Abstract
Introduction
There is much literature about the role of 2-[18F]FDG PET/CT in patients with breast cancer (BC). However, there exists no international guideline with involvement of the nuclear medicine societies about this subject.
Purpose
To provide an organized, international, state-of-the-art, and multidisciplinary guideline, led by experts of two nuclear medicine societies (EANM and SNMMI) and representation of important societies in the field of BC (ACR, ESSO, ESTRO, EUSOBI/ESR, and EUSOMA).
Methods
Literature review and expert discussion were performed with the aim of collecting updated information regarding the role of 2-[18F]FDG PET/CT in patients with no special type (NST) BC and summarizing its indications according to scientific evidence. Recommendations were scored according to the National Institute for Health and Care Excellence (NICE) criteria.
Results
Quantitative PET features (SUV, MTV, TLG) are valuable prognostic parameters. In baseline staging, 2-[18F]FDG PET/CT plays a role from stage IIB through stage IV. When assessing response to therapy, 2-[18F]FDG PET/CT should be performed on certified scanners, and reported either according to PERCIST, EORTC PET, or EANM immunotherapy response criteria, as appropriate. 2-[18F]FDG PET/CT may be useful to assess early metabolic response, particularly in non-metastatic triple-negative and HER2+ tumours. 2-[18F]FDG PET/CT is useful to detect the site and extent of recurrence when conventional imaging methods are equivocal and when there is clinical and/or laboratorial suspicion of relapse. Recent developments are promising.
Conclusion
2-[18F]FDG PET/CT is extremely useful in BC management, as supported by extensive evidence of its utility compared to other imaging modalities in several clinical scenarios.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Objectives
The aim of this guideline is to provide information regarding the role of 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography (2-[18F]FDG PET/CT) in patients with no special type breast cancer (NST BC), summarizing its indications according to updated literature evidence.
This guideline should be viewed as a dynamic document in a constantly evolving field, rather than a definitive document or summary of existing protocols. Moreover, modifications may be considered according to local legislation and regulations.
Methodology
The idea for this document was formulated by a subset of members of the EANM Oncology and Theranostics Committee. The concept of this guideline was approved by the EANM Board; then, the SNMMI was invited and named its representatives. The EANM board approved suggested experts and recommended others with the aim of creating a multidisciplinary team of experts. The final version received the comments from the EANM national societies and SNMMI public comments and was endorsed by the American College of Radiology (ACR), European Society of Surgical Oncology (ESSO), European Society for Radiotherapy and Oncology (ESTRO), European Society of Breast Imaging/European Society of Radiology (EUSOBI/ESR), and European Society of Breast Cancer Specialists (EUSOMA).
This guideline provides practical recommendations to be applied in clinical practice. All sections were written according to updated literature and state-of-the-art information and then critically verified by the writing committee.
For the writing section “Indications for 2-[18F]FDG PET/CT in no special type breast cancer”, the literature search was based on the international Appraisal of Guidelines, Research and Evaluation (AGREE) tool [1]. We used the 23 topics from the AGREE II reporting checklist. In summary, the following steps were taken:
-
1. Clinical indications for 2-[18F]FDG PET/CT were defined by a multidisciplinary team of experts. The clinical indications were the topics for the literature search.
-
2. Keywords were selected for each indication to search the literature in the following databases: PubMed, Embase, Web of Science, Cochrane Library, and Emcare through January 2022. Preclinical studies, case reports, images of interest, abstracts-only presented in congresses, and editorials were excluded.
-
3. Relevant papers were selected for each indication based on title and abstract. Non-English papers were excluded.
-
4. Papers were critically analyzed before being included. Additional recent publications were also included whenever they were considered important by the writing committee.
-
5. Recommendations for performing 2-[18F]FDG PET/CT were discussed in online meetings and were summarized in boxes in the “Metabolic response criteria” (Box nº 1) and “Indications for 2-[18F]FDG PET/CT in no special type breast cancer” (Box nº 2 to 8) sections.
-
6. For each summary box, the level of evidence and grade of recommendation was provided, following the National Institute for Health and Clinical Excellence (NICE) criteria (Table 1), as previously used in EANM documents [2,3,4], as follows:
-
7. Finally, the writing committee composed by a multidisciplinary team of experts votes on the scores for each recommendation’s level of evidence and grade of recommendation stating if they agree, disagree, or abstain.
-
8. The level of evidence, grade of recommendation, and percentage of agreement are provided for each recommendation in the respective box. The percentage of agreement was at least 85% for each recommendation.
Introduction
According to GLOBOCAN 2020, female breast cancer (BC) was the leading cause of cancer incidence worldwide (2.3 million new cases, corresponding to 11.7% of all cancers) and it was the fifth leading cause of cancer mortality (685,000 deaths) [5].
The histology of the majority of diagnosed BC (75–80%) is no special type (NST) that corresponds to invasive ductal carcinoma (IDC) in the previous nomenclature; the second most common histology (10–15%) is invasive lobular carcinoma (ILC). Up to 5% of BC is considered “special types” due to distinctive characteristics as well as particular cellular and molecular behaviours. These “special types” include medullary, apocrine, neuroendocrine, mucinous, tubular, and metaplastic carcinomas [6,7,8,9,10,11]. BC is a heterogeneous disease with different biological subtypes depending on the expression of hormone receptors (HR), including oestrogen receptors (ER) and/or progesterone receptors (PR), and the levels of the human epidermal growth factor receptor 2 (HER2). There are four main subtypes of BC, although different classifications exist with small differences between them: Luminal A-like (HR + /HER2 − and low-grade/low proliferation), Luminal B-like (HR + /HER2 − and high-grade/high proliferation) – luminal-like correspond to 65% of cases, HER2 + (HR + or − /HER2 +) in 15–20%, and triple negative (HR − /HER2 −) in 10–15%.
BC usually disseminates loco-regionally to the ipsilateral axillary lymph nodes but can also spread loco-regionally to ipsilateral internal mammary or supraclavicular lymph nodes (up to stage N3c) [12, 13]. However, dissemination to contralateral axillary, internal mammary, or supraclavicular lymph nodes or to ipsilateral or contralateral cervical lymph nodes is regarded as distant metastasis (or stage M1). BC can potentially spread to any organ, but the most common sites are the skeleton, liver, lung, and brain. Different from NST, metastatic ILC more commonly features sclerotic bone metastases (sometimes of miliary type without FDG uptake) and metastases to the gastrointestinal tract and serosa. Factors associated with the presence of distant metastases at an earlier stage are younger age at diagnosis and triple-negative tumours [14, 15]. Each subtype has different biological behaviour in terms of survival, recurrence, and typical patterns of metastatic spread [16,17,18,19]. For example, bone metastases are more common in patients with HR + BC, whereas visceral metastases occur more often in HR- tumours [20]. In this regard, triple-negative breast cancers (TNBC) present with visceral metastases more often, predominantly intrapulmonary [17, 21, 22]. The HER2-enriched subtypes usually metastasize to the lung, liver, and brain and less often to the skeleton [23].
The 8th edition of the American Joint Committee on Cancer (AJCC) includes two staging systems: (1) the anatomic stage, which includes the characteristics of the primary tumour (T), nodal status (N), and distant metastasis (M), and is then further subdivided into clinical and pathologic anatomic stage, and (2) the prognostic stage, adding tumour grade, HR status, HER2 expression, and multigene panel testing results to the anatomic stage [12].
Currently, there is extensive evidence that 2-[18F]FDG PET/CT can be useful in BC management, including initial staging, assessing neoadjuvant systemic treatment response, assessing treatment response in the metastatic setting, searching for loco-regional or metastatic recurrence, and re-staging after therapy, as well as radiation therapy (RT) planning. The 2-[18F]FDG avidity of BC is related to the histologic type, receptor status (ER, PR, and HER2), tumour grade, proliferation index (Ki-67 index), and tumour size. It has been demonstrated that, in general, (1) NST histology has higher 2-[18F]FDG avidity than ILC, (2) TNBC has higher 2-[18F]FDG avidity than ER + tumours, and (3) grade 3 cancers have higher 2-[18F]FDG avidity than lower-grade malignancies [24,25,26,27]. 2-[18F]FDG uptake is also related to microvasculature density for delivering nutrients (and 2-[18F]FDG), glucose transporter 1 (GLUT1) for transportation of the tracer into the cell, hexokinase for tracer entering into glycolysis, number of viable neoplastic cells per volume, number of lymphocytes, and hypoxia-inducible factor 1-alpha (HIF-1a) upregulation of GLUT1 [28]. Consequently, high 2-[18F]FDG uptake correlates with tumour aggressiveness and is associated with a worse prognosis [3, 29,30,31]. A retrospective study reported that 2-[18F]FDG PET/CT was more likely to reveal unsuspected distant metastases in patients with stage III NST (22%) compared to patients with stage III ILC (11%) [32].
