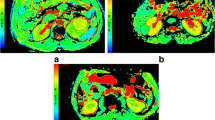
Abstract
Objective
To quantitatively compare the diagnostic values of conventional diffusion-weighted imaging (DWI), intravoxel incoherent motion (IVIM), and diffusion kurtosis imaging (DKI) in differentiating between malignant and benign renal tumors.
Methods
Multiple b value DWIs and DKIs were performed in 180 patients with renal tumors, which were divided into clear cell renal cell carcinoma (ccRCC), non-ccRCC, and benign renal tumor group. The apparent diffusion coefficient (ADC), true diffusivity (D), pseudo-diffusion coefficient (D*), perfusion fraction (f), mean kurtosis (MK), and mean diffusivity (MD) maps were calculated. The diagnostic efficacy of various diffusion parameters for predicting malignant renal tumors was compared.
Results
The ADC, D, and MD values of ccRCCs were higher, while D*, f, and MK values were lower than those of benign renal tumors (all p < 0.025). The D* and f values of non-ccRCCs were lower than those of benign renal tumors (p = 0.002 and p < 0.001, respectively). The difference of ADC, D, MD, and MK values between non-ccRCCs and benign renal tumors was not statistically significant (p > 0.05). The ADC, D, MD, and f values of ccRCCs were higher, while MK values were lower than those of non-ccRCCs (all p < 0.001). The AUC values of ADC, D, D*, f, MK, and MD were 0.849, 0.891, 0.708, 0.656, 0.862, and 0.838 for differentiating ccRCCs from benign renal tumors, respectively. The AUC values of D* and f were 0.772 and 0.866 for discrimination between non-ccRCCs and benign renal tumors, respectively.
Conclusion
IVIM parameters are the best, while DWI and DKI parameters have similar performance in differentiating malignant and benign renal tumors.
Key Points
• The D value is the best parameter for differentiating ccRCC from benign renal tumors.
• The f value is the best parameter for differentiating non-ccRCC from benign renal tumors.
• Conventional DWI and DKI have similar performance in differentiating malignant and benign renal tumors.





Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Areas under the curves
- ccRCC:
-
Clear cell renal cell carcinoma
- CI:
-
Confidence interval
- D :
-
True diffusivity
- D* :
-
Pseudo-diffusion coefficient
- DKI:
-
Diffusion kurtosis imaging
- DWI:
-
Diffusion-weighted imaging
- f :
-
Perfusion fraction
- ICC:
-
Intraclass correlation coefficient
- IVIM:
-
Intravoxel incoherent motion
- MD:
-
Mean diffusivity
- MK:
-
Mean kurtosis
- non-ccRCC:
-
Non-clear cell renal cell carcinoma
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- SPIR:
-
Spectral presaturation inversion recovery
- TE:
-
Echo time
- TR:
-
Repetition time
References
Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK (2006) Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst 98:1331–1334
Kutikov A, Fossett LK, Ramchandani P et al (2006) Incidence of benign pathologic findings at partial nephrectomy for solitary renal mass presumed to be renal cell carcinoma on preoperative imaging. Urology 68:737–740
Fujii Y, Komai Y, Saito K et al (2008) Incidence of benign pathologic lesions at partial nephrectomy for presumed RCC renal masses: Japanese dual-center experience with 176 consecutive patients. Urology 72:598–602
Lee SH, Park SU, Rha KH et al (2010) Trends in the incidence of benign pathological lesions at partial nephrectomy for presumed renal cell carcinoma in renal masses on preoperative computed tomography imaging: a single institute experience with 290 consecutive patients. Int J Urol 17:512–516
Bauman TM, Potretzke AM, Wright AJ, Knight BA, Vetter JM, Figenshau RS (2017) Partial nephrectomy for presumed renal-cell carcinoma: incidence, predictors, and perioperative outcomes of benign lesions. J Endourol 31:412–417
Forbes CM, Rendon RA, Finelli A et al (2016) Disease progression and kidney function after partial vs. radical nephrectomy for T1 renal cancer. Urol Oncol 34:417–486
