Abstract
Purpose
To investigate [11C]acetate PET-surrogate parameter of fatty acid synthase activity—as suitable tool for diagnosis and monitoring of liver steatosis.
Methods
In this retrospective study, data were obtained from 83 prostatic carcinoma patients from 1/2008 to 1/2014. Mean HU was calculated from unenhanced CT of all patients from liver with liver HU less than 40 as threshold for liver steatosis. SUVmax of the liver and of the blood pool in thoracic aorta (as background for calculation of a liver/background ratio [SUVl/b]) was measured. t test was used with a P < 0.05 considered as statistically significant difference and ROC analysis was used for calculating specificity and sensitivity.
Results
19/83 patients (20%) had diagnosis of hepatic steatosis according to CT. Uptake of [11C]acetate was significantly higher in patients with hepatic steatosis as compared to control group (SUVmax 7.96 ± 2.0 vs. 5.48 ± 2.3 [P < 0.001]). There was also a significant correlation between both SUVmax (r = − 0.52, P < 0.001) and SUVl/b (r = − 0.59, P < 0.001) with the density (HU) of the liver. In ROC analysis for detection of liver steatosis SUVmax (threshold: 5.86) had a sensitivity of 94% and specificity of 69% with an AUC of 0.81. Increasing body mass index is correlated with the severity of steatosis.
Conclusion
We showed for the first time that hepatic steatosis associates with increased [11C]acetate uptake. Also, severity of steatosis correlates with [11C]acetate uptake. [11C]acetate uptake PET seems promising for the assessment of liver steatosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Non-alcoholic fatty liver disease (NAFLD) is becoming rapidly the most frequent chronic liver disease in adult and children worldwide [1]. The incidence of disease especially in developed countries is increasing parallel to obesity and metabolic disease [2, 3] and therefore represents the hepatic manifestation of the metabolic syndrome. The US National Health and Nutrition Examination Survey (NHANES) has reported that the proportion of NAFLD among chronic liver diseases has increased from 47% to 75% between 1998 and 2008 [4]. In the US, UK and European countries NAFLD is the most common cause of chronic liver disease. NAFLD is defined by accumulation of lipid in more than 5–10% of liver weight which is not caused by excessive consumption of alcohol [5]. About 20% of patients with NAFLD can progress to non-alcoholic steato-hepatitis (NASH) which is characterized by the presence of inflammation and other signs of progressive liver injury in addition to steatosis. In laboratory chemistry, elevations of liver enzymes (AST, ALT and probably G-GT) may be observed, but poorly reflects disease activity and may even be within the normal range. The disease spectrum can further progress to liver fibrosis and liver cirrhosis [3, 6]. Alarmingly, NASH has become already the leading cause of hepatocellular carcinoma (HCC) in some European countries and NASH-associated HCC occurs in an increasing rate in pre-cirrhotic stages of NASH, posing additional challenges for screening strategies [7, 8].
The standard diagnosis of NASH is made by liver biopsy and histopathological staging [9, 10]. Currently different imaging methods are used for evaluation and quantification of NAFLD, as biopsy is invasive with risk of complications, semi-quantitative and prone to sample variability. Fibroscan™ as a method for measuring liver stiffness and Fibroscan™ CAP® for the quantification of liver steatosis are becoming widely used. However, these measures do not reflect intrahepatic metabolic alterations [11]. Therefore, a non-invasive diagnostic tool for monitoring the extent of lipid accumulation and for diagnosis of NASH is urgently needed. Ultrasonography as the most accessible tool for evaluating NAFLD is limited by operator dependency, lack of quantitative information as well as not sufficient sensitivity and specificity [12,13,14].
Computer-tomography (CT) can quantify the density of liver tissue, which is reduced by lipid accumulation. However, CT seems also to be not sensitive enough for robust diagnosis of mild steatosis [14]. It has been reported that CT has about 97% sensitivity and 76% specificity for fat infiltration more than 33% (that would be in the range of moderate). Magnetic resonance imaging (MRI) has been recently used for diagnosis of NAFLD. In some studies it has been shown that it correlates with histology data but sequences and values are vendor dependent, so its use is hampered for follow-up or diagnosis of disease severity [15,16,17]. Relative liver enhancement of gadoxetic acid may also distinguish patients with NASH among NAFLD individuals with a good diagnostic accuracy as demonstrated in a Viennese cohort of NAFLD patients [18].