Considering there are limited data about 2-[18F]FDG specifically in the ILC subtype, the recommendations written in this document are applicable to NST. In the “Other developments and future applications” section, radiopharmaceuticals other than 2-[18F]FDG are referred to and those may be more useful to study patients with ILC. We recognize the need to have guideline/recommendations about the lobular subtype, and this may be a future project.
Several studies have documented 2-[18F]FDG PET/CT’s utility compared to other imaging modalities including bone scan and contrast-enhanced CT (ceCT). However, the superiority of 2-[18F]FDG PET/CT is still unclear in comparison with whole-body magnetic resonance imaging (wbMRI) [33,34,35,36].
In Table 2, we observe that baseline 2-[18F]FDG PET/CT enabled overall upstaging, when compared with conventional imaging modalities, in more than 19% of patients from IIB onwards: stage IIB (19%), IIIA (34%), IIIB (41%), and IIIC (35%). The percentage of stage modification due to 2-[18F]FDG PET findings is weaker in stage IIA patients but not negligible (13% in Table 2). Three studies from Table 2 also evaluated the percentage of upstaging based on the identification of distant metastasis only (meaning the exclusion of regional lymph node metastasis) and revealed staging modification in 10% of patients initially staged IIB, 20% in stage IIIA, 25% in stage IIIB, and 32% in stage IIIC [37,38,39].
These same authors showed that 2-[18F]FDG PET/CT can provide valuable information for nodal staging in around a quarter of patients, leading to upstaging in 17–24% of patients (mainly due to the identification of N3 disease) [37,38,39]. In one study, modifications included downstaging in 16% of patients [38].
A prospective and randomized clinical trial published in 2023 analyzed 369 patients with NST BC stage IIB (25%) or III (75%) staged with 2-[18F]FDG PET/CT or conventional imaging (bone scan, CT of the chest/abdomen and pelvis) [40]. 2-[18F]FDG PET/CT identified more distant metastases than conventional modalities, resulting in upstaging to stage IV for 12% more patients (23% vs 11%). Consequently, this led to changes in therapy decision and reduction in the number of patients initially considered for combined modality therapy (chemotherapy, surgical resection, and radiotherapy) aimed at curative intent [40].
As referred to above, despite the commonly reported upstaging after 2-[18F]FDG PET/CT due to the identification of N3 or distant metastases, Cochet et al. [38] also evaluated the percentage of downstaging. This prospective study compared staging with conventional imaging and 2-[18F]FDG PET/CT in 142 patients. They observed that 21% of patients were upstaged after 2-[18F]FDG PET/CT (including 8% whose stage changed from stage II/III to stage IV—detailed in Table 2) and 16% were downstaged (including 3% that were initially classified as stage IV, but changed to stage II/III), with high or medium impact on clinical management in 13% of patients (mainly because the intent to treat was modified from curative to palliative or vice-versa) [38].
A systematic review and meta-analysis from 2021 analyzed the impact of 2-[18F]FDG PET (3 studies), PET/CT (25 studies), and PET/MRI (1 study) and found a pooled 25% change in staging that resulted in an 18% change in management [41]. Literature shows a better diagnostic accuracy of 2-[18F]FDG PET/CT to detect distant metastases of NST BC compared to the combination of conventional imaging, due to its higher sensitivity (97–99% vs 56–75%) and specificity (95–99% vs 88–99%), as summarized in Table 3.
Overall, several studies have demonstrated a good diagnostic accuracy of 2-[18F]FDG PET/CT to detect distant metastases, compared to conventional imaging, in particular due to its high sensitivity (Table 2 and 3).
Additionally, 2-[18F]FDG PET/CT seems to play a role in the context of personalized medicine, emerging as a useful imaging modality for response assessment, allowing for early identification of non-responding tumours, providing information regarding adverse therapeutic effects, and defining the right moment to implement changes in therapeutic approach or shift to a subsequent line of treatment with benefits of disease control and cost-effectiveness [48, 49].
2-[18F]FDG PET/CT preparation and acquisition
Patient preparation should follow the “FDG PET/CT EANM procedural guidelines for tumour imaging version 2.0” and the American “Society of Nuclear Medicine and Molecular Imaging (SNMMI) procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0” [50, 51]. Several studies have shown that the metabolic flare reaction occurs between 7 and 10 days after the start of endocrine therapy, and this effect has been observed not only with tamoxifen, but also with fulvestrant and anti-aromatases [52, 53]. Therefore, it is recommended to perform 2-[18F]FDG PET/CT after this time interval [53, 54]. Based on routine clinical practice, our expert consensus panel suggests performing 2-[18F]FDG PET/CT at least 10 days (15 days if possible) after the last dose of systemic therapy (chemotherapy or endocrine therapy) to avoid the effects of the flare phenomenon or stunning reaction. This is an expert opinion-based recommendation, and not based on scientific evidence yet. Interruption of ongoing targeted therapy for therapeutic evaluation is not recommended [55]. Because of the inflammatory effect, the recommendation is to wait at least 3 months after the end of radiotherapy to search for a recurrence in the radiotherapy field.
It is also important to report whether the patient is/was taking corticosteroids when scanned; has received growth factors; was treated with radiation therapy with specification of the treated volumes or recently underwent invasive procedures, including biopsy or surgery, because it will influence 2-[18F]FDG uptake; and has implications in image interpretation [50].
It is recommended that patients fast for at least 4 h before radiopharmaceutical administration and be properly hydrated, serum glucose level should be < 200 mg/dl, and patients should rest in a quiet and warm environment during the 2-[18F]FDG-uptake time that should last 60 min (± 5 min) before image acquisition. Concurrent ceCT imaging can be considered to improve lesion detection on CT, mainly when evaluating response to treatment in patient with metastatic disease. Additionally, it may enable easier comparative imaging with follow-up CT scans. If intravenous contrast administration is performed, kidney function and history of contrast allergy should be verified before the injection and, in such cases, measures (i.e. reinforced hydration and specific medications) should be taken according to radiological protocols [56] as well as local guidelines and regulations.
The standard 2-[18F]FDG PET/CT starts after bladder voiding. The patient is usually in the supine position with the arms above the head, and the acquisition includes the mid-thighs to the skull.
When RT planning is considered, and to better evaluate breasts, a flat table-top and an additional scan in the prone hanging breast position may be used in the imaging acquisition [50, 57]. It is recommended to use support devices for the arms and knees to improve patient comfort and reproducibility. It is important to remember that positioning may be modified and adapted, and analgesic medication may be offered, when necessary, to improve patient comfort.
Ideally, patients should undergo a pre-treatment PET scan (baseline study) and a scan after the end of treatment (final study), performed on the same PET scanner, to evaluate response to therapy; interim PET scan may also be helpful to direct response adapted treatment protocols [58] and should be performed with the same PET scanner used in the baseline study.
False positive findings
The most common cause of false positive 2-[18F]FDG-PET findings in the breast and surrounding tissues are due to (1) benign lesions that include a wide range of diagnoses such as fibroadenoma, intraductal papilloma, as well as reactive, hyperplastic, and metaplastic processes, such as fibrocystic changes and apocrine metaplasia; (2) infection and inflammation (mastitis, fat necrosis, fungal infection, granulomatous processes, such as tuberculosis and sarcoidosis, ruptured breast implant or silicone-related reaction); (3) post-surgery (seroma, muscle uptake); and (4) physiologic (e.g. brown fat activation, lactational changes result in diffuse increased uptake in the breasts) [59, 60].
