Abstract
Purpose
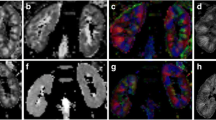
To evaluate the potential of 3 T magnetic resonance diffusion kurtosis imaging (DKI) in assessing the renal damage in early-stage of chronic kidney disease (CKD) patients with normal or slightly changed functional index, using histopathology as reference standard.
Methods
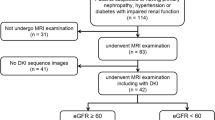
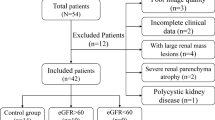
49 CKD patients and 18 healthy volunteers were recruited in this study. CKD patients were divided into two groups based on estimated glomerular filtration rate (eGFR): Study group I (eGFR ≥ 90 ml/min/1.73 m2 [n = 20]) and Study group II (eGFR < 90 ml/min/1.73 m2 [n = 29]). DKI was performed in all participants. The DKI parameters (mean kurtosis [MK], mean diffusivity [MD], fractional anisotropy [FA]) of renal cortex and medulla were measured. The differences of parenchymal MD, MK and FA values among the different groups were compared. The correlations between DKI parameters and clinicopathological characteristics were assessed. Diagnostic performance of DKI to assess renal damage in early-stage of CKD was analyzed.
Results
The cortex MD and MK showed significant difference among three groups (P < 0.05): trend of cortex MD: Study group II < Study group I < control group; trend of cortex MK: control group < Study group I < Study group II. The cortex MD and MK and medulla FA were correlated with eGFR and Interstitial fibrosis/Tubular atrophy score (0.3 < r < 0.5). Cortex MD and MK yielded an AUC of 0.752 for differentiating healthy volunteers from CKD patients with eGFR ≥ 90 ml/min/1.73 m2.
Conclusion
DKI shows potential in non-invasive and multi-parameter quantitative assessment of renal damage in early-stage of CKD patients and provide additional information for changes in renal function and histopathology.





Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Obrador GT, Levin A (2019) CKD hotspots: challenges and areas of opportunity. Semin Nephrol 39(3):308–314. https://doi.org/10.1016/j.semnephrol.2019.02.009
Prigent A (2008) Monitoring renal function and limitations of renal function tests. Semin Nucl Med 38(1):32–46. https://doi.org/10.1053/j.semnuclmed.2007.09.003
Pokhrel A, Agrawal RK, Baral A, Rajbhandari A, Hada R (2018) Percutaneous renal biopsy: comparison of blind and real-time ultrasound guided technique. J Nepal Health Res Counc 16(1):66–72
Uppot RN, Harisinghani MG, Gervais DA (2010) Imaging-guided percutaneous renal biopsy: rationale and approach. AJR Am J Roentgenol 194(6):1443–1449. https://doi.org/10.2214/AJR.10.4427
Hostetter TH, Rennke HG, Brenner BM (1982) Compensatory renal hemodynamic injury: a final common pathway of residual nephron destruction. Am J Kidney Dis 1(5):310–314. https://doi.org/10.1016/s0272-6386(82)80032-2
Jensen JH, Helpern JA (2010) MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed 23(7):698–710. https://doi.org/10.1002/nbm.1518
Dai Y, Yao Q, Wu G, Wu D, Wu L, Zhu L, Xue R, Xu J (2016) Characterization of clear cell renal cell carcinoma with diffusion kurtosis imaging: correlation between diffusion kurtosis parameters and tumor cellularity. NMR Biomed 29(7):873–881. https://doi.org/10.1002/nbm.3535
Rosenkrantz AB, Padhani AR, Chenevert TL, Koh DM, De Keyzer F, Taouli B, Le Bihan D (2015) Body diffusion kurtosis imaging: basic principles, applications, and considerations for clinical practice. J Magn Reson Imaging 42(5):1190–1202. https://doi.org/10.1002/jmri.24985
Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K (2005) Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 53(6):1432–1440. https://doi.org/10.1002/mrm.20508
Huang Y, Chen X, Zhang Z, Yan L, Pan D, Liang C, Liu Z (2015) MRI quantification of non-Gaussian water diffusion in normal human kidney: a diffusional kurtosis imaging study. NMR Biomed 28(2):154–161. https://doi.org/10.1002/nbm.3235
Kjølby BF, Khan AR, Chuhutin A, Pedersen L, Jensen JB, Jakobsen S, Zeidler D, Sangill R, Nyengaard JR, Jespersen SN, Hansen B (2016) Fast diffusion kurtosis imaging of fibrotic mouse kidneys. NMR Biomed 29(12):1709–1719. https://doi.org/10.1002/nbm.3623
Liang P, Li S, Yuan G, He K, Li A, Hu D, Li Z, Xu C (2022) Noninvasive assessment of clinical and pathological characteristics of patients with IgA nephropathy by diffusion kurtosis imaging. Insights Imaging 13(1):18. https://doi.org/10.1186/s13244-022-01158-y
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
Mao W, Ding Y, Ding X, Fu C, Zeng M, Zhou J (2021) Diffusion kurtosis imaging for the assessment of renal fibrosis of chronic kidney disease: a preliminary study. Magn Reson Imaging 80:113–120. https://doi.org/10.1016/j.mri.2021.05.002
Sethi S, D’Agati VD, Nast CC et al (2017) A proposal for standardized grading of chronic changes in native kidney biopsy specimens. Kidney Int 91:787–789. https://doi.org/10.1016/j.kint.2017.01.002
Katafuchi R, Kiyoshi Y, Oh Y, Uesugi N, Ikeda K, Yanase T, Fujimi S (1998) Glomerular score as a prognosticator in IgA nephropathy: its usefulness and limitation. Clin Nephrol 49(1):1–8