Positron emission tomography (PET) imaging with and without CT has emerged as a diagnostic tool for liver disease, in particular for hepatic lesions. Only few studies used PET imaging for evaluation and quantification of liver fat density and its correlation with obesity and/or coronary disease, all of them focusing on glucose metabolism measured with 2-[18F]fluoro-2-deoxy-d-glucose ([18F]FDG). However, results are contradictory and not convincing so far [19, 20].
Recently, several studies have highlighted the role of acetate as one of the most central and dynamic metabolites in intermediary lipid metabolism [21]. Acetate or acetic acid in human cells is converted into acetyl-Co A and, therefore, is involved in synthesis of cholesterol and fatty acids and plays a fundamental role in cell growth and proliferation processes [21]. A close correlation between [11C]acetate uptake and fatty acid synthesis has already been shown [22]. Consequently, it has been used to assess fatty acid production in cancers and heart tissue [23,24,25,26]. Despite the drawback of limited availability, [11C]acetate can be considered as promising tool in many diseases connected to lipid accumulation. Therefore, we hypothesize that [11C]acetate uptake is significantly increased in steatosis as compared to normal liver parenchyma.
Materials and methods
Subjects
The institutional review board of the Medical University of Vienna has approved the study. The study retrospectively reviewed 126 consecutive patients with known prostate carcinoma who have had [11C]acetate PET/CT scanning at the Medical University of Vienna between January 2008 and January 2014. Only patients with present non-enhanced CT were enrolled in this study. Additionally, conventional diagnostic workup including a thorough history, body mass index (BMI) and laboratory data (including ALT, AST and Gamma GT) were selected and assessed retrospectively. In most patients alcohol abuse could be ruled out. However, in some cases no information about alcohol consumption was available. The indication of PET–CT for all included patients was biochemical relapse of prostate cancer and patients who received chemotherapies at the time of PET/CT, known liver disease (e.g., viral or auto-immune hepatitis) and also patients with liver metastasis or a significant tumor burden with [11C]acetate uptake were excluded from the study.
[11C]acetate PET/CT
[11C]acetate was prepared according to a well-established method [27]. All PET/CT scans were obtained using combined PET/CT scanner (Siemens Biograph TruePoint 64). According to the routine protocol all patients were asked to fast for at least 6 h before the examination and then received an intra-venous injection of [11C]acetate of 8 MBq/kg of body weight. After 20 min patients were scanned from thorax and abdomen. PET images were reconstructed using the TruX algorithm, with four iterations per 21 subsets, a 5-mm-slice thickness and a 168 × 168 matrix. Helical CT acquisitions were performed with 4 D care dose protocol and the following parameters: a tube current of 230 effective mAs, a tube voltage of 120 kVp, a collimation of 24 × 1.2 mm, a pitch of 0.813, and a scanning time of 0.5 s per rotation. For review, the CT images were reconstructed with a section thickness of 5 mm in 3-mm increments.
PET, non-enhanced CT and fused PET/CT images were generated and reviewed on the computer by a specialized physician, and co-registered images were displayed on special workstation system using Hybrid Viewer (HERMES Medical solutions, Stockholm, Sweden).
Data analysis
PET uptake values from liver, spleen as well as blood pool of thoracic aorta and CT HU of the liver were measured by drawing three region of interest with a diameter of at least 3 cm in the center of the organ for liver and 1.5 cm for the blood pool in the thoracic aorta. Continuous data (such as BMI, SUVmax, HU) are reported with medians, range and standard deviations while categorical ones are mentioned with counts and percentage. As defined in previous studies fatty liver has been defined as mean liver attenuation measured in HU less than 40 [28,29,30]. Therefore, the steatosis group was defined as study subjects with HU < 40 and the rest have been considered as control group. The maximum standard uptake value (SUVmax) of the thoracic aorta has been considered as reference value for calculation of liver/background SUV ratio (SUVl/b). It has been measured by adding 3 manually drawn ROI to a VOI (Fig. 1).
Fatty liver has been defined as mean liver attenuation measured in Hounsfield Units (HU) < 40. [11C]acetate uptake (A) and CT HU (B) values of the liver were measured by drawing three region of interest (ROI) with a diameter of at least 3 cm within the liver. Three ROIs into the blood pool within the thoracic aorta (C) to measure the SUVmean as background activity for standardization of liver uptake (calculation of liver/background SUV ratio)
Correlation between the steatosis patients according HU definition, SUVmax and SUVl/b was performed using Pearson correlation test and a P value < 0.05 was considered statistically significant. For comparison of different values of the patient group and control group, we used a t test for independent samples in combination with a Levene test for equality of variances. A P value < 0.05 was considered as statistically significant.
