Abstract
Background
Hepatic steatosis is the most common chronic hepatic disease. Imaging diagnosis of hepatic steatosis has been evaluated as an alternative to invasive histological diagnosis.
Study aims
The study aimed to assess the effect of hepatic steatosis on Flourine-18 fluorodeoxyglucose (18F-FDG) uptakes in cancer patients.
Patients and Methods
Blood samples were collected from 50 cancer patients and analyzed to calculate fatty liver index and Hepatic steatosis index (HIS). Hepatic steatosis examined using high-resolution ultrasound and positron emission tomography—computed tomography (PET-CT). Linear attenuation coefficient, standardized-uptake value (SUV) mean (SUV mean), and SUV maximum (SUVmax) were measured. Accordingly, patients were divided equally into non-fatty liver, and fatty liver groups.
Results
A significant increase in SUVmax and SUV mean was observed in the fatty liver group more than in the non-fatty liver group. HSI significantly increased in the fatty liver group compared to the non-fatty liver group. Liver tissue uptake FDG was significantly correlated with HSI values. SUV max significantly correlated with body mass index (BMI) in the non-fatty group only.
Conclusion
Hepatic changes in cancer patients affect the liver metabolic activity and thus the 18 F-FDG uptake. Therefore, further corrections should be considered when the liver is used as a comparator for PET-CT scans of cancer patients.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Fatty liver disease reflects a wide spectrum of conditions characterized histologically by excessive accumulation of triglycerides and cholesterols within the cytoplasm of > 5% of hepatocytes (Sanyal et al. 2011). Hepatic steatosis is caused by an abnormal and excessive intracellular accumulation of fat (mostly triglycerides) in hepatocytes. It is a common radiologic finding. Fat accumulation in the liver occurs in six patterns: diffuse, regional, localized, subcapsular, multifocal, and perivascular (Hu et al. 2022).
Fatty infiltration of the liver is further subdivided into alcoholic fatty liver disease and non-alcoholic fatty liver disease (NAFLD) (Özülker and Özülker 2019).
NAFLD or MAFLD (metabolic associated fatty liver disease) includes two pathological entities; simple steatosis and non-alcoholic steatohepatitis (NASH) (Salomon et al. 2018).
NAFLD is a complex disease that involves multiple organs and diverse mechanisms and results from the interplay between metabolic and environmental factors with genetic and epigenetic predispositions (Juanola et al. 2021).
In a small number of cases NAFLD develop into NASH and progress towards end stage liver diseases (Keramida et al. 2020).
In Egypt, NAFLD “remains unknown due to the lack of large population-based studies” (Alboraie et al. 2019). Liver biopsy is the gold standard to differentiate NASH from simple steatosis and identify the advanced hepatic fibrosis but it is invasive, poorly acceptable, expensive, and has sampling variability (Castera et al. 2019).
Noninvasive techniques include quantification of serum biomarkers and measurement of liver stiffness, using either ultrasound- or magnetic resonance-based elastography are investigated (Castera et al. 2019).
Algorithms based on serum biomarkers such as Fatty Liver Index (FLI) and Hepatic Steatosis Index (HSI) were extensively used. FLI value varies between 0 and 100 (A FLI < 30 rules out and a FLI ≥ 60 rules in fatty liver) and was used as an accurate predictor for fatty liver in the general population (Bedogni et al. 2006). HSI is an efficient simple index based on standard laboratory tests and anthropometric parameters used as a screening tool for NAFLD (an HSI < 30.0 rules out and an HSI > 36.0 rules in NAFLD) (Lee et al. 2010).
The diagnostic accuracy of serum biomarkers is suggested to be improved by combining them with different approaches such as imaging modalities (Castera et al. 2019).
Conventional imaging techniques [Ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI)] have low to moderate accuracy to identify liver fibrosis (Allan et al. 2010; Lo et al. 2017). Sonography and unenhanced CT effectively detect steatosis if fatty infiltration is > 10% and > 30%; respectively (Obika and Noguchi 2012; Ballestri et al. 2017; Hajong et al. 2018). Fluorodeoxyglucose positron emission tomography/CT (FDG-PET/CT) plays a specific role in assessing diagnosis, staging, and monitoring therapeutic response in oncology imaging (Luk et al. 2013).
The standardized uptake values (SUVs) are used to eliminate the variability resulting from differences in patient size and the amount of the injected FDG (Lin et al. 2011).