In addition, changes in metabolic activity at non-cancer sites related to cancer treatments and other medical conditions and interventions may cause imaging misinterpretation at extramammary sites. Some examples include the following: (1) recent vaccinations, particularly to COVID-19, which can result in increased uptake in axillary, subpectoral, and neck nodes; (2) bone marrow repopulation in relation to systemic therapy or granulocyte colony-stimulating factor; (3) inflammation and/or immune-related adverse events such as mucositis, colitis, pneumonitis, thyroiditis, pancreatitis, adrenalitis, and hypophysitis; (4) systemic inflammatory diseases, in particular granulomatosis, sarcoidosis, and sarcoid-like reactions; (5) osteonecrosis of the jaw; (6) fracture and post-procedural inflammation.
False negative findings
Common causes of false negative 2-[18F]FDG-PET findings are as follows [26, 59, 60]: (1) small lesions measuring ≤ 10 mm (or 4–5 mm in digital PET scanners [61]), (2) low-grade tumours, (3) certain histologic subtypes (e.g. lobular, tubular, carcinoma in situ, and neuroendocrine differentiation), (4) low tumour cell density (in necrotic tissue, fibrotic scar, cystic lesions, and mucinous component), (5) artefacts (for example lesions located close to prosthetic devices, or adjacent to areas of high 2-[18F]FDG accumulation, such as activated brown fat or bone marrow, brain, myocardium, bladder), (6) suboptimal technique (e.g. elevated blood glucose or 2-[18F]FDG injection without adequate fasting), (7) PET/CT procedure (e.g. patient movement or breathing artefacts), and (8) recent or ongoing therapy.
Incidental breast findings with 2-[18F]FDG uptake and additional procedures
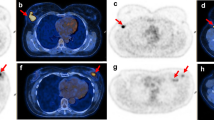
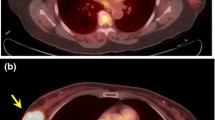
When a 2-[18F]FDG-avid breast lesion is incidentally detected in a study performed for reasons other than BC, further characterization with diagnostic mammography and/or breast US should be performed because these lesions are malignant in 30–40% of cases [62, 63]. Several aetiologies have been described, including unsuspected BC, lymphoma, and metastases [59, 64,65,66,67].
In the context of breast metastases, the most common aetiologies of non-mammary cancers include haematopoietic malignancies (50%), epithelial cancers (23%), such as lung and gastrointestinal adenocarcinomas and squamous cell carcinomas, and melanomas (21%) [68].
A systematic review and meta-analysis from 2019 [63] concluded that the pooled prevalence of focal incidental breast uptake on 2-[18F]FDG PET/CT in women was 0.61%, and in this group of patients, the pooled prevalence of malignancy was 38.7%, with invasive ductal carcinoma being the most commonly detected cancer. In case of focal incidental breast uptake without a known correlate, irrespective of CT appearance, the patient should undergo further evaluation with physical examination, breast imaging, and possible biopsy.
Metabolic response criteria
Summary box nº 1 • 2-[18F]FDG PET/CT should be reported according to PERCIST or to the EORTC PET response criteria [69,70,71] (III-92%/C-85%) • In patients on immunotherapy, 2-[18F]FDG PET/CT should be reported according to the respective EANM guidelines [72] (IV-100%/D-92%) • Quantitative features are imaging biomarkers and valuable tools for prognostication (I-92%/A-92%) |
Metabolic therapy response assessment should be performed according to either the European Organisation for Research and Treatment in Cancer (EORTC) PET response criteria or PET Response Criteria in Solid Tumors (PERCIST) [69,70,71, 73]. For patients with BC undergoing immunotherapy, the recently published EANM/SNMMI/AZZSNM guideline is recommended for response assessment [72].
The use of quantitative 2-[18F]FDG PET/CT as an imaging biomarker has proven to be a valuable tool in treatment response assessment and prognostication [74, 75], with an important predictive role in the prognosis of patients with locally advanced or metastatic BC [58, 76,77,78,79]. Such metrics include the standardized uptake values (SUV) using either the body weight (SUVbw) or the lean body mass (SUL) for normalization, metabolically active tumour volume (MATV or MTV), and total lesion glycolysis (TLG), defined as MATV × SUVmean. In this regard, in a meta-analysis by Diao et al., metrics such as the maximum standardized uptake value (SUVmax—maximal voxel intensity in a defined volume of interest) in the primary tumour were related to a higher risk of recurrence or disease progression in this group of patients. Furthermore, SUVmax showed significant prognostic value in patients with NST [80]. Prospective studies have shown that SUV, MTV, and TLG correlated with response to treatment and prognosis [81, 82].
Pak et al. demonstrated in their meta-analysis that volumetric parameters obtained from 2-[18F]FDG PET/CT were significant prognostic factors for outcome in patients with BC. They concluded that patients with a high MTV and TLG from the primary tumour have a higher risk of adverse events and patients with a high TLG from whole-body tumour burden have a higher risk of death, therefore suggesting that these volumetric parameters should be routinely used when reporting scans [83].
However, no specific cut-off values for these metrics can be recommended currently, as these values differ widely among the data published [78]. To overcome this limitation, compliance with harmonizing standards, such as the EANM Research GmbH (EARL) or American College of Radiology (ACR)/Intersocietal Accreditation Commission (IAC) accreditation, aiming at using 2-[18F]FDG PET/CT as a quantitative imaging biomarker [84,85,86], is recommended.
Indications for 2-[18f]FDG PET/CT in no special type breast cancer
Each indication contains sub-headings with updated information about the role of 2-[18F]FDG PET/CT. Considering that most of the papers were meta-analyses, systematic reviews, and prospective studies, recommendations were graded according to available level of evidence and scored according to NICE grading. The recommendations for performing 2-[18F]FDG PET/CT are summarized in boxes at the beginning of each Sect. 2-[18F]FDG PET/CT indications were organized in the following three parts:
-
A-
Baseline staging
-
B-
Assessment of treatment response
-
C-
Assessment of recurrence
Baseline staging
Although, 2-[18F]FDG avidity of BC is related to receptor status (ER, PR, and HER2), tumour grade, and proliferation index (see above), the recommendations given hereafter are valuable for NST BC whatever the BC molecular subtype and the tumour biological characteristics. In a prospective study of 254 patients, the rates of extra-axillary lymph node metastases on 2-[18F]FDG PET/CT were higher in grade 3 than in low-grade tumours (p = 0.004) and in triple-negative or HER2 + tumours compared to ER + /HER2 − tumours (p = 0.01) [37]. Despite the rate of distant metastases not being related to tumour grade or BC subtype, which has also been found by other studies [14, 37, 43], the location of metastases differed according to primary tumour subtype (for example, triple negative and HER2 + tumours had more extra-skeletal metastases than ER + /HER2 − tumours) [37].
Therefore, recommendations hereafter were performed according to the BC clinical stage. In this section, the clinical stage is defined according to the 8th edition of AJCC classification (Table 4). The clinical stage is based on clinical examination and locoregional imaging (breast/axilla ultrasound, mammography ± breast MRI) performed before 2-[18F]FDG PET/CT.
This section is divided into the following parts:
-
1-Stage I
-
2-Stage IIA
-
3-Stages IIB and III
-
4-Stage IV
Stage I
Summary box nº 2 • 2-[18F]FDG PET/CT is not recommended in stage I (II-100%/B-100%) |
Patients with small tumour ≤ 2cm (T1 of the TNM classification) are usually treated with primary surgery combined with sentinel node biopsy. PET has limited spatial resolution (approximately 5–6 mm) and its performance is inferior to that of the sentinel node biopsy [88].
SUVmax values are lower in stage I than in higher stages [89]. In addition, the risk of distant metastases in T1N0 disease (stage I) is very low and PET imaging may lead to false positive findings. In a multicentre study of 325 women with operable BC, 2-[18F]FDG PET (without a CT component) suggested distant metastases in 13 patients, only three (0.9%) were confirmed as metastatic disease and 10 (3.0%) were false positives [90]. Therefore, 2-[18F]FDG PET/CT is not indicated for staging patients with stage I [91].