Sensitivity and specificity of [11C]acetate PET as a diagnostic tool for liver steatosis has been calculated using ROC analysis (receiver operating characteristic).
Results
According to the inclusion criteria 43/126 patients were excluded from the study due to: liver lesions, known hepatitis B and/or C, incomplete laboratory data, lack of unenhanced CT, and additional disease or therapies that may affect liver parenchymal enhancement. The study population included 83 patients (mean age 68.9, range 48–94). 19/83 patients were defined as steatosis patients and 64 patients as controls. This data indicates a prevalence of steatosis in about 20% in our study population.
We observed a statistically significant difference in BMI between steatosis and control group, consistent with NAFLD as the most likely etiology: mean BMI measure from steatosis group was 32.3 ± 1, whereas in control group was 26.8 ± 0.5.
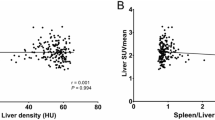
The mean SUVmax of the liver in the control group (5.48 ± 2.3) was significantly lower than the SUVmax of the steatosis group (7.96 ± 2.0; P < 0.001; Fig. 2). The mean SUVl/b in the steatosis group was significantly higher as compared to the control group (7.17 ± 1.7 vs. 4.78 ± 2.2; P < 0.001). Increasing SUVmax of the liver had a statistically significant negative correlation with decreasing mean HU (r = − 0.52; P < 0.001). The SUVl/b and the HU liver showed a significant correlation, too (r = − 0.59; P < 0.001). This relationship is shown in Fig. 3.
Liver enzymes in fatty liver group were higher than in controls, but with the exception of AST the difference was not statistically significant (Table 1). According to available follow-up data from 15/19 patients (4 patients without follow-up data) of the control group with high [11C]acetate uptake but no signs of steatosis on CT, about 47% (9/19 patients) developed elevated liver enzymes and/or report of liver steatosis in abdominal CT/ultrasonography during follow-up.
The sensitivity of [11C]acetate PET/CT for diagnosis of steatosis patients was analyzed using ROC analysis. The value of steatosis diagnosis by using SUVmax alone, as well as using SUVl/b is shown in Fig. 4. SUVmax (AUC 0.81) had a sensitivity of 94% and specificity of 69%, whereas SUVl/b (AUC 0.84) had a sensitivity of 77% and specificity of 82%. The biochemical as well as clinical characteristics and imaging information of study participants categorized by presence or absence of steatosis are presented in Table 1.
For further analysis, we excluded all patients with a borderline HU value of the liver between 40 and 45 (n = 13). We aimed for a more robust differentiation of patients and tried to exclude patients with borderline fatty involvement of the liver. In the remaining patients, SUVmax (7.96 vs. 4.89; P < 0.001) and SUVl/b (7.17 vs. 4.27; P < 0.001) of the steatosis group was significantly higher as compared to the control group. HU of the liver and SUVmax (R = − 0.60; P < 0.001) and SUVl/b (R = − 0.57; P < 0.001) correlated significantly. Using ROC analysis SUVmax had a sensitivity of 94% with a specificity of 82% (AUC = 0.89; threshold SUVmax 5.86). SUVl/b had a sensitivity of 100% and a specificity of 80% (AUC = 0.91, threshold SUVmax 5.08) (Fig. 4).
Discussion
According to recent guidelines published by the American Gastroenterological Association in patients with the incidental findings of fatty liver (mostly in ultrasound), liver biopsy is not recommended in the absence of risk factors such as diabetes mellitus, hyperlipidemia, hypertension and/or central obesity. These patients should be followed by an imaging method and by monitoring of biochemical data [31]. Several imaging methods are available for diagnosis and follow-up of NAFLD, all of them with specific drawbacks. Ultrasound is machine and operator dependent and especially in patients with central obesity and thick adipose tissue, it cannot be used properly [32]. CT has been shown to have less accuracy in detecting mild steatosis in comparison to more advanced steatosis [28]. MRI is prone to artifacts and has difficulties in providing reliable vendor-independent quantitative data. As mentioned before, despite its increasing use, Fibroscan does not reflect the metabolic situation [11]. Further on, results of Fibroscan show a wide variation in sensitivity ranging from 77% to 100% and specificity ranging from 78% to 98% [33].