Then the mean value within a fixed size of a region of interest (ROI), SUV maximum (SUVmax), the use of “reference tissue” SUVmax values and normalization of lesion/target SUV measures to those of selected reference tissues were introduced to reduce variability of SUV measurements (Fletcher and Kinahan 2010). Liver was among the tissues that have been advocated as reference tissue and showed the least inter-patient coefficient of variance (0.21) (Fletcher and Kinahan 2010).
Several studies investigated the possible effect of fatty infiltration on liver SUVs (Abikhzer et al. 2011; Kim et al. 2011; Keramida et al. 2014; Keramida et al. 2014; Liu et al. 2015; McDermott et al. 2019; Rozenblum et al. 2020; Seraj et al. 2019).
Frequently, liver FDG uptake is used as the benchmark for diagnosis, treatment assessment, prognosis, and quality control in PET/CT imaging. A number of factors, including age, blood sugar, body mass index (BMI), incubation time, and hepatic steatosis, affect the liver’s capacity to absorb FDG. There are several factors that could affect the SUV-measured FDG uptake, including weight, plasma glucose level, interval length, partial volume effects, and recovery factor. It has been demonstrated that overweight or obesity and non-alcoholic fatty liver disease are linked, and that the metabolic syndrome is characterized by alterations in normal glucose metabolism (Ahmed et al. 2022). However, the relation between fatty infiltration of the liver and FDG uptake in terms of SUVmax and SUV mean values remains unresolved. Therefore, the current study was designed to assess the effect of hepatic steatosis on 18F-FDG uptake in PET-CT examinations and to evaluate the efficiency of using the liver as an internal reference organ in the Egyptian cancer patients.
Patients and methods
The current study included 50 subjects (with no definite focal fatty changes or focal fatty sparing areas) from those who refereed to perform PET-CT examination in Ayadi Al-Mostakbal Oncology Center (Alexandria, Egypt) from 1st of June 2020 to 20th of December 2020. Subjects were recruited according to the rules of Ayadi Al-Mostakbal ethical committee for conduction of medical research on human subjects and informed consents “were obtained from each patient included in the study.
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) as reflected in a priori approval by the institution’s human research committee”. Other liver diseases, such as alcoholic liver diseases, and viral liver diseases are excluded. Deoxygenated blood sample were collected from patients before injection of the 18F-FDG to analyze the alanine amino transferase (ALT), aspartate amino transferase (AST), gamma-glutamyl transferase (GGT), fasting plasma glucose (FPG), triglycerides (TGs) which were measured using fully automated (Roche, Cobas c 311 analyzer, India) and values were used to calculate FLI and HSI.
Hepatic steatosis patients were identified by using High Resolution US (HRUS; General Electric model LOGIQ S7, USA, C1-5-D broad- spectrum convex transducer with field of view 70° and frequency range 1.8-5 MHz) and non-contrast computed tomography (NCCT). Patients were scanned separately on an integrated PET- CT scanner with 2D image acquisition after injection of 18F-FDG (without contrast) (Siemens Biograph 64-slice PET scanner, Germany, 120 KVp/50 mA-Care dose; slice thickness 5 mm; pitch 0.8; rotational speed 0.5/sec, convolution kernel B19f PET, very smooth) then Digital Imaging and Communications in Medicine (DICOM) images were extracted from PET-CT scan and sent to workstation. Both of the attenuation coefficient value of the liver and spleen tissues were measured using region of interest (ROI) avoiding any lesions, biliary, vascular and artifacts. SUV mean, and SUVmax of the liver were also measured on PET scan. Accordingly, patients were subdivided into two groups:
-
1.
Control group (25 patients) with a mean liver attenuation value ≥ the mean spleen attenuation value.
-
2.
Diffuse fatty liver (hepatic steatosis) group (25 patients) with a mean liver attenuation value < the mean spleen attenuation value.
Results
US revealed absence of fatty liver in 50% (25/50) of patients and variable grades of fatty liver status in 50% (25/50) of patients [grade 1 (mild): 13 (26%); grade 2 (moderate): 8 (16%); and grade 3 (sever); 4 (8%)].
Based on the CT the HU liver to spleen ratio was < 1 in 25 patients (11 of grade zero, 9 grade 1, 3 grade 2, and 2 grade 3 as detected by US) designated as fatty liver group. While, HU liver to spleen ratio was ≥ 1 in 25 patients (14 of grade zero, 4 grade 1, 5 grade 2, and 2 grade 3 as detected by US) designated as non-fatty liver group.