Stage IIA
Summary box nº 3 • 2-[18F]FDG PET/CT may be useful in patients with clinical stage IIA (T1N1 or T2N0), but there is not enough strong data to recommend routine use in this subgroup (III-100%/C-100%) |
In Table 2, the percentage of stage modification due to 2-[18F]FDG PET findings is weaker in stage IIA patients, but not negligible (13%). In the retrospective study by Lebon et al., distant metastases were detected by FDG-PET/CT in 15% of stage IIB patients and in 11% of stage IIA patients [44]. In another recent retrospective study, however, PET/CT demonstrated distant disease in 9.8% of stage IIB BC patients, but in only 0.8% of those with stage IIA [92]. In the study by Groheux et al., stage IIA was mainly represented by tumours classified as T2N0. PET showed pathological foci in 4.5% of women (2.3% distant metastases and 2.3% extra-axillary nodes) [37]. Larger studies in which the performance of PET is examined in subcategories of patients with T2N0 disease, such as those with large tumours (T2 > 3 cm) would be useful [93].
Stage IIB and stage III
Summary box nº 4 • 2-[18F]FDG PET/CT can be recommended for baseline staging of stage IIB (preferably before surgery) and stage III (including inflammatory BC) (II-100%/B-100%) • 2-[18F]FDG PET/CT can be done instead of, and not in combination with, conventional imaging modalities for staging (combination of bone scan, chest X-ray or CT-chest, and ultrasound of the liver or CT-abdomen) (II-100%/B-100%) • 2-[18F]FDG PET/CT is recommended in baseline treatment planning and may improve RT planning (III-100%/C-100%) |
Role of 2-[18F]FDG PET/CT in baseline staging of stage IIB
Two recent papers about clinical management, including a good clinical practice guideline and a meta-analysis, which were published in 2020 and 2021, respectively, concluded that 2-[18F]FDG PET/CT can be recommended for initial staging to identify distant metastases in patients with clinical stage ≥ IIB BC [3, 93]. Several studies support this indication (Table 2), including a retrospective multicentre study [39], which included 195 patients with clinical stage IIA–IIIC, and compared 2-[18F]FDG PET/CT and conventional imaging modalities (combination of bone scan, chest X-ray or CT-chest, and ultrasound of the liver or CT-abdomen). The authors showed that 2-[18F]FDG PET/CT before neoadjuvant therapy enabled the identification of more extensive disease in 37% of patients, including more nodal metastases in 23% and distant metastases in 14%. Additionally, the same authors concluded that due to the high detection rate, comparable cost ($1604.37 vs $1679.94), lower radiation dose (14 mSv vs 21 mSv), and greater convenience, 2-[18F]FDG PET/CT should be considered as an alternative to conventional imaging modalities. Another study in 2020 revealed that 2-[18F]FDG PET/CT decreased the false-positive rate by 50% compared with conventional imaging and, therefore, decreased the necessity for further work-up due to incidental findings, preventing delay of treatment in a cost-effective manner [94]. In a prospective study to evaluate the role of 2-[18F]FDG PET/CT in the initial staging of 142 patients with ≥ T2 BC, when compared with conventional imaging modalities (mammogram and/or breast US, bone scan, abdominal US and/or CT, X-rays and/or CT of the chest), 21% of patients were upstaged by 2-[18F]FDG PET/CT (including 8% from stage II or III to stage IV) and 16% were down-staged (including 3% from stage IV to stage II or III) [38]. Several other studies have also highlighted the role of 2-[18F]FDG PET/CT in early-stage disease [74, 95], with the percentage change in staging after 2-[18F]FDG PET/CT by initial TNM summarized in Table 2.
2-[18F]FDG PET/CT may add value in the assessment of lymph node regions not easily accessible by US, including internal mammary and mediastinal lymph nodes [96]. A prospective study verified that 2-[18F]FDG PET/CT provided useful information in 13% of patients with clinical T3N0, T2N1, or T3N1 disease, because of the detection of extra-axillary regional lymph nodes in 6.5% and distant metastases in 9% of patients [97].
Finally, besides the higher sensitivity and specificity of 2-[18F]FDG PET/CT for staging of distant disease compared to combined conventional staging, performing a hybrid study in a single visit may be more convenient for the patient.
Role of 2-[18F]FDG PET/CT in baseline staging in stage III (including inflammatory BC)
According to the National Comprehensive Cancer Network (NCCN), LABC corresponds to AJCC stages IIIC, IIIB, and IIIA (except for T3N1 tumours) [98]. LABC has at least one of the following characteristics: T4 or N2 or N3 (Table 4). 2-[18F]FDG PET/CT has been shown to be very effective for staging of LABC and inflammatory BC (T4d).
In LABC, the percentage of patients with extra-axillary lymph node involvement detected by 2-[18F]FDG PET/CT varies between 10 and 29% [99,100,101]. Considering the prevalence of axillary lymph node involvement reaches up to 80% in this setting, the usefulness of 2-[18F]FDG PET/CT is related to its capability of detecting lymph node metastases [100, 102]. Nevertheless, based on the available literature, a negative axilla on 2-[18F]FDG PET/CT does not exclude the need for a sentinel lymph node biopsy. Moreover, studies have shown 2-[18F]FDG PET/CT detects distant metastases in 6–26% of patients with LABC [101, 103,104,105].
Furthermore, as previously mentioned, the recent prospective multicenter study by Dayes et al. [40] demonstrated that the proportion of patients initially staged as IIB and III, who were subsequently upstaged to stage IV following 2-[18F]FDG PET-CT, was significantly higher compared to those evaluated using conventional imaging modalities (23% vs 11%), thereby influencing treatment decisions.
Inflammatory BC is a distinct and aggressive subtype of BC characterized by rapid onset and a high propensity for metastatic disease at presentation, with approximately 80% of patients presenting with axillary lymph node involvement and 40% with distant metastases. Many guidelines recommend incorporating 2-[18F]FDG PET/CT in the staging of inflammatory BC [98, 106].
2-[18F]FDG PET/CT improves nodal staging in inflammatory BC, as demonstrated by a retrospective study correlating SUVmax of regional lymph nodes with histopathology, reporting a sensitivity of 89% and specificity of 99% [107]. Furthermore, it surpasses conventional imaging methods (including mammography, breast/regional lymph node ultrasound, whole-body bone scan, chest X-ray or CT, and abdominal ultrasound or CT) in detecting distant metastases, achieving sensitivity rates of 96–100% and specificity rates of 91–98%, compared to the combined conventional imaging sensitivity of 60–84% and specificity of 67–83% [107,108,109,110,111,112,113,114].
Role of 2-[18F]FDG PET/CT in the baseline treatment planning of stage IIB and III
In patients with BC and confirmed axillary nodal involvement, 2-[18F]FDG PET/CT is useful prior to surgery or neoadjuvant therapy because it identifies distant metastases ranging from 6 to 26% of cases [4]. Furthermore, it is useful for patient selection and planning of RT volumes.
In a prospective study from 2021, patients with high-risk primary BC were staged with 2-[18F]FDG PET/CT. Distant metastases were identified in 23% of patients, and more advanced loco-regional disease in 16% [115]. This led to more extensive RT and stage modification in 40% of patients [115]. Therefore, the authors concluded that 2-[18F]FDG PET/CT should be considered for initial staging in high-risk primary BC to improve treatment planning.
Another prospective trial evaluated the diagnostic performance of pre-treatment 2-[18F]FDG PET/CT and its impact on RT in patients with LABC [116]. The authors observed 2-[18F]FDG PET/CT detected 20% more distant metastatic disease or nodal disease outside conventional RT fields not visualized on conventional imaging (including mammography and breast US, bone scan, and CT chest, abdomen, and pelvis scans), leading to a change in RT plan [116].
2-[18F]FDG PET/CT changed regional RT planning in patients with involved axillary lymph nodes in around 20% of cases [117, 118]. Also, it improved the RT procedure of involved internal mammary lymph nodes, allowing for conformal 3-dimensional (3D) planning and dose-escalation [119, 120].
Regarding inflammatory BC, 2-[18F]FDG PET/CT led to a change in RT plan in 18% of patients and in overall treatment in approximately 10% of patients [121]. Other authors stated that 2-[18F]FDG PET/CT changed the initial staging and, consequently, the treatment strategy in up to 50% of patients with inflammatory BC [4, 122]. Pre-treatment 2-[18F]FDG PET/CT has also provided valuable information on disease extent evaluation, leading to changes in post-mastectomy RT target volumes and radiation doses in approximately 20% of patients [113, 123].