Jewett MA, Mattar K, Basiuk J et al (2011) Active surveillance of small renal masses: progression patterns of early stage kidney cancer. Eur Urol 60:39–44
Malayeri AA, El KR, Zaheer A et al (2011) Principles and applications of diffusion-weighted imaging in cancer detection, staging, and treatment follow-up. Radiographics 31:1773–1791
Ye J, Kumar BS, Li XB, Li HM, Zhou YW, Liu LQ (2017) Clinical applications of diffusion-weighted magnetic resonance imaging in diagnosis of renal lesions - a systematic review. Clin Physiol Funct Imaging 37:459–473
Li Y, Wang Y, Qin J, Wu J, Dai X, Xu J (2018) Meta-analysis of diffusion-weighted imaging in the differential diagnosis of renal lesions. Clin Imaging 52:264–272
Le Bihan D, Turner R (1992) The capillary network: a link between IVIM and classical perfusion. Magn Reson Med 27:171–178
Zhang JL, Sigmund EE, Chandarana H et al (2010) Variability of renal apparent diffusion coefficients: limitations of the monoexponential model for diffusion quantification. Radiology 254:783–792
Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M (1986) MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 161:401–407
Rosenkrantz AB, Padhani AR, Chenevert TL et al (2015) Body diffusion kurtosis imaging: basic principles, applications, and considerations for clinical practice. J Magn Reson Imaging 42:1190–1202
Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K (2005) Diffusional kurtosis imaging: the quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 53:1432–1440
Chandarana H, Kang SK, Wong S et al (2012) Diffusion-weighted intravoxel incoherent motion imaging of renal tumors with histopathologic correlation. Invest Radiol 47:688–696
Gaing B, Sigmund EE, Huang WC et al (2015) Subtype differentiation of renal tumors using voxel-based histogram analysis of intravoxel incoherent motion parameters. Invest Radiol 50:144–152
Shen L, Zhou L, Liu X, Yang X (2017) Comparison of biexponential and monoexponential DWI in evaluation of Fuhrman grading of clear cell renal cell carcinoma. Diagn Interv Radiol 23:100–105
Zhu Q, Zhu W, Ye J, Wu J, Chen W, Hao Z (2019) Value of intravoxel incoherent motion for differential diagnosis of renal tumors. Acta Radiol 60:382–387
Ding Y, Zeng M, Rao S, Chen C, Fu C, Zhou J (2016) Comparison of biexponential and monoexponential model of diffusion-weighted imaging for distinguishing between common renal cell carcinoma and fat poor angiomyolipoma. Korean J Radiol 17:853–863
Dai Y, Yao Q, Wu G et al (2016) Characterization of clear cell renal cell carcinoma with diffusion kurtosis imaging: correlation between diffusion kurtosis parameters and tumor cellularity. NMR Biomed 29:873–881
Iima M, Kataoka M, Kanao S et al (2018) Intravoxel incoherent motion and quantitative non-Gaussian diffusion MR imaging: evaluation of the diagnostic and prognostic value of several markers of malignant and benign breast lesions. Radiology 287:432–441
Xiao Z, Zhong Y, Tang Z et al (2018) Standard diffusion-weighted, diffusion kurtosis and intravoxel incoherent motion MR imaging of sinonasal malignancies: correlations with Ki-67 proliferation status. Eur Radiol 28:2923–2933
Wan Q, Deng YS, Lei Q et al (2019) Differentiating between malignant and benign solid solitary pulmonary lesions: are intravoxel incoherent motion and diffusion kurtosis imaging superior to conventional diffusion-weighted imaging? Eur Radiol 29:1607–1615
Lu Y, Jansen JF, Mazaheri Y, Stambuk HE, Koutcher JA, Shukla-Dave A (2012) Extension of the intravoxel incoherent motion model to non-Gaussian diffusion in head and neck cancer. J Magn Reson Imaging 36:1088–1096
Luciani A, Vignaud A, Cavet M et al (2008) Liver cirrhosis: intravoxel incoherent motion MR imaging--pilot study. Radiology 249:891–899
Wang WT, Yang L, Yang ZX et al (2018) Assessment of microvascular invasion of hepatocellular carcinoma with diffusion kurtosis imaging. Radiology 286:571–580