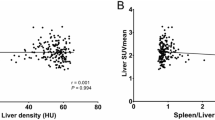
No significant difference was observed between the two groups regarding age (p = 0.286) or sex (p = 0.564), or any of the anthropometric parameters [weight (Kg; p = 0.104), height (cm; p = 0.611, BMI (Kg/m2; p = 0.055), waist circumference (cm; p = 0.159), body surface area (BSA: cm2; p = 0.170). About 64% and 52% of fatty liver and non-fatty liver groups were obese; respectively. Also, BSA increased in 60% and 44% in fatty liver and non-fatty liver groups; respectively. The primary tumor type varied between nineteen types of tumors in both groups. The main tumors detected in fatty liver groups were colon, Hodgkin lymphoma (HL), breast, uterus, neurodegenerative, ovarian, testicular, axillary, bone, lung and nasal tumors (from higher to lower frequency). While in nonfatty liver groups tumors were mainly HL, colon, non-Hodgkin lymphoma (NHL), axillary, breast, lung, urinary bladder (UB), Neck, thymic, and gastric tumors (from higher to lower frequency). ALT, AST, GGT, TG, and FPG were increased in 16% (4/25), 24% (6/25), 16% (4/25), 32% (8/25), and 44% (11/25) of fatty liver group and in 8% (2/25), 28% (7/25), 32% (8/25), 20% (5/25), and 12% (3/25) of nonfatty liver group; respectively. Liver enzyme ratio (AST/ALT ratio) < 1 indicates NAFLD where ALT, AST, and GGT are high (Hall & Cash, 2012). AST/ALT ratio indicated NAFLD in 64 and 56% of fatty and non-fatty liver groups; respectively. However, no significant difference was detected between fatty and non-fatty groups regarding ALT (p = 0.165) or AST (p = 0.478) or GGT (p = 0.620). Although diabetes was evident in each group, no significant difference was observed in FPG (p = 0.109) between the two groups. Despite the insignificant difference between the two groups regarding the level of TG (p = 0.145), the triglyceride index (TyG index) suggested insulin resistance (IR) in 96% and 80% of fatty and non-fatty liver groups; respectively. It also suggested a high likelihood of NAFLD in 92% and 76% of fatty and non-fatty liver groups. However, FLI assured fatty liver in 84% and 64% of fatty and non-fatty liver groups; respectively. NAFLD was ruled in by HSI in 88% and 64% of fatty and non-fatty liver groups; respectively. HSI significantly increased in fatty liver group compared to non-fatty liver group (p = 0.049). Both FLI and HSI showed a significant direct correlation with BMI in both groups (Table 1). Fibrosis-4 (FIB-4) score (Seraj et al. 2019) indicated fibrosis in (20/50) and 40% of fatty and non-fatty liver groups; respectively. Albumin level was available only for two patients in the fatty liver group and NAFLD fibrosis (NFS) score (Ahmed et al. 2022) indicated F3-F4 (advanced/sever) fibrosis in both of the two patients. All patients with high FIB-4 and NFS received either chemotherapy or radiotherapy. Hounsfield unit (HU) of liver and spleen of fatty and non- fatty liver cases are shown in Fig. 1a. A significant decrease in HU and HU liver to HU spleen ratio was observed (p < 0.001) of fatty liver group in comparison to their respective values in the non-fatty liver group (Table 2). While no significant difference was detected in HU of spleen in non-fatty liver group compared to fatty liver group (Table 2). The SUV in liver tissue using 6 cm2 ROI is shown in Fig. 1 b. (Table 3). A significant direct correlation between SUVmax and BMI was observed in non-fatty group only (Table 1). SUVmax significantly correlated with 18F–FDG dose in non-fatty group only (Table 4). While, no correlation was observed between SUV and HU liver/ HU spleen ratio (Table 5).
Discussion
The current study provided an evaluation of liver attenuation using a liver/spleen ratio < 1 to define the prevalence of liver fat (Fig. 2). These measures are easy to obtain on CT scans where images of the liver and spleen are available. Our results emphasize that caution should be taken when liver is used as a comparator during PET-CT scan oncological studies. Hepatic steatosis causes a statistically significant increase in liver metabolic activity as measured by SUV mean and SUVmax values in fatty liver patients using CT-18F-FDG PET scan than in non-fatty liver patients. These metabolic changes might reflect an increase in the inflammatory process of the liver tissue. BMI correlated with SUV and FDG dose in non-fatty liver group and these findings require further investigations. Several imaging modalities can detect fatty liver status. The US was reported to have moderate sensitivity (65%) and specificity (81%) in detecting mild hepatic steatosis while it has good sensitivity (84.8%) and specificity (93.6%) in detecting moderate to severe hepatic steatosis (Angulo et al. 2007).