Stage IV
Summary box nº 5 • 2-[18F]FDG PET/CT can be useful for determining the extent of metastatic disease (outside the brain) and improving treatment planning (III-100%/C-100%) • 2-[18F]FDG PET/CT can be done instead of, and not in addition to separate conventional imaging modalities (combination of bone scan, chest X-ray or CT-chest, and ultrasound of the liver or CT-abdomen) (II-100%/B-100%) |
The presence, extent, and localization of distant metastases are key prognostic factors in BC patients and play a central role in therapeutic decision-making. Studies have not focused on patients with known stage IV disease, but on patients with LABC, in whom 2-[18F]FDG PET/CT has shown to perform better than bone scans or CT scans in assessing metastatic disease.
In the study from Choi et al. [124], encompassing a group of 154 consecutive patients, the sensitivity and specificity in detecting distant metastasis was 61.5% and 99.2%, respectively, for conventional imaging, and 100% and 96.4%, respectively, for 2-[18F]FDG PET/CT.
The bone is the most frequent site of metastases in patients with BC. In the study from van Es et al. [125], baseline ceCT, bone scan, and 2-[18F]FDG PET for all patients included in the IMPACT-MBC study were reviewed for bone lesions. In total, 3473 unequivocal bone lesions were identified in 102 evaluated patients (39% by ceCT, 26% by bone scan, and 87% by 2-[18F]FDG PET). Additional bone lesions on 2-[18F]FDG PET plus ceCT compared with bone scan plus ceCT led to a change in MBC management recommendations in 16% of patients [125].
Several other studies showed that bone scan is not useful when 2-[18F]FDG PET/CT is performed [37, 111, 126]. In particular, PET/CT was more sensitive and more specific than bone scan or ceCT for detecting lytic or mixed bone metastases and bone marrow involvement [126]. 2-[18F]FDG uptake was more variable in osteoblastic metastases, and careful reading of CT data from PET/CT may help detect them [13]. In a study of 23 BC patients with bone metastases, PET/CT detected more lesions than bone scan (mean, 14.1 vs. 7.8 lesions, respectively, p < 0.01) [127]. [18F]NaF PET/CT can also be of added value in the case of sclerotic bone lesions [128] (see “Other radiopharmaceuticals” section).
2-[18F]FDG PET/CT also performs well in detecting distant lymph nodes, pleural, hepatic, splenic, adrenal, and pelvic metastases. In 117 patients with LABC, 2-[18F]FDG PET/CT detected distant metastases in 43 patients (37%) [111]. The sensitivity and specificity of PET/CT were 100% and 97.7% for the detection of bone lesions (compared with 76.7% and 94.2%, respectively, for planar bone scan); 100% and 99.1%, respectively, for the detection of pleural metastases (vs. 50% and 100% for dedicated CT); and 85.7% and 98.2%, respectively, for the detection of pulmonary metastases (vs. 100% and 98.2% for dedicated chest CT) [111]. In this study, PET was therefore less sensitive than chest CT for the detection of small lung nodules, which could be explained by the partial volume effect and respiratory motion. Regarding distant lymph node involvement, 2-[18F]FDG PET/CT detected supra-diaphragmatic distant lymph nodes in 18 patients and infra-diaphragmatic nodes in four patients. Among 117 patients, 10 were diagnosed with liver metastases, and 2-[18F]FDG PET/CT confirmed the nine cases detected by abdominal CT and/or liver ultrasound and enabled the identification of one additional patient [111].
Assessment of treatment response
Assessment of neoadjuvant treatment response in non-metastatic breast cancer
Summary box nº 6 • 2-[18F]FDG PET/CT may be used to assess early metabolic response in non-metastatic breast cancer, particularly in TNBC and HER2 + (II-100%/ B-100%) |
Considering 2-[18F]FDG PET/CT provides different information depending on timing and therapeutic procedure, the following paragraphs describe its potential use in the early evaluation of metabolic response in patients under neoadjuvant therapy, as well as response evaluation after completion of it. While results are promising, further evidence is needed to support this recommendation.
Metabolic response at early response evaluation in the primary tumour to primary systemic therapy
2-[18F]FDG PET/CT is associated with good sensitivity, but lower specificity, to predict early histopathological response to neoadjuvant therapy (as early as the first cycle of treatment), independent of BC subtypes. Metabolic changes after systemic therapy, measured by 2-[18F]FDG PET/CT, usually predict treatment response earlier than anatomic changes [3, 58]. Many clinical trials found the ability to distinguish responding from non-responding tumours, with the potential for personalized adaptation of therapy courses [55, 58, 129, 130].
In four meta-analyses, regrouping 920 [131], 781 [132], 745 [133], and 1119 [134] patients, PET sensitivity and specificity in predicting the pathological response early were respectively 84% and 66% in the first, 84% and 71% in the second, 81% and 79% in the third, and 82% and 79% in the fourth meta-analysis. Overall, the sensitivity was in the order of 80–85% and the specificity was somewhat lower. A fifth meta-analysis, comparing PET to MRI to predict pCR, showed that PET was more sensitive and MRI more specific [135]. Finally, a most recent meta-analysis of 1630 patients (assessing interim and post-treatment 2-[18F]FDG PET scans) suggested that the use of 2-[18F]FDG PET or PET/CT for evaluation of response to neoadjuvant treatment provides significant predictive value for disease recurrence and survival in BC patients [136].
Overall, these meta-analyses also highlighted large disparities between studies. In most studies, a cut-off for the reduction in the primary tumour SUVmax value (ΔSUVmax) has been used to discriminate metabolic responders (decrease in SUV above the threshold) from non-responders. The cut-off value that offers the best prediction of pathological complete response (pCR) at the end of the neoadjuvant treatment has most often been determined from the area under the receiver operating characteristic (ROC) curves analysis. Unfortunately, the optimal cut-off varied between the studies. The tumour subtype and the treatment used should be considered to assess response in patients with BC treated by neoadjuvant treatment. Triple-negative tumours have higher FDG uptake than the others. The type of treatment is also crucial. In triple-negative tumours, a team observed that the SUV decreased significantly more with dose-dense and dose-intense treatment than with conventional dose chemotherapy regimen [137]. An ancillary study in the NeoALTTO trial showed that SUV reduced more with lapatinib + trastuzumab than with trastuzumab alone [55].
Early assessment of neoadjuvant treatment in triple-negative tumour
Pathological complete response (pCR) is associated with better survival and is therefore the objective in patients with TNBC [138]. Several studies evaluated the value of 2-[18F]FDG PET for early prediction of pCR in patients with TNBC [137, 139,140,141,142,143,144,145]. In 78 patients with TNBC, the change in SUVmax after two cycles of neoadjuvant chemotherapy was strongly correlated with pCR at surgery. [137]. These patients were followed up and the risk of recurrence was greater when the SUVmax of the primary tumour did not decrease or only slightly decreased after two cycles of neoadjuvant treatment [137].
Early assessment of neoadjuvant treatment in HER2 overexpressing tumour
TBCRC 026 [146] evaluated patients with stage II or III HER2 + BC for reduction of metabolic activity under neoadjuvant treatment. 2-[18F]FDG PET/CT was performed at baseline and 15 days after therapy initiation of pertuzumab and trastuzumab. The difference between the groups who obtained pCR at the end of the neoadjuvant treatment and those who did not was documented by a median percent reduction in SULmax (63.8% vs 41.8%) and SULmax reduction ≥ 40% (83% vs 52%).
The multicentre randomized phase 2 study AVATAXHER included 142 patients who initially received standard therapy combining docetaxel and trastuzumab and evaluated the role of 2-[18F]FDG PET in modulating neoadjuvant treatment according to the metabolic response after one cycle [129]. In the case of poor response after one cycle (low or absence of SUV decrease), a randomization was performed: arm A received bevacizumab in addition to the initial treatment starting from cycle-3, while arm B continued the initial treatment. At treatment completion, the pCR rates were respectively 37/69 (53.6%) for responding patients (high SUV decrease after one cycle), 21/48 (43.8%) for arm A, and 6/25 (24.0%) for arm B. Thus, a change in treatment led to an increase in the pCR rate in poor responders. Unfortunately, long-term follow-up of this patient cohort showed that improvement of pCR did not modify disease-free survival [147].
