE, Ramirez G, Weiss AJ, Hill G Jr (1971) Combination phase I-II study of imidazole carboxamide (NCS 45388). Oncology 26:310
Wen Y-J, Ling M, Bailey-Wood R, Lim SH (1998) Idiotypic protein-pulsed adherent peripheral blood mononuclear cell-derived dendritic cells prime immune system in multiple myeloma. Clin Cancer Res 4:957
Yi Q, Desikan R, Barlogie B, Munshi N (2002) Optimizing dendritic cell-based immunotherapy in multiple myeloma. Br J Haematol 117:297
Yu JS, Wheeler CJ, Zeltzer PM, Ying H, Finger DN, Lee PK, Yong WH, Incardona F, Thompson RC, Riedinger MS, Zhang W, Prins RM, Black KL (2001) Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res 61:842
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cranmer, L.D., Trevor, K.T. & Hersh, E.M. Clinical applications of dendritic cell vaccination in the treatment of cancer. Cancer Immunol Immunother 53, 275–306 (2004). https://doi.org/10.1007/s00262-003-0432-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-003-0432-5