Wu XF, Liang X, Wang XC, Qin JB, Zhang L, Tan Y, Zhang H (2021) Differentiating high-grade glioma recurrence from pseudoprogression: comparing diffusion kurtosis imaging and diffusion tensor imaging. Eur J Radiol 135:109445. https://doi.org/10.1016/j.ejrad.2020.109445
Liang P, Chen Y, Li S, Xu C, Yuan G, Hu D, Kamel I, Zhang Y, Li Z (2021) Noninvasive assessment of kidney dysfunction in children by using blood oxygenation level-dependent MRI and intravoxel incoherent motion diffusion-weighted imaging. Insights Imaging 12(1):146. https://doi.org/10.1186/s13244-021-01091-6
Kriz W (1981) Structural organization of the renal medulla: comparative and functional aspects. Am J Physiol 241(1):R3-16. https://doi.org/10.1152/ajpregu.1981.241.1.R3
Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM (1981) Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol 241(1):F85-93. https://doi.org/10.1152/ajprenal.1981.241.1.F85
Gaudiano C, Clementi V, Busato F, Corcioni B, Orrei MG, Ferramosca E, Fabbri E, Berardi P, Santoro A, Golfieri R (2013) Diffusion tensor imaging and tractography of the kidneys: assessment of chronic parenchymal diseases. Eur Radiol 23(6):1678–1685. https://doi.org/10.1007/s00330-012-2749-y
Tang X, Wen Q, Zhou Q, Yang Q, Chen W, Yu X (2022) Prognostic significance of the extent of tubulointerstitial lesions in patients with IgA nephropathy. Int Urol Nephrol. https://doi.org/10.1007/s11255-022-03286-2
Yang W, Mou S, Xu Y, Du J, Xu L, Li F, Li H (2018) Contrast-enhanced ultrasonography for assessment of tubular atrophy/interstitial fibrosis in immunoglobulin A nephropathy: a preliminary clinical study. Abdom Radiol (NY) 43(6):1423–1431. https://doi.org/10.1007/s00261-017-1301-6
Turner CM, Arulkumaran N, Singer M, Unwin RJ, Tam FW (2014) Is the inflammasome a potential therapeutic target in renal disease? BMC Nephrol 15:21. https://doi.org/10.1186/1471-2369-15-21
Qian Q (2017) Inflammation: a key contributor to the genesis and progression of chronic kidney disease. Contrib Nephrol 191:72–83. https://doi.org/10.1159/000479257
Schelling JR (2016) Tubular atrophy in the pathogenesis of chronic kidney disease progression. Pediatr Nephrol 31(5):693–706. https://doi.org/10.1007/s00467-015-3169-4
Zhang T, Lu Y, Yang B, Zhang C, Li J, Liu H, Wang H, Wang D (2020) Diffusion metrics for staging pancreatic fibrosis and correlating with epithelial-mesenchymal transition markers in a chronic pancreatitis rat model at 11.7T MRI. J Magn Reson Imaging 52(1):197–206. https://doi.org/10.1002/jmri.26995
Zhou H, Zhang J, Zhang XM, Chen T, Hu J, Jing Z, Jian S (2021) Noninvasive evaluation of early diabetic nephropathy using diffusion kurtosis imaging: an experimental study. Eur Radiol 31(4):2281–2288. https://doi.org/10.1007/s00330-020-07322-6
Cai XR, Yu J, Zhou QC, Du B, Feng YZ, Liu XL (2016) Use of intravoxel incoherent motion MRI to assess renal fibrosis in a rat model of unilateral ureteral obstruction. J Magn Reson Imaging 44(3):698–706. https://doi.org/10.1002/jmri.25172
Mao W, Ding Y, Ding X, Wang Y, Fu C, Zeng M, Zhou J (2021) Pathological assessment of chronic kidney disease with DWI: Is there an added value for diffusion kurtosis imaging? J Magn Reson Imaging 54(2):508–517. https://doi.org/10.1002/jmri.27569
Liang P, Yuan G, Li S, He K, Peng Y, Hu D, Li Z, Ma Z, Xu C (2023) Non-invasive evaluation of the pathological and functional characteristics of chronic kidney disease by diffusion kurtosis imaging and intravoxel incoherent motion imaging: comparison with conventional DWI. Br J Radiol 96(1141):20220644. https://doi.org/10.1259/bjr.20220644
Funding
This work was supported by grants from the Medical Health Science and Technology Project of Zhejiang Province (Grant no. 2019PY066) and Basic Public Welfare Research Program of Zhejiang Province (Grant no. GF18H180031) and Zhejiang Traditional Chinese Medical Scientific Technology (Grant no. 2020ZA080).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. YC designed and performed the research; Jiazhen Lin wrote the original draft; CZ and JY collected the cases and assisted in pathological diagnosis; FC and XL provided technical and financial support; HQ performed the data analysis; YZ revised manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of Hangzhou TCM Hospital Affiliated to Zhejiang Chinese Medical University (no. 2018KY002). All the patients (or their guardians in the case of minors) provided their written informed consent before data collection.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, J., Zhu, C., Cui, F. et al. Based on functional and histopathological correlations: is diffusion kurtosis imaging valuable for noninvasive assessment of renal damage in early-stage of chronic kidney disease?. Int Urol Nephrol 56, 263–273 (2024). https://doi.org/10.1007/s11255-023-03632-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03632-y