More recently, in the PHERGain multicentre, randomized, open-label, non-comparative, phase 2 study, 356 patients with HER2 + early-stage BC were included [130, 148]. 2-[18F]FDG PET identified patients who were likely to benefit from chemotherapy-free dual HER2 blockade with trastuzumab and pertuzumab and a reduced negative impact on global health status [130].
Early assessment of neoadjuvant treatment in HR-positive tumour without HER2 overexpression
The majority of HR + tumours have weak 2-[18F]FDG uptake. The chemosensitivity of these tumours is variable but limited and pCR is rarely reached [149, 150]. In the study from Humbert et al. [141], evaluating the predictive value of 2-[18F]FDG PET by BC subgroups, pCR was obtained in only one of the 53 patients with a luminal BC. Therefore, 2-[18F]FDG PET/CT seems to have limited value to predict pCR in this subgroup.
Metabolic response after primary systemic therapy
A 2020 meta-analysis [136] included 17 studies assessing interim and post-treatment 2-[18F]FDG PET scans and described that the pooled hazard ratio of metabolic responses on disease-free survival and OS was 0.21 and 0.20 for interim PET scans and 0.31 and 0.26 for post-treatment PET scans, respectively. However, several studies have shown that 2-[18F]FDG PET is not very sensitive at the end of treatment to reveal the residual primary tumour tissue [151, 152]. 2-[18F]FDG PET/CT shows a tendency toward underestimation of the residual tumour [153]; therefore, MRI performs better in this context [151, 154].
Two systematic reviews [155, 156] analyzed the diagnostic performance of clinical examination, axillary US, breast MRI, and whole-body 2-[18F]FDG PET/CT and found that, currently, there is no accurate non-invasive technique to identify patients with a complete axillary response after neoadjuvant therapy. Given the ongoing debate about optimal axillary management after neoadjuvant therapy, sentinel lymph node biopsy with or without the removal of marked initially positive lymph nodes should be considered in patients with an imaging-based negative axilla.
However, at the end of neoadjuvant chemotherapy, 2-[18F]FDG PET/CT can be useful to perform a whole-body examination, to exclude metabolically active regional lymph nodes or distant metastases before breast surgery.
Assessing treatment response in metastatic breast cancer
Summary box nº 7 • 2-[18F]FDG PET/CT may play a role in monitoring treatment response in metastatic breast cancer (III-100%/C-85%) • 2-[18F]FDG PET/CT may be particularly useful to assess bone metastases and enable early response to treatment evaluation (III-100%/C-100%) |
Several studies have demonstrated the usefulness of 2-[18F]FDG PET/CT to assess response to systemic therapy in metastatic BC, and some have shown its superiority compared to CT, including a systematic literature review from 2019 and a prospective clinical study from 2023 [157,158,159,160].
In patients with multiple distant metastases, 2-[18F]FDG PET/CT allows for an earlier detection of progression, when compared with conventional imaging, enabling a change in treatment with a potential impact on survival [161]. The results of a prospective clinical study (MESTAR) of 87 patients with metastatic BC revealed that disease progression was detected first by 2-[18F]FDG PET/CT in half of the patients, with a mean time of 6 months earlier than on ceCT [159]. Furthermore, tumour response on 2-[18F]FDG PET/CT was significantly associated with progression-free survival and disease-specific survival [160]. A retrospective study of 300 patients with metastatic BC (86% presented with multiple metastases) verified that 2-[18F]FDG PET/CT improved patient management with a survival benefit of 14–24 months when 2-[18F]FDG PET/CT was used alone or in combination with CT to evaluate treatment response [162].
In the specific context of bone metastases, decreased 2-[18F]FDG uptake and increased bone sclerosis on CT images are predictors of good response to therapy [163]. Several retrospective studies have reported that 2-[18F]FDG PET/CT is superior to CT or bone scan to assess response to therapy [164, 165]. A prospective study of 31 patients verified an overall similar diagnostic performance between 2-[18F]FDG PET/CT and wbMRI [166]. However, contrary to MRI, 2-[18F]FDG uptake was associated with progression-free survival [166]. A prospective study of 23 women with biopsy-proven ER-positive bone-only or bone-dominant metastatic BC supported the usefulness of the early 2-[18F]FDG PET/CT evaluation (4 weeks after starting new endocrine therapy) to predict treatment failure. The authors reported that patients with good response on early 2-[18F]FDG PET/CT evaluation tend to have a longer progression-free survival, OS, and time to skeletal-related events, compared with non-responders [167]. The results of this study informed the inclusion of a 4-week time point in the ongoing phase 2 ECOG-ACRIN 1183 (FEATURE) trial.
Assessment of recurrence
Summary box nº 8 • 2-[18F]FDG PET/CT is useful to detect the site and extent of recurrence when conventional imaging methods are equivocal (I-85%/A-100%) • 2-[18F]FDG PET/CT can be recommended: o In patients with signs or symptoms suggestive of metastatic disease (I-92%/A-100%) o In patients with rising serum tumour markers (II-92%/B-85%) o To guide site of biopsy (IV-100%/D-100%) o To improve RT planning (III-100%/C-100%) • 2-[18F]FDG PET/CT can substitute for CT and/or bone scan in the detection of bone metastases (II-100%/B-100%) |
2-[18F]FDG PET/CT was compared prospectively with thoraco-abdominal ceCT and bone scan (all scans were performed within approximately 10 days) in 100 patients with suspected BC recurrence [168]. Twenty-two percent of patients were diagnosed with distant recurrence, 19% were classified as having local recurrence only, and in 59%, no recurrence was found. For distant recurrence, the area under the ROC curve was 0.99 for 2-[18F]FDG PET/CT, 0.84 for ceCT, and 0.86 for the combination of ceCT and bone scan [168]. Other guidelines suggest 2-[18F]FDG PET/CT may be useful to detect the site of recurrence when conventional imaging methods provide equivocal results [98, 169].
Locoregional recurrence
Radiological techniques are the standard imaging modalities for assessing locoregional recurrence, and, in particular, breast MRI plays an important role. 2-[18F]FDG PET/CT is particularly useful in the differentiation of post-treatment fibrosis from viable tumour tissue [98]. It may identify isolated loco-regional lesions [98, 169], particularly in aberrant lymph drainage locations due to previous surgery and/or RT, enabling targeted treatment.
2-[18F]FDG PET/CT impacted the clinical management of recurrent locoregional disease in 51–69% of patients [170].
Distant recurrence
High diagnostic accuracy of 2-[18F]FDG PET/CT for metastases identification has been shown prospectively, with an area under the ROC curve of 0.99 for 2-[18F]FDG PET/CT, 0.84 for thoraco-abdominal ceCT, and 0.86 for the combination of ceCT and bone scan [168]. Even in patients with early-stage BC, but presenting symptoms suspicious for first distant metastases, 2-[18F]FDG PET/CT was evaluated prospectively against biopsy-verified local recurrence in 225 women. 2-[18F]FDG PET/CT showed high diagnostic accuracy in detecting recurrence with a sensitivity, specificity, and area under the ROC curve of 1.00, 0.88, and 0.98, respectively [171].
Considering bone lesions, 2-[18F]FDG PET/CT allows earlier detection of bone metastases and identifies non-measurable lesions by morphologic examinations [48]. Its improved sensitivity may be due to the capability of detecting rapid osteolytic growth, as well as metastatic tumour cells within the bone marrow, before there is sufficient osteoblastic reaction detectable by bone-specific tracers or on CT imaging [170]. Furthermore, lesion-based sensitivity of 2-[18F]FDG PET/CT is comparable to the combination of bone scan and low-dose CT (98.2% vs. 98.6%, respectively), but is significantly higher than low-dose CT (80%) or bone scan (76%) alone [172]. Therefore, it is an equivalent substitution for CT and/or bone scan, meaning that if bone metastases are detected on 2-[18F]FDG PET/CT, the bone scan is not needed for confirmation [98, 173, 174].
A retrospective study of patients with suspicion of BC relapse verified that, when compared with conventional imaging (including mammography, CT, MRI, and bone scintigraphy), 2-[18F]FDG PET/CT changed treatment modality or intent in 48% and led to modifications in the treatment regimen (namely the RT volume or dose fractionation) in 9% [175].