In the present study, CT was able to detect deposition of fats in 11 cases (grade zero) that were missed by the US as well as excluding fatty deposition in 11 cases (grade 1: 2; grade 2: 5; and grade 3: 2 cases) that were defied as fatty liver cases by the US. The overall agreement for US and CT has 56%. CT attenuation can be affected by tissue density, attenuation, and scanning parameters, and tiny fractions of hepatic fat may be undetected by CT” (Angulo et al. 2007; Hernaez et al. 2011). However, Steatosis can be detected if liver HU is ≤ 40 or liver HU is at least 10 less than the HU spleen (Bohte et al. 2011; Pirmoazen et al. 2020). Accordingly, steatosis was detected in 84% of the fatty liver group (21/25) where liver HU was ≤ 40) and 12 (48%) of those cases had liver HU is at least 10 less than HU spleen. In contrast, no cases were detected with these criteria among the non-fatty liver group. Further multicentral studies using various imaging modalities in comparison to the golden standard “liver biopsy” are mandatory to validate the most accurate non- invasive modality. In our study and others (Zhang et al. 2017; Kodama et al. 2007), no significant correlation was detected between age or gender and fatty liver status despite the sample size. Although Pak et al. (2012) reported that “high BMI (≥ 25) is an independent, dose-dependent risk factor for the fatty liver”, our results and Liu et al. (2015) showed no significant difference between the fatty and non-fatty liver groups regarding the weight, and height (the main components for calculating BMI). Contrary to Liu et al. (2015), we could not detect any significant difference between the fatty and non-fatty liver groups regarding the BMI although the percentage of subjects with ≥ 25 is higher in the fatty liver group than in the non-fatty liver group (80 versus 56%). This would emphasize the fact that not all patients with FLD are obese but they often have metabolic syndrome and IR as obesity-associated risk factors (Pak et al. 2012; Fan et al. 2018). Therefore, it was proposed to change the name of nonalcoholic fatty liver disease (NAFLD) to obesity-associated fatty liver disease (OAFLD) and metabolic- associated FLD (MAFLD) (Pak et al. 2012; Fan et al. 2018). Pak et al. (2012) identified three main diagnostic criteria for MAFLD clinical evidence of metabolic dysregulation; (I) overweight/obesity, (II) type 2 diabetes, and (III) clinical evidence of metabolic dysregulation. The presence of one criterion is sufficient to diagnose MAFLD. In our groups, the prevalence of obesity, type 2 diabetes, and metabolic dysregulation represented by IR (as detected by the TyG index) exceeded 50, 20, and 80%; respectively. Abdominal obesity, hypertension, dyslipidemia, and hyperglycemia were also considered among the metabolic syndrome (MS) associated with MAFLD (Softic and Kahn 2019). In our groups, abdominal obesity (as reflected by WC) showed an insignificant difference. Regardless of the higher percentage of hyperglycemia and dyslipidemia (as measured by TG) in the fatty liver group than in the non-fatty group (44 versus 12% & 32 versus 20%; respectively), no significant difference was observed as well. This would emphasize the complexity of the disease and involvements of genetic, epigenetic, environmental, metabolic factors, geographic location, the involvement of multiple organs, and various mechanisms (Alharthi and Eslam 2021). Liver enzymes (ALT, AST, and GGT) are considered indicators of liver injury. Elevated liver transaminases are common in NAFLD with evidence of metabolic syndrome [high WC, elevated blood pressure, high serum TG levels and low serum high-density lipoprotein (HDL) levels, hyperglycemia, or evidence of IR] (Alam and Fahim 2021). Juanola et al. (2021) showed a significant difference between fatty and non-fatty liver cohort regarding ALT (p = 0.021), AST (p = 0.016), GGT (p = 0.009), and AST/ALT ratio (p = 0.028). Apart from the fact that ALT, AST, GGT, and AST/ALT ratio elevations are detected in some patients of our two studied groups, no significant difference was observed. However, it is recommended to follow up with patients with elevated ALT, AST, and GGT since they correlate with the fibrosis progression in NAFLD (Oh et al. 2017; Sanyal et al. 2015; Canbakan et al. 