We proposed [11C]acetate PET as a marker for hepatic steatosis, because of its involvement in free fatty acid metabolism cascade, which has a reasonable relevance and, therefore, can be used especially in high-risk patients for diagnosis, risk stratification and follow-up. About 25% of fatty acids for hepatic triglyceride accumulating in NAFLD result from increased de novo lipogenesis which is driven by insulin and dietary factors including fructose [34]. In our study, not surprisingly, we have had a high incidence of steatosis as an incidental finding in the study population (20%) [35] that underlines the necessity of an optimum imaging method for primary diagnosis as well as follow-up evaluation of a population at risk. With these results, for the first time we could demonstrate the utility of [11C]acetate PET for detecting fatty accumulation in the liver. In our study, liver SUVmax and SUVl/b had a high accuracy in detecting fatty infiltration with liver HU as gold standard (sensitivity of 94% and specificity of 69% for liver SUVmax and sensitivity of 77%, and specificity of 82% for SUVl/b). Furthermore, we demonstrated that the severity of fatty infiltration according CT findings was correlated with increasing [11C]acetate uptake. As expected the fatty liver group had significantly higher BMI in comparison to control group and a trend of higher liver enzymes was observed in fatty liver group that both correlated with findings in relationship between fatty liver and obesity/inflammation. When excluding patients with a borderline density of the liver (HU between 40 and 45) for further analysis [11C]acetate had an even higher AUC for diagnosis of fatty liver disease. Excluding patients with a borderline liver density in CT was done for this additional analysis as a value of 40 HU for discriminating patients with fatty liver disease is quite arbitrary and the transition to fatty liver disease is smooth. We suggested that patients with a liver HU of 40–45 might present with increased fat in the liver without fulfilling the CT-based criteria and excluding these patients might allow for better discrimination of patients with and without fatty liver disease.
During recent years, few studies were published analyzing [18F]FDG uptake in the liver for detecting fatty infiltration. The results were controversial: in a retrospective study Jonathan et al. [19] could not find any significant difference in [18F]FDG uptake between a fatty liver group and a control group, whereas Qazi et al. [36] described a significantly higher [18F]FDG uptake in the fatty liver group compared with healthy controls. From the metabolic point of view, increasing glucose metabolism in patients with fatty infiltration—especially with mild or no inflammation—seems not to be likely. Also considering the high percentage of patients with NAFLD suffering from diabetes mellitus or metabolic syndrome a basic disturbance in glucose uptake in whole body organs as well as the liver must be assumed. Another study has proven this glucose uptake disturbance theory by demonstrating an inverse association between liver fat content and [18F]FDG uptake in type 2 diabetic patients [37]. In this study, [18F]FDG was injected 90 min after insulin stimulation in diabetic patients. This could be relevant for type 2 diabetic patients and patients with metabolic syndrome, although the correlation of insulin resistance with the fat content of the liver is questionable.
Due to the lack of biopsy-proved studies the accuracy of PET, CT or MRI in detecting mild-to-moderate grades of NAFLD is not clear. Although there are studies showing that assessment of liver fat by CT attenuation is unreliable and is insensitive for detecting mild steatosis, the reported sensitivity and specificity of unenhanced CT for detecting moderate-to-severe steatosis (> 30% on histology) is about 73% and 95%, respectively [38]. Therefore, the gold standard for diagnosis of NAFLD has limitations in its accuracy itself. Indeed, we noted some patients with high [11C]acetate uptake in the control group. Interestingly, 47% of these patients with increased [11C]acetate uptake developed NAFLD later on. Therefore, we hypothesize that an increased [11C]acetate uptake might precede development of morphologically visible liver steatosis. In addition, our study population were oncologic patients, consisting of males only with somehow high age range and a higher likelihood of presence of chronic diseases; all these factors might affect normal parenchyma uptake in patients. For establishing a normal range of liver [11C]acetate uptake or a reliable cut-off value for NAFLD, a healthy control group would be needed. Another clear limitation of the study was the lack of histopathologic data as the detection of fatty liver was retrospectively done and can be considered as an incidental finding.