Several studies, including a meta-analysis [176], have demonstrated that when there are equivocal results or findings suspicious for recurrence on conventional imaging (CT, MRI, ultrasound, bone scan, and mammography) or when tumour markers (cancer antigen 15.3, CA15.3, or carcinoembryonic antigen, CEA) increase during follow-up, the inclusion of 2-[18F]FDG PET/CT in the diagnostic algorithm of BC relapse changes clinical management in 40–50% of patients. The pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, diagnostic odds ratio, and AUC of 2-[18F]FDG PET or PET/CT to detect recurrent disease are 0.90, 0.81, 4.64, 0.12, 46.52, and 0.94, respectively [177]. 2-[18F]FDG PET/CT has also demonstrated a high PPV (0.97) and accuracy (0.83–0.86) in patients with suspected recurrent disease because of increased CA15.3 or CEA [178, 179]. In 561 consecutive patients, the median CA 15.3 value was 35.0 U/mL in cases in which no distant metastases were detected, and it was 58.9 U/mL in cases in which metastases were detected by 2-[18F]FDG PET/CT (p < 0.001) [180]. The median CEA value was 6.6 U/mL in cases without metastases and 12.4 U/mL in cases with metastases (p < 0.001) [180]. It should be noted that in cases of clinical suspicion, 2-[18F]FDG PET/CT can also reveal recurrence even in cases of negative tumour markers [181, 182]. In the case of a known recurrence (identified with clinical examination and/or conventional imaging), 2-[18F]FDG PET/CT is also useful to determine whether the recurrence is isolated or to classify it as either oligo- or multi-metastatic disease. Additionally, 2-[18F]FDG PET/CT plays a role to guide the site of biopsy in a variety of organs, as it increases the detection rate and improves diagnostic accuracy [183].
Summary of indications for 2-[18F]FDG PET/CT in no special type breast cancer
In baseline staging, 2-[18F]FDG PET/CT plays a role from stage IIB through stage IV. 2-[18F]FDG PET/CT is possibly useful in patients with clinical stage IIA (T1N1 or T2N0), but there are not enough data to recommend its routine use.
Whenever possible, quantitative features (such as SUV, MTV, and TLG) should be evaluated and included in the report, because there is robust evidence indicating that these are important imaging biomarkers and valuable prognostic parameters.
When assessing response to therapy, 2-[18F]FDG PET/CT should be performed on EARL or ACR/IAC certified PET/CT scanners, and scans should be reported either according to PERCIST, EORTC PET response criteria, or EANM immunotherapy response criteria, as appropriate. 2-[18F]FDG PET/CT may be useful to assess early metabolic response, particularly in non-metastatic triple-negative and HER2 + tumours.
2-[18F]FDG PET/CT is also useful to detect the site and extent of recurrence when conventional imaging methods are equivocal and when there is clinical and/or laboratorial suspicion of relapse.
The concise summary of the 2-[18F]FDG PET/CT indications according to the clinical scenario defined in this guideline is presented in Table 5 below. Recommendations with level of evidence/grade of recommendation I/A or II/B are highlighted in bold. The remaining recommendations, particularly the ones scored as III/C need further investigation. In our opinion, it would be particularly useful to have more evidence about the role of 2-[18F]FDG PET/CT in assessing response to therapy in the metastatic setting and in RT planning in different clinical scenarios. We consider this the first attempt to define and organize 2-[18F]FDG PET/CT indications for patients with NST BC, but future work is needed to clarify scenarios still lacking robust scientific evidence.
Other developments and future applications
Other radiopharmaceuticals
While 2-[18F]FDG has by far been the most widely utilized PET agent, multiple other radiotracers have an impact or might have a future impact on the care of patients with BC.
16α-18F-Fluoro-17β-fluoroestradiol ([18F]FES)
[18F]FES is a radiolabeled oestrogen analogue that binds to ER. It enables non-invasive, whole-body, and rapid study of functional ER on one single imaging examination. It shows a good correlation with ER immunohistochemistry and enables the detection of ER-positive tumours. [18F]FES was FDA-approved in 2020 as a diagnostic agent for the detection of ER-positive lesions as an adjunct to biopsy in patients with recurrent or metastatic BC. Its sensitivity is high in the bone, lymph nodes, and brain. However, liver evaluation is limited due to its physiologic biliary excretion.
The recently published Appropriate Use Criteria for [18F]FES endorses the following as the most appropriate uses of [18F]FES: (1) to assess ER functionality when endocrine therapy is considered either at initial diagnosis of metastatic BC or after progression of disease on endocrine therapy, (2) to assess the ER status of lesions that are difficult or dangerous to biopsy, and (3) to assess the ER status of lesions when other tests are inconclusive [184]. Additional clinical scenarios which may be appropriate to use [18F]FES include systemic staging of ILC and low-grade NST BC and subtypes of BC which may have low 2-[18F]FDG uptake.
[18F]Sodium fluoride (NaF)
NCCN Guidelines continue to allow for [18F]NaF PET to be utilized in lieu of bone scan with single photon agents, because the tracer uptake is based on the same principle but has a higher sensitivity and resolution, providing 3D information [98]. A prospective study of 28 patients with bone-dominant MBC evaluated before changing therapy and 4 months later concluded that change in 2-[18F]FDG parameters predicted time to skeletal-related events (tSRE) and time to progression (TTP), but not OS [185]. Change in [18F]NaF PET/CT parameters was associated with OS; however, it was not useful for predicting TTP or tSRE [185]. Further strong studies are needed to compare both radiopharmaceuticals.
[18F]Fluciclovine (FACBC)
FDG may have reduced sensitivity for primary and metastatic ILC [28, 32, 186, 187], and there is preliminary evidence that metabolic imaging with amino acid agents such as FACBC may be more sensitive than glucose agents in this setting [188,189,190].
Fibroblast activation protein inhibitor (FAPI)
Fibroblast activation protein (FAP) has been found to be overexpressed in cancer-associated fibroblasts of multiple cancer types. PET tracers targeting FAP have been utilized for imaging patients with BC with retrospective evidence that they may outperform FDG in some patients [191,192,193,194].
Human epidermal growth factor receptors (HER)
HER-targeted agents provide another opportunity for PET to guide targeted therapy. HER2-targeted therapies can prolong survival in patients with HER2 + malignancies [195]; however, spatial and temporal heterogeneity of HER2 may lead to suboptimal use of these therapies [196, 197]. HER2-targeted PET imaging with radiolabeled antibodies and antibody fragments has demonstrated the ability to help select patients that may best benefit from HER2-targeted therapies [198,199,200,201,202,203,204], even among patients with tumours previously presumed to lack HER2 expression [205]. Recent work demonstrates that patients with low HER2 expression on immunohistochemistry, but previously classified HER2-, may respond to newer HER2-targeted therapies [206, 207]. The successful treatment of HER2-low breast cancer raises new opportunities and areas of investigation for HER2-targeted imaging.
PET/MRI
In most soft tissues, the sensitivity and specificity of CT are outperformed by MRI. In BC staging, MRI is superior to CT for detecting lymph node involvement as well as brain, bone, and liver metastases [208,209,210]. The information that can be obtained with MRI is not only anatomic in nature, but also allows functional evaluation. Enhancement patterns, for example, provide insight in perfusion characteristics, whereas diffusion-weighted imaging provides insight into the free movement of water molecules (Brownian motion), which in cancer is usually related to the cellular density of lesions.
PET/MRI aims to fuse the functional information of PET with the functional and anatomic information obtained with MRI. Attenuation correction is done by segmenting the body in different structures (soft tissue, fat, bone, lung), although the exact procedures are vendor and protocol-specific [211]. Still, quantification of PET signals in PET/MRI is more cumbersome than from PET/CT [212, 213], and measurement of SUVs is therefore less robust, although a strong correlation has been shown in BC metastases [214]. Furthermore, PET/MRI is still time-consuming compared to standard MRI. For evaluation of the breasts, PET/MRI can best be performed in the prone position, using a dedicated breast coil. However, studies have thus far only suggested a modest increase in specificity compared to MRI alone for evaluation of the primary tumour [215,216,217]. Some authors have suggested that the combination of both modalities may improve the diagnostic performance to assess local BC pCR after neoadjuvant therapy [135].