2007). Also, the coexistence of elevated transaminases with metabolic syndrome, type 2 diabetes, hypertension, a subclinical hypothyroidism was reported recently and protection of liver function in those patients is recommended (Kleiner et al. 2019). NAFLD is divided into the nonalcoholic fatty liver (hepatic steatosis without inflammation) and nonalcoholic steatohepatitis (hepatocyte injury with ballooning of cells, inflammation, and in severe cases, fibrosis) that might progress to cirrhosis and hepatocellular carcinoma (HCC). Therefore, it is challenging to determine patients with a high risk of progression. Recent guidelines suggested a screening system for NAFLD including the use of liver function biomarkers, and variable indices (Giri et al. 2022; Jiang et al. 2021; Tokushige et al. 2021; Kang et al. 2021). AST/ ALT ratio, TyG index, FLI, and HIS reflected NAFLD in our groups with variable percentages (AST/ALT: 64 versus 56%; TyG index: 92 versus 76%; FLI: 84 versus 64%; HSI: 88 versus 64% in fatty and non-fatty liver groups; respectively). The four indices showed a weak agreement of 48% in fatty and 32% in non-fatty liver groups. FLI and HSI showed a significant direct correlation with BMI. Some of our cases in both groups showed liver fibrosis as detected using FIB-4 and NFS scores (when available). It is evident now that obesity affects various cellular responses that enhance hepatic metabolism, liver injury, and NAFLD progression while hider liver regeneration (Kitae et al. 2019). Since it was shown that chemotherapy leads to the number of cycles dependent fatty liver changes in patients with lymphoma (Oh et al. 2017), it is important to mention that all our patients with high FIB-4 and NFS received either chemotherapy or radiotherapy (patients with lymphoma were five in the fatty liver group and ten in the non-fatty liver groups). Interestingly, our results showed a significant correlation between SUVmax and each of 18F–FDG dose and BMI in nonfatty group only which may be due to the type of tumor and treatments used in this group. Thus, the link between fatty liver changes and treatment regimens in cancer therapy warrants intensive future investigations.
Although Della (2020) showed that FDG has limited access to adipose tissue, our fatty liver groups showed significant increased FDG dose than non-fatty liver group. Also, our results showed no correlation was observed between SUV and HU liver/ HU spleen ratio. A possible explanation would be that the FDG accumulated in local fat-induced micro-inflammatory foci. FDG was shown to accumulate at the sites of infection and inflammation due to increased glycolytic activity of inflammatory cells (such as neutrophils, lymphocytes, and macrophages) that use glucose as an energy source only after activation during the metabolic burst (Salama et al. 2020; Christen et al. 2010). This is supported by the fact that both SUVmax, SUV mean, and HSI significantly increased in our fatty liver group. Stumpe and Strobel (2006) also suggested that the increased SUVmax and SUV mean would be due to higher metabolic activity of liver infiltrating inflammatory cells. In the presence of hepatic inflammation, hepatic FDG uptake postulated to be increased as a result of irreversible FDG accumulation I inflammatory cells superimposed on reversible hepatocyte uptake suggesting that FDG-PET could be developed as a potential imaging approach for an early detection of NASH (Hu et al. 2022; Juanola et al. 2021; Glaudemans et al. 2013). Moreover, elevated serum GGT and TGs (as markers of hepatic inflammation and injury) shown to associate with increased hepatic glucose uptake (Bural et al. 2010). GGT elevated in 16% and 32% of our fatty and non-fatty liver groups, while TG elevated in 32% and 20% of them, respectively. Paul (2020) showed that hepatic FDG uptake is also associated with future cardiovascular and cardio-cerebrovascular events in asymptomatic individuals with NAFLD. In conclusion further corrections should be considered when the liver is used as a comparator for PET-CT scans of cancer patients. Future studies are mandatory to understand the correlation between BMI and both SUV and FDG dose in non-fatty liver cancer patients.
Availability of data and materials
Available when request by send an email to correspond author.