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Moch H, Humphrey PA, Ulbright TM, Reuter VE (2016) WHO classification of tumours of the urinary system and male genital organs, 4th edn. IARC, Lyon
Squillaci E, Manenti G, Cova M et al (2004) Correlation of diffusion-weighted MR imaging with cellularity of renal tumours. Anticancer Res 24:4175–4179
Tanaka H, Yoshida S, Fujii Y et al (2011) Diffusion-weighted magnetic resonance imaging in the differentiation of angiomyolipoma with minimal fat from clear cell renal cell carcinoma. Int J Urol 18:727–730
Agnello F, Roy C, Bazille G et al (2013) Small solid renal masses: characterization by diffusion-weighted MRI at 3 T. Clin Radiol 68:e301–e308
Sasamori H, Saiki M, Suyama J, Ohgiya Y, Hirose M, Gokan T (2014) Utility of apparent diffusion coefficients in the evaluation of solid renal tumors at 3T. Magn Reson Med Sci 13:89–95
Chandarana H, Lee VS, Hecht E, Taouli B, Sigmund EE (2011) Comparison of biexponential and monoexponential model of diffusion weighted imaging in evaluation of renal lesions: preliminary experience. Invest Radiol 46:285–291
Zhang J, Lefkowitz RA, Ishill NM et al (2007) Solid renal cortical tumors: differentiation with CT. Radiology 244:494–504
Sasiwimonphan K, Takahashi N, Leibovich BC, Carter RE, Atwell TD, Kawashima A (2012) Small (<4 cm) renal mass: differentiation of angiomyolipoma without visible fat from renal cell carcinoma utilizing MR imaging. Radiology 263:160–168
Vargas HA, Chaim J, Lefkowitz RA et al (2012) Renal cortical tumors: use of multiphasic contrast-enhanced MR imaging to differentiate benign and malignant histologic subtypes. Radiology 264:779–788
Koh DM, Collins DJ, Orton MR (2011) Intravoxel incoherent motion in body diffusion-weighted MRI: reality and challenges. AJR Am J Roentgenol 196:1351–1361
Pan J, Zhang H, Man F et al (2018) Measurement and scan reproducibility of parameters of intravoxel incoherent motion in renal tumor and normal renal parenchyma: a preliminary research at 3.0 T MR. Abdom Radiol (NY) 43:1739–1748
Li HM, Zhao SH, Qiang JW et al (2017) Diffusion kurtosis imaging for differentiating borderline from malignant epithelial ovarian tumors: a correlation with Ki-67 expression. J Magn Reson Imaging 46:1499–1506
Roethke MC, Kuder TA, Kuru TH et al (2015) Evaluation of diffusion kurtosis imaging versus standard diffusion imaging for detection and grading of peripheral zone prostate cancer. Invest Radiol 50:483–489
Tamura C, Shinmoto H, Soga S et al (2014) Diffusion kurtosis imaging study of prostate cancer: preliminary findings. J Magn Reson Imaging 40:723–729
Das SK, Yang DJ, Wang JL, Zhang C, Yang HF (2017) Non-Gaussian diffusion imaging for malignant and benign pulmonary nodule differentiation: a preliminary study. Acta Radiol 58:19–26
Yang L, Rao S, Wang W et al (2018) Staging liver fibrosis with DWI: is there an added value for diffusion kurtosis imaging? Eur Radiol 28:3041–3049
Acknowledgements
We thank Caixia Fu (Siemens Healthcare) for providing the prototype diffusion sequence used in this study and for the excellent technical support.
Funding
This study has received funding by the Educational Specialist Training Fund (No.002) from Zhongshan Hospital, Fudan University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Jianjun Zhou.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• cross-sectional study/diagnostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jianjun Zhou and Mengsu Zeng have the same contribution to the design and guidance of this manuscript. As a result, they are listed as co-corresponding authors of this article.
Rights and permissions
About this article
Cite this article
Ding, Y., Tan, Q., Mao, W. et al. Differentiating between malignant and benign renal tumors: do IVIM and diffusion kurtosis imaging perform better than DWI?. Eur Radiol 29, 6930–6939 (2019). https://doi.org/10.1007/s00330-019-06240-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06240-6