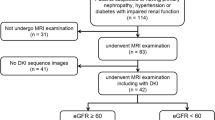
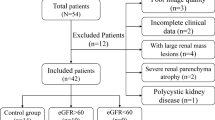
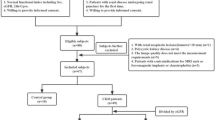
Abstract
Purpose
This study used various diffusion-weighted imaging (DWI) models (including monoexponential, biexponential, stretched-exponential and kurtosis models) in renal magnetic resonance imaging (MRI) to compare whether there were differences in each diffusion parameter between patients with primary aldosteronism (PA) and healthy volunteers.
Materials and methods
Twenty-two (female:male, 14:8; age, 48 ± 10 years) patients with PA and 22 age- and sex-matched healthy controls (HCs) underwent MRI examinations of the kidneys. The independent-sample t test or the Mann‒Whitney U test was used to detect differences in the diffusion metrics of the kidneys between the two groups. Univariable and multivariable linear regression were applied to analyze the correlations between diffusion parameters and the clinical indicators.
Results
The mean diffusivity (MD, p < 0.001) and radial diffusivity (Dr, p < 0.001) values in the medulla were lower in the PA group than in the HC group. The medullary fractional anisotropy (FA, p < 0.001) was higher than that of HCs. The FA (p < 0.001) and axial diffusivity (Da, p < 0.001) values in the cortex were lower in the PA group. The cortical α (anomalous exponent term, p = 0.016) was higher in the PA patients than in the HCs. Linear regression analysis showed that log(plasma aldosterone concentration) and the estimated glomerular filtration rate (eGFR) were correlated with medullary FA.
Conclusion
The stretched-exponential model (cortical α) and the kurtosis model (FA, MD and Dr in the medulla and FA and Da in the cortex) showed significant differences between PA patients and healthy volunteers and may have potential for noninvasive renal assessment in PA patients.
Graphical abstract




Similar content being viewed by others
References
Funder JW, Carey RM, Mantero F, et al. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2016;101(5): 1889-1916.
Onohara T, Takagi T, Yoshida K, et al. Assessment of postoperative renal function after adrenalectomy in patients with primary aldosteronism. Int J Urol 2019;26(2):229–233.
Hollenberg NK. Aldosterone in the development and progression of renal injury. Kidney Int 2004;66(1):1–9.
Kim DH, Kwon HJ, Ji SA, et al. Risk factors for renal impairment revealed after unilateral adrenalectomy in patients with primary aldosteronism. Medicine 2016;95(27): e3930.
Monticone S, Sconfienza E, D'Ascenzo F, et al. Renal damage in primary aldosteronism: a systematic review and meta-analysis. J Hypertens 2020;38(1):3–12.
Lai L, Chen J, Hao CM, Lin S, Gu Y. Aldosterone promotes fibronectin production through a Smad2-dependent TGF-beta 1 pathway in mesangial cells. Biochem Biophys Res Commun 2006;348(1):70–75.
Reincke M, Rump LC, Quinkler M, et al. Risk factors associated with a low glomerular filtration rate in primary aldosteronism. J Clin Endocrinol Metab. 2009;94(3):869-875.
Kobayashi H, Abe M, Nakamura Y, et al. Association Between Acute Fall in Estimated Glomerular Filtration Rate After Treatment for Primary Aldosteronism and Long-Term Decline in Renal Function. Hypertension. 2019;74(3):630-638.
Lu YC, Liu KL, Wu VC, et al. Factors associated with renal function change after unilateral adrenalectomy in patients with primary aldosteronism. Int J Urol. 2022;29(8):831-837.
Ding Y, Tan Q, Mao W, et al. Differentiating between malignant and benign renal tumors: do IVIM and diffusion kurtosis imaging perform better than DWI? Eur Radiol 2019;29(12):6930–6939.
Mao W, Zhou J, Zeng M, et al. Chronic kidney disease: Pathological and functional evaluation with intravoxel incoherent motion diffusion-weighted imaging. J Magn Reson Imaging 2018;47(5):1251–1259.
Zhu Q, Ye J, Zhu W, Wu J, Chen W, Ling J. Functional magnetic resonance imaging for distinguishing type of papillary renal cell carcinoma: a preliminary study. Br J Radiol 2021;94(1126):20201315.
Pentang G, Lanzman RS, Heusch P, et al. Diffusion kurtosis imaging of the human kidney: A feasibility study. Magn Reson Imaging 2014;32(5):413–420.
Zhu Q, Xu Q, Dou W, et al. Diffusion kurtosis imaging features of renal cell carcinoma: a preliminary study. Br J Radiol 2021;94(1122):20201374.
Fu J, Ye J, Zhu W, Wu J, Chen W, Zhu Q. Magnetic resonance diffusion kurtosis imaging in differential diagnosis of benign and malignant renal tumors. Cancer Imaging 2021;21(1):6.
Zheng X, Li M, Wang P, et al. Assessment of chronic allograft injury in renal transplantation using diffusional kurtosis imaging. BMC Med Imaging 2021;21(1):63.
Liu Y, Zhang GM, Peng X, Li X, Sun H, Chen L. Diffusion kurtosis imaging as an imaging biomarker for predicting prognosis in chronic kidney disease patients. Nephrol Dial Transplant 2022;37(8):1451–1460.