Abbreviations
- ALT:
-
Alanine amino transferase
- AST:
-
Aspartate amino transferase
- BMI:
-
Body mass index
- BSA:
-
Body surface area
- CT:
-
Computed tomography
- DICOM:
-
Digital imaging and communications in medicine
- FDG-PET/CT:
-
Fluorodeoxyglucose positron emission tomography/CT
- FIB-4 score:
-
Fibrosis-4 score
- FLI:
-
Fatty liver index
- FPG:
-
Fasting plasma glucose
- GGT:
-
Gamma-glutamyl transferase
- HCC:
-
Hepatocellular carcinoma
- HDL:
-
High-density lipoprotein
- HL:
-
Hodgkin lymphoma
- HRUS:
-
High-resolution ultrasound
- HSI:
-
Hepatic steatosis index
- HU:
-
Hounsfield unit
- IR:
-
Insulin resistance
- MAFLD:
-
Metabolic associated fatty liver disease
- MRI:
-
Magnetic resonance imaging
- MS:
-
Metabolic syndrome
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatohepatitis
- NCCT:
-
Non-contrast computed tomography
- NFS score:
-
NAFLD fibrosis score
- NHL:
-
Non-Hodgkin lymphoma
- OAFLD:
-
Obesity-associated fatty liver disease
- ROI:
-
Region of interest
- SUVs:
-
Standardized uptake values
- SUVmax:
-
SUV maximum
- TGs:
-
Triglycerides
- TyG index:
-
Triglyceride index
- UB:
-
Urinary bladder
- US:
-
Ultrasonography
- WC:
-
Waist circumference
References
Abikhzer G, Alabed YZ, Azoulay L, Assayag J, Rush C (2011) Altered hepatic metabolic activity in patients with hepatic steatosis on FDG PET/CT. Am J Roentgenol 196(1):176–180
Ahmed A, Ali M, Salah H et al (2022) Evaluation of uptake values of FDG: body surface area Vs. body weight correction. Radiat Phys Chem 201:110482. https://doi.org/10.1016/j.radphyschem.2022.110482
Alam S, Fahim SM (2021) Transition of an acronym from nonalcoholic fatty liver disease to metabolic dysfunction-associated fatty liver disease. World J Hepatol 13(10):1203–1207
Alboraie M, Youssef N, Sherief AF, Afify S, Wifi MN, Omran D, Hafez E, Omar H, Eltabbakh M, Abdellah M, El Badry M (2019) Egyptian liver library: an indexed database for liver disease evidence in Egypt. Arab J Gastroenterol 20(2):109–113
Alharthi J, Eslam M (2021) Metabolic associated fatty liver disease (MAFLD): a milestone in the history of fatty liver disease. Hepatobiliary Surg Nutrit 10(5):696
Allan R, Thoirs K, Phillips M (2010) Accuracy of ultrasound to identify chronic liver disease. World J Gastroenterol WJG 16(28):3510
Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, Lindor K (2007) The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 45(4):846–854
Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Targher G, Lonardo A (2017) Ultrasonographic fatty liver indicator detects mild steatosis and correlates with metabolic/ histological parameters in various liver diseases. Metabolism 1(72):57–65
Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C (2006) The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 6(1):1–7
Bohte AE, Van Werven JR, Bipat S, Stoker J (2011) The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol 21(1):87–97
Bural GG, Torigian DA, Burke A, Houseni M, Alkhawaldeh K, Cucchiara A, Basu S, Alavi A (2010) Quantitative assessment of the hepatic metabolic volume product in patients with diffuse hepatic steatosis and normal controls through use of FDG-PET and MR imaging: a novel concept. Mol Imag Biol 12(3):233–239
Canbakan B, Senturk HA, Tahan V, Hatemi I, Balci H, Toptas T, Sonsuz A, Velet M, Aydin S, Dirican A, Ozgulle S (2007) Clinical, biochemical and histological correlations in a group of non-drinker subjects with non-alcoholic fatty liver disease. Acta Gastro-Enterol Belgica 1(70):277–284
Castera L, Friedrich-Rust M, Loomba R (2019) Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 156(5):1264–1281
Christen T, Sheikine Y, Rocha VZ, Hurwitz S, Goldfine AB, Di Carli M, Libby P (2010) Increased glucose uptake in visceral versus subcutaneous adipose tissue revealed by PET imaging. JACC Cardiovascular Imaging 3(8):843–51
Della TS (2020) Non-alcoholic fatty liver disease as a canonical example of metabolic inflammatory-based liver disease showing a sex-specific prevalence: relevance of estrogen signaling. Front Endocrinol 18(11):572490
Fan R, Wang J, Du J (2018) Association between body mass index and fatty liver risk: a dose-response analysis. Sci Rep 8(1):1–7
Fletcher JW, Kinahan PE (2010) PET/CT standardized uptake values (SUVs) in clinical practice and assessing response to therapy. NIH Public Access 31(6):496–505