Finally, our results apply to a cross section of patients in a single period of time. Therefore, we could not demonstrate the utility of [11C]acetate PET for reliable non-invasive monitoring of NAFLD; however, the increasing uptake with increasing fat content is an indicator for its potential.
As mentioned before, [11C]acetate PET provides a functioning image from the fatty acid content of the liver cells, which might allow for future non-invasive analysis of interventions against NAFLD. Some studies have shown that dynamic PET imaging possibly including kinetic modeling may give us more accurate information, for example in diagnosis of focal, multifocal or geographic liver steatosis. In a recent study, [11C]acetate was used for detection of FNH and primary HCC and the accuracy of diagnosis was significantly increased when dynamic imaging protocols were used [39].
In conclusion, the presented data demonstrated a significant association between liver attenuation—and therefore liver fat content—and [11C]acetate uptake for the first time. We can conclude that [11C]acetate PET can be considered as a promising and reasonable imaging biomarker for early diagnosis and follow-up of patients with fatty liver. This should be the basis for further studies to evaluate the accuracy of [11C]acetate PET as a diagnostic tool for detection of different grades of fatty liver disease (mild, moderate and severe), for evaluation of follow-up situations, prognostic risk stratification, and monitoring therapeutic interventions.
References
Bedogni G, Miglioli L, Masutti F, et al. (2005) Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology 42:44–52
Adams LA, Lymp JF, Sauver JS, et al. (2005) The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 129:113–121
Marchesini G, Brizi M, Bianchi G, et al. (2001) Nonalcoholic fatty liver disease a feature of the metabolic syndrome. Diabetes 50:1844–1850
Weiß J, Rau M, Geier A (2014) Non-alcoholic fatty liver disease: epidemiology, clinical course, investigation, and treatment. Deutsches Ärzteblatt International. 111:447
Paschos P, Paletas K (2009) Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia. 13:9
Fassio E, Álvarez E, Domínguez N, Landeira G, Longo C (2004) Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology 40:820–826
Perumpail RB, Wong RJ, Ahmed A, Harrison SA (2015) Hepatocellular carcinoma in the setting of non-cirrhotic nonalcoholic fatty liver disease and the metabolic syndrome: US experience. Dig Dis Sci 60:3142–3148
White DL, Kanwal F, El-Serag HB (2012) Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol 10(1342–59):e2
Kleiner DE, Brunt EM, Van Natta M, et al. (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41:1313–1321
Bedossa P, Poitou C, Veyrie N, et al. (2012) Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology 56:1751–1759
Myers RP, Pollett A, Kirsch R, et al. (2012) Controlled Attenuation Parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int 32:902–910
Nascimbeni F, Pais R, Bellentani S, et al. (2013) From NAFLD in clinical practice to answers from guidelines. J Hepatol 59:859–871
Ghoshal G, Lavarello RJ, Kemmerer JP, Miller RJ, Oelze ML (2012) Ex vivo study of quantitative ultrasound parameters in fatty rabbit livers. Ultrasound Med Biol 38:2238–2248
Sasso M, Miette V, Sandrin L, Beaugrand M (2012) The controlled attenuation parameter (CAP): a novel tool for the non-invasive evaluation of steatosis using Fibroscan®. Clin Res Hepatol Gastroenterol 36:13–20
Springer F, Machann J, Claussen CD, Schick F, Schwenzer NF (2010) Liver fat content determined by magnetic resonance imaging and spectroscopy. World J Gastroenterol 16:1560
Permutt Z, Le TA, Peterson M, et al. (2012) Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease-MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther 36:22–29
Tang A, Tan J, Sun M, et al. (2013) Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology 267:422–431
Bastati N, Feier D, Wibmer A, et al. (2014) Noninvasive differentiation of simple steatosis and steatohepatitis by using gadoxetic acid–enhanced mr imaging in patients with nonalcoholic fatty liver disease: a proof-of-concept study. Radiology 271:739–747
Abele JT, Fung CI (2010) Effect of hepatic steatosis on liver FDG uptake measured in mean standard uptake values 1. Radiology 254:917–924
Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G (2010) A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol 53:372–384