Mao W, Ding Y, Ding X, Fu C, Zeng M, Zhou J. Diffusion kurtosis imaging for the assessment of renal fibrosis of chronic kidney disease: A preliminary study. Magn Reson Imaging 2021;80:113–120.
Wang B, Wang Y, Li L, et al. Diffusion kurtosis imaging and arterial spin labeling for the noninvasive evaluation of persistent post-contrast acute kidney injury. Magn Reson Imaging 2022; 87:47–55.
Zhang J, Suo S, Liu G, et al. Comparison of Monoexponential, Biexponential, Stretched-Exponential, and Kurtosis Models of Diffusion-Weighted Imaging in Differentiation of Renal Solid Masses. Korean J Radiol 2019;20(5):791–800.
Liu Y, Wang X, Cui Y, et al. Comparative Study of Monoexponential, Intravoxel Incoherent Motion, Kurtosis, and IVIM-Kurtosis Models for the Diagnosis and Aggressiveness Assessment of Prostate Cancer. Front Oncol 2020;10:1763.
Bai Y, Lin Y, Tian J, et al. Grading of Gliomas by Using Monoexponential, Biexponential, and Stretched Exponential Diffusion-weighted MR Imaging and Diffusion Kurtosis MR Imaging. Radiology 2016;278(2):496–504.
Song J, Lu Y, Wang X, et al. A comparative study of four diffusion-weighted imaging models in the diagnosis of cervical cancer. Acta Radiol 2022;63(4):536–544.
Wu T, Ren Y, Wang W, et al. Left Ventricular Remodeling in Patients with Primary Aldosteronism: A Prospective Cardiac Magnetic Resonance Imaging Study. Korean J Radiol 2021;22(10):1619–1627.
Li H, Liang L, Li A, et al. Monoexponential, biexponential, and stretched exponential diffusion-weighted imaging models: Quantitative biomarkers for differentiating renal clear cell carcinoma and minimal fat angiomyolipoma. J Magn Reson Imaging 2017;46(1):240–247.
Huang Y, Chen X, Zhang Z, et al. MRI quantification of non-Gaussian water diffusion in normal human kidney: a diffusional kurtosis imaging study. NMR Biomed 2015;28(2):154–161.
Rosenkrantz AB, Padhani AR, Chenevert TL, et al. Body diffusion kurtosis imaging: Basic principles, applications, and considerations for clinical practice. J Magn Reson Imaging 2015;42(5):1190–1202.
Nakamura Y, Kobayashi H, Tanaka S, Hatanaka Y, Fukuda N, Abe M. Association between plasma aldosterone and markers of tubular and glomerular damage in primary aldosteronism. Clin Endocrinol 2021; 94(6):920–926.
Liu Y, Zhang GMY, Peng X, Wen Y, Ye W, Zheng K, et al. Diffusional kurtosis imaging in assessing renal function and pathology of IgA nephropathy: a preliminary clinical study Clinical. Clin Radiol 2018;73(9):818–826.
Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed 2010;23(7):698–710.
Feng Y-Z, Ye YJ, Cheng ZY, Hu JJ, Zhang C-B, Qian L, et al. Non-invasive assessment of early stage diabetic nephropathy by DTI and BOLD MRI. Br J Radiol 2020;93(1105):20190562.
Liu Z, Xu Y, Zhang J, et al. Chronic kidney disease: pathological and functional assessment with diffusion tensor imaging at 3T MR. Eur Radiol 2015;25(3):652–660.
Li XS, Zhang QJ, Zhu J, et al. Assessment of kidney function in chronic kidney disease by combining diffusion tensor imaging and total kidney volume. Int Urol Nephrol 2022;54(2):385–393.
Chen X, Xiao W, Li X, He J, Huang X, Tan Y. In vivo evaluation of renal function using diffusion weighted imaging and diffusion tensor imaging in type 2 diabetics with normoalbuminuria versus microalbuminuria. Front Med 2014;8(4):471–476.
Saini S, Kumar V, Koteshwara P. Role of Diffusion Tensor Imaging in renal parenchymal changes. Indian J Radiol Imaging 2018; 28(2):175–181.
Rogers HJ, Verhagen MV, Clark CA, Hales PW. Comparison of models of diffusion in Wilms' tumours and normal contralateral renal tissue. MAGMA 2021;34(2):261–271.
Kusunoki M, Kikuchi K, Togao O, et al. Differentiation of high-grade from low-grade diffuse gliomas using diffusion-weighted imaging: a comparative study of mono-, bi-, and stretched-exponential diffusion models. Neuroradiology 2020;62(7):815–823.
Liu X, Zhou L, Peng W, Wang H, Zhang Y. Comparison of Stretched-Exponential and Monoexponential Model Diffusion-Weighted Imaging in Prostate Cancer and Normal Tissues. J Magn Reson Imaging 2015; 42(4):1078–1085.
Funding
This study was supported by the 1.3.5 Project for disciplines of excellence, West China Hospital, Sichuan University [Grant No. ZYGD18022].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wen, D., Xu, C., Deng, L. et al. Monoexponential, biexponential, stretched-exponential and kurtosis models of diffusion-weighted imaging in kidney assessment: comparison between patients with primary aldosteronism and healthy controls. Abdom Radiol 48, 1340–1349 (2023). https://doi.org/10.1007/s00261-023-03833-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-023-03833-0