Giri S, Agarwal D, Afzalpurkar S (2022) GGT dynamic for advanced fibrosis in NAFLD–novel but not convincing. J Gastroenterol Hepatol. https://doi.org/10.1111/jgh.15908
Glaudemans AW, de Vries EF, Galli F, Dierckx RA, Slart RH, Signore A (2013) The use of F-FDG-PET/CT for diagnosis and treatment monitoring of inflammatory and infectious diseases. Clin Develop Immunol. https://doi.org/10.1155/2013/623036
Hajong R, Dhal MR, Naku N, Kapa B (2018) Incidence of nonalcoholic fatty liver disease in patients undergoing laparoscopic cholecystectomy. J Fam Med Prim Care 7(6):1375
Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, Clark JM (2011) Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta- analysis. Hepatology 54(3):1082–1090
Hu N, Su SJ, Li JY et al (2022) Hepatic steatosis with mass effect: a case report. World J Clin Cases 10(30):11066–11073. https://doi.org/10.12998/wjcc.v10.i30.11066
Jiang W, Liu CH, Wu D, Wang YJ, Tang H (2021) Abnormal transaminase and lipid profiles in coexisting diseases in patients with fatty liver: a population study in Sichuan. Biosci Rep. https://doi.org/10.1042/BSR20211769
Juanola O, Martínez-López S, Francés R, Gómez-Hurtado I (2021) Non-alcoholic fatty liver disease: metabolic, genetic, epigenetic and environmental risk factors. Int J Environ Res Public Health 18(10):5227
Kang SH, Lee HW, Yoo JJ, Cho Y, Kim SU, Lee TH, Jang BK, Kim SG, Ahn SB, Kim H, Jun DW (2021) KASL clinical practice guidelines: management of nonalcoholic fatty liver disease. Clin Mol Hepatol 27(3):363
Keramida G, Potts J, Bush J, Verma S, Dizdarevic S, Peters AM (2014) Accumulation of 18F-FDG in the liver in hepatic steatosis. Am J Roentgenol 203(3):643–648
Keramida G, Potts J, Bush J, Dizdarevic S, Peters AM (2014) Hepatic steatosis is associated with increased hepatic FDG uptake. Eur J Radiol 83(5):751–755
Keramida G, Roldao Pereira L, Kaya G, Peters AM (2020) Hepatic and splenic 18F-FDG blood clearance rates (Ki) in hepatic steatosis and diabetes mellitus. Clin Physiol Funct Imaging 40(2):99–105
Kim YH, Kim JY, Jang SJ, Chung HW, Jang KS, Paik SS, Song SY, Choi YY (2011) F-18 FDG uptake in focal fatty infiltration of liver mimicking hepatic malignancy on PET/CT images. Clin Nucl Med 36(12):1146–1148
Kitae A, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M (2019) The triglyceride and glucose index is a predictor of incident nonalcoholic fatty liver disease: a population- based cohort study. Can J Gastroenterol Hepatol 7:2019
Kleiner DE, Brunt EM, Wilson LA, Behling C, Guy C, Contos M, Cummings O, Yeh M, Gill R, Chalasani N, Neuschwander-Tetri BA (2019) Association of histologic disease activity with progression of nonalcoholic fatty liver disease. JAMA Netw Open 2(10):e1912565
Kodama Y, Ng C, Wu TT, Ayers G, Curley S, Abdalla E, Vauthey N, Chamsangavej C (2007) Comparison of CT methods for determining the fat content of the liver. Am J Roentgenol 188:1307–1312
Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, Kim YJ, Yoon JH, Cho SH, Sung MW, Lee HS (2010) Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis 42(7):503–508
Lin CY, Lin WY, Lin CC, Shih CM, Jeng LB, Kao CH (2011) The negative impact of fatty liver on maximum standard uptake value of liver on FDG PET. Clin Imaging 35(6):437–441
Liu G, Li Y, Hu P, Cheng D, Shi H (2015) The combined effects of serum lipids, BMI, and fatty liver on 18F-FDG uptake in the liver in a large population from China: an 18F-FDG- PET/CT study. Nucl Med Commun 36(7):709–716
Lo GC, Besa C, King MJ, Kang M, Stueck A, Thung S, Wagner M, Smith AD, Taouli B (2017) Feasibility and reproducibility of liver surface nodularity quantification for the assessment of liver cirrhosis using CT and MRI. European J Radiol Open 1(4):95–100
Luk WH, San Au-Yeung AW, Loke TK (2013) Imaging patterns of liver uptakes on PET scan: pearls and pitfalls. Nuclear Med Rev 16(2):75–81
McDermott S, Kilcoyne A, Wang Y, Scott JA, Halpern EF, Ackman JB (2019) Comparison of the 18F-FDG avidity at PET of benign and malignant pure ground-glass opacities: a paradox? Clin Radiol 74(3):187–195
Obika M, Noguchi H (2012) Diagnosis and evaluation of nonalcoholic fatty liver disease. Exper Diabet Res. https://doi.org/10.1155/2012/145754
Oh RC, Hustead TR, Ali SM, Pantsari MW (2017) Mildly elevated liver transaminase levels: causes and evaluation. Am Fam Physician 96(11):709–715
Özülker T, Özülker F (2019) Assessment of the effect of fat infiltration on hepatic FDG uptake. European Arch Med Res 35(1):27–32