Lyssiotis CA, Cantley LC (2014) Acetate fuels the cancer engine. Cell 159:1492–1494
Molloy JW, Calcagno CJ, Williams CD, et al. (2012) Association of coffee and caffeine consumption with fatty liver disease, nonalcoholic steatohepatitis, and degree of hepatic fibrosis. Hepatology 55:429–436
Befroy DE, Perry RJ, Jain N, et al. (2014) Direct assessment of hepatic mitochondrial oxidative and anaplerotic fluxes in humans using dynamic 13C magnetic resonance spectroscopy. Nat Med 20:98–102
Grassi I, Nanni C, Allegri V, et al. (2012) The clinical use of PET with 11C-acetate. Am J Nucl Med Mol Imaging 2:33
Pickhardt PJ, Hahn L, del Rio AM, et al. (2014) Natural history of hepatic steatosis: observed outcomes for subsequent liver and cardiovascular complications. Am J Roentgenol 202:752–758
Porenta G, Cherry S, Czernin J, et al. (1999) Noninvasive determination of myocardial blood flow, oxygen consumption and efficiency in normal humans by carbon-11 acetate positron emission tomography imaging. Eur J Nucl Med 26:1465–1574
Mitterhauser M, Wadsak W, Krcal A, et al. (2004) New aspects on the preparation of [11C]acetate: a simple and fast approach via distillation. Appl Radiat Isot 61:1147–1150. https://doi.org/10.1016/j.apradiso.2004.03.060
Limanond P, Raman SS, Lassman C, et al. (2004) Macrovesicular hepatic steatosis in living related liver donors: correlation between CT and histologic findings 1. Radiology 230:276–280
Park YS, Park SH, Lee SS, et al. (2011) Biopsy-proven nonsteatotic liver in adults: estimation of reference range for difference in attenuation between the liver and the spleen at nonenhanced CT. Radiology 258:760–766
Zeb I, Li D, Nasir K, et al. (2012) Computed tomography scans in the evaluation of fatty liver disease in a population based study: the multi-ethnic study of atherosclerosis. Acad Radiol 19:811–818
Chalasani N, Younossi Z, Lavine JE, et al. (2012) The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 55:2005–2023
Mishra P, Younossi ZM (2007) Abdominal ultrasound for diagnosis of nonalcoholic fatty liver disease (NAFLD). Am J Gastroenterol 102:2716–2717
Friedrich-Rust M, Ong MF, Martens S, et al. (2008) Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology 134(960–74):e8
Fuchs CD, Claudel T, Trauner M (2014) Role of metabolic lipases and lipolytic metabolites in the pathogenesis of NAFLD. Trends Endocrinol Metab 25:576–585
Blachier M, Leleu H, Peck-Radosavljevic M, Valla D-C, Roudot-Thoraval F (2013) The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol 58:593–608
Qazi F, Oliver D, Nguyen N, Osman M (2008) Fatty liver: impact on metabolic activity as detected with 18F FDG-PET/CT. Soc Nucl Med Ann Meet Abstr 49:263P
Borra R, Lautamäki R, Parkkola R, et al. (2008) Inverse association between liver fat content and hepatic glucose uptake in patients with type 2 diabetes mellitus. Metabolism 57:1445–1451
Rofsky NM, Fleishaker H (1995) CT and MRI of diffuse liver disease. Semin Ultrasound CT MRI 16:16–33
Huo L, Dang Y, Lv J, Xing H, Li F (2014) Application of dual phase imaging of 11C-acetate positron emission tomography on differential diagnosis of small hepatic lesions. PLoS ONE 9:e96517
Acknowledgements
Open access funding provided by Medical University of Vienna. The authors thank the team of radiation technologist at the Medical University of Vienna for performance of the PET scans as well as the Medical University of Vienna for technical support with the radiosynthesis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The institutional review board of the Medical University of Vienna has approved the study. The study is in accordance with the declaration of Helsinki. Due to the retrospective study design the ethical committee waived the requirement for informed consent. No financial support was received for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Supplemental Fig.
ROC analysis excluding all patients with a liver HU from 40 to 45. In this population sensitivity, specificity and AUC are clearly higher than in the total population (SUVmax: sensitivity 94%, specificity 82%, AUC = 0.89; SUVl/b: sensitivity 100%, specificity 76%, AUC = 0.88). Supplementary material 1 (JPEG 385 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nejabat, M., Leisser, A., Karanikas, G. et al. [11C]acetate PET as a tool for diagnosis of liver steatosis. Abdom Radiol 43, 2963–2969 (2018). https://doi.org/10.1007/s00261-018-1558-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-018-1558-4