Pak K, Kim SJ, Kim IJ, Kim K, Kim H, Kim SJ (2012) Hepatic FDG uptake is not associated with hepatic steatosis but with visceral fat volume in cancer screening. Nucl Med Mol Imaging 46(3):176–181
Paul J (2020) Recent advances in non-invasive diagnosis and medical management of non-alcoholic fatty liver disease in adult. Egyptian Liver J 10(1):1–8
Pirmoazen AM, Khurana A, El Kaffas A, Kamaya A (2020) Quantitative ultrasound approaches for diagnosis and monitoring hepatic steatosis in nonalcoholic fatty liver disease. Theranostics 10(9):4277
Rozenblum L, Mokrane FZ, Yeh R, Sinigaglia M, Besson FL, Seban RD, Zadro C, Dierickx L, Chougnet CN, Partouche E, Revel-Mouroz P (2020) Imaging-guided precision medicine in non-resectable gastro-entero-pancreatic neuroendocrine tumors: a step-by-step approach. Eur J Radiol 1(122):108743
Salama AI, Mohamed Houseni M, Elsheshiny AA (2020) Effect of chemotherapy on liver metabolism as measured by PET/CT scan. Egyptian J Biomed Eng Biophys 21(1):75–85
Salomon T, Nganoa C, Gac AC, Fruchart C, Damaj G, Aide N, Lasnon C (2018) Assessment of alteration in liver 18F–FDG uptake due to steatosis in lymphoma patients and its impact on the Deauville score. Eur J Nucl Med Mol Imaging 45(6):941–950
Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, Ratziu V, McCullough A (2011) Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology 54:344–353
Sanyal D, Mukherjee P, Raychaudhuri M, Ghosh S, Mukherjee S, Chowdhury S (2015) Profile of liver enzymes in non-alcoholic fatty liver disease in patients with impaired glucose tolerance and newly detected untreated type 2 diabetes. Indian J Endocrinol Metab 19(5):597–601
Seraj SM, Al-Zaghal A, Zadeh MZ, Jahangiri P, Pournazari K, Raynor WY, Werner TJ, Høilund-Carlsen PF, Alavi A, Hunt SJ (2019) Dynamics of fluorine-18-fluorodeoxyglucose uptake in the liver and its correlation with hepatic fat content and BMI. Nucl Med Commun 40(5):545–551
Softic S, Kahn RC (2019) Fatty liver disease: is it nonalcoholic fatty liver disease or obesity-associated fatty liver disease? Eur J Gastroenterol Hepatol 31(1):143
Stumpe KD, Strobel K (2006) ^ sup 18^ F FDG-PET imaging in musculoskeletal infection. Q J Nucl Med Mol Imaging 50(2):131
Tokushige K, Ikejima K, Ono M, Eguchi Y, Kamada Y, Itoh Y, Akuta N, Yoneda M, Iwasa M, Yoneda M, Otsuka M (2021) Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. J Gastroenterol 56(11):951–963
Zhang X, Gao X, Zhang P, Guo Y, Lin H, Diao X, Liu Y, Dong C, Hu Y, Chen S, Chen X (2017) Dynamic mechanical analysis to assess viscoelasticity of liver tissue in a rat model of nonalcoholic fatty liver disease. Med Eng Phys 1(44):79–86
Acknowledgements
The authors would like to thank all the members’ staff of the Ayadi El Mostakbal Hospital for their kind assistance and cooperation, and especially the nuclear imaging department. Also, they would like to give special thanks to Dr.Ehab Mahmoud, for his constant moral support and encouragement.
Funding
No funded.
Author information
Authors and Affiliations
Contributions
MAA: Quantitative and qualitative measurements of research cases, Participate in writing the research paper. EEA: Selecting cancer patients, Acceptance of the plan established for positron emission tomography and the method of drawing blood samples for cancer patients. MM: Sharing thesis scientific idea, Extracting significances from statistical analysis of research data. MMES: Diagnosis and quantification of diagnostic PET CT scans for cancer patients. MZES: Supervising on the Laboratory analyzes procedures, Quantitative and qualitative measurements of research cases, Participate in writing the research paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was obtained from the Medical Research Institute, Alexandria University, Egypt.
Informed consent
Subjects were recruited according to the rules of Ayadi Al-Mostakbal ethical committee for conduction of medical research on human subjects and informed consents were obtained from each patient included in the study.
Competing interests
No Competing interests between authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ali, M.A., El-Abd, E., Morsi, M. et al. The effect of hepatic steatosis on 18F-FDG uptake in PET-CT examinations of cancer Egyptian patients. European J Hybrid Imaging 7, 19 (2023). https://doi.org/10.1186/s41824-023-00173-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41824-023-00173-6