For nodal staging 2-[18F]FDG PET/MRI outperforms MRI alone [218, 219] and is, in a meta-analysis, similar to 2-[18F]FDG PET/CT [220]. Due to the higher sensitivity of MRI for bone and soft tissue lesions, early studies showed an improved sensitivity of 2-[18F]FDG PET/MRI over PET/CT for hepatic and bone metastases [221, 222]. Currently, CT still outperforms MRI for the evaluation of lung parenchyma, and consequently, assessment of lung metastases is more difficult with PET/MRI than with PET/CT.
A meta-analysis showed excellent diagnostic performance of 2-[18F]FDG PET/MRI for both nodal staging (sensitivity 94%, specificity 90%) and distant staging (sensitivity 98%, specificity 96%) [223]. According to the currently available preliminary data, PET/MRI can safely be used as an alternative to PET/CT for current indications. The improved multi-factorial functional information that can be obtained with PET/MRI potentially further characterizes cancerous lesions, which might in turn lead to more tailored therapies [224, 225]. However, these approaches, whether based on clinical evaluation or radiomics, have currently not yet left the research domain, and their impact in clinical practice remains to be proved.
PET LINAC
Image-guided radiation therapy (IGRT) entails RT delivery guided by images to verify patient positioning, conventionally using X-ray imaging. With the transition from field-based RT to volume-based RT, more advanced techniques became available, including cone-beam CT scanning. This allows matching the position of the primary tumour during treatment delivery with the treatment-planning CT scan, including adaptation to changes including movements and tumour growth or shrinkage.
The next level of IGRT followed the introduction of the MRI-LINACs, facilitating anatomical/geographic position verification and functional imaging, allowing for adaptation of the dose distribution within the target volume [226, 227]. Similarly, recently proposed as a new concept, the combination of a PET with a linear accelerator offers advantages by generating biological/functional information, ambitiously called biology-guided RT (https://reflexion.com/). The PET/LINAC combination can visualize and subsequently deliver treatment to multiple cancer sites during one single session.
Currently, the physical integration of a linear accelerator with either MRI or PET into one single unit is challenged by an optimized sequential workflow of functional and anatomical imaging on separate machines [228, 229]. The scientific question to be resolved became thereby whether simultaneous or sequential functional imaging will be superior. Likely, this may depend on the indication, depending on factors such as tumour biology and mobility.
Positron emission mammography (PEM) and dedicated breast PET (dbPET)
There are several dedicated breast PET systems that make use of breast compression, also known as positron emission mammography (PEM), and ring-shaped scanners for imaging uncompressed hanging breasts in the prone position, known as dedicated breast PET systems (dbPET). These systems visualize 2-[18F]FDG uptake or other tracer uptake in a small field of view. The advantages of such systems over whole-body PET/CT are their higher spatial resolution (1.6 mm), shorter imaging time, reduced attenuation, and higher count sensitivity, allowing PET-guided biopsy and resulting in a higher sensitivity to detect primary breast malignancies than whole-body 2-[18F]FDG PET/CT (identification rate of 95% vs. 87%). The diagnostic performance of PEM/dbPET is higher than that of X-ray mammography or US, and comparable to that of MRI in the identification of invasive BC. Furthermore, its diagnostic performance is not affected by dense breast tissue. Due to the limited field of view, PEM/dbPET can miss small deeply located lesions closer than 2 cm to the chest wall, next to the pectoral muscle, or in the axillary region [230, 231].
Metastasis-directed treatment in oligometastatic breast cancer
2-[18F]FDG PET/CT is useful for RT planning in patients with oligometastatic disease, particularly to exclude other metastatic sites prior to curative intent radioablation [232,233,234,235].
Currently, there exist no validated biomarkers for response evaluation after ablative RT in patients with oligometastatic disease, particularly in the context of BC. Nevertheless, a cohort study published in 2012 concluded that 2-[18F]FDG PET/CT performed 1.2 months post-therapy enabled response monitoring to SBRT in non-measurable metastases by CT and it could be used as a potential imaging biomarker [235]. 2-[18F]FDG PET/CT has the advantage of identifying oligometastatic disease resistant to treatment at a very early phase, allowing for locoregional ablative treatment which often offers a favourable clinical impact [232].
With the goal of optimizing surveillance imaging protocols, a joint initiative between the ESTRO and the EORTC has launched a prospective, large-scale observational study for oligometastatic patients entitled Oligocare (NCT03818503). The preliminary results suggest that 2-[18F]FDG PET/CT is favoured in BC (with wbMRI or PET/MR as alternatives) but needs to be supplemented by liver-specific MRI [232, 236]. The final conclusions of these clinical trials will improve the understanding of this topic.
Image analysis and quantification
Textural features that measure tumour heterogeneity and changes in the surrounding stroma have also emerged as potential prognostic imaging biomarkers in BC studies [237, 238]. Nevertheless, despite encouraging results, studies are far from providing definitive conclusions. This is mainly due to variations in acquisition, reconstruction, segmentation, and radiomic processing between studies, which makes its clinical application challenging [239]. This underlines the importance of using harmonization programs (e.g. EARL certification). Furthermore, radiomics analysis should also follow the definitions of the Image Biomarker Standardization Initiative (IBSI) [240].
Currently, there is no consensus regarding the optimal metabolic parameter and time to perform 2-[18F]FDG PET/CT for response monitoring [58]. The optimal timing for PET/CT imaging depends on tumour phenotype and therapeutic schemes as well as on local protocols and reimbursement considerations [58, 137, 141].
A systematic review from 2019 evaluated response to first- or second-line systemic therapy in patients with metastatic BC [158]. The authors highlighted one study that compared the use of RECIST (with ceCT) and PERCIST (with 2-[18F]FDG PET/CT) and demonstrated that 40% of non-responding tumours based on RECIST were responders on PERCIST criteria, demonstrating that metabolic assessment with 2-[18F]FDG PET/CT may be a better predictor of both progression-free and disease-specific survival than CT in patients with metastatic BC [157, 158]. Despite the extensive literature on the use of (semi)quantitative PET parameters in oncology, specific studies about BC are lacking.
Data availability
The datasets generated during and/or analyzed during the current document are available from the corresponding author on reasonable request.
Abbreviations
- 2-[18F]FDG:
-
2-[18F]Fluoro-2-deoxy-D-glucose
- BC:
-
Breast cancer
- CA15.3:
-
Cancer antigen 15.3
- CA125:
-
Cancer antigen 125
- CEA:
-
Carcinoembryonic antigen
- ceCT:
-
Contrast-enhanced CT
- CT:
-
Computed tomography
- EANM:
-
European Association of Nuclear Medicine
- ER:
-
Oestrogen receptor
- ESMO:
-
European Society of Medical Oncology
- ESTRO:
-
European Society for Radiotherapy and Oncology
- HIF-1a:
-
Hypoxia-inducible factor 1-alpha
- HR + or HR:
-
Hormone (oestrogen or progesterone) receptors present or absent
- IDC:
-
Invasive ductal carcinoma
- IGRT:
-
Image-guided radiation therapy
- ILC:
-
Invasive lobular carcinoma
- IMRT:
-
Intensity-modulated radiation therapy
- MTV:
-
Metabolic tumour volume
- MRI:
-
Magnetic resonance imaging
- NCCN:
-
National Comprehensive Cancer Network
- OS:
-
Overall survival
- PEM:
-
Positron emission mammography
- PET:
-
Positron emission tomography
- PPV:
-
Positive predictive value
- PR:
-
Progesterone receptor
- RCT:
-
Randomized controlled trials
- ROC:
-
Receiver operating characteristic
- RT:
-
Radiation therapy
- SBRT:
-
Stereotactic body radiation therapy
- SEER:
-
Surveillance, Epidemiology, and End Results Program
- SNMMI:
-
Society of Nuclear Medicine and Molecular Imaging
- SULmax:
-
SUVmax corrected for lean body mass
- SUVmax:
-
Maximum standardized uptake value
- TLG:
-
Total Lesion glycolysis
- TNBC:
-
Triple-negative breast cancer
- tSRE:
-
Time to skeletal-related events
- TTP:
-
Time to progression
- US:
-
Ultrasonography
- wbMRI:
-
Whole-body MRI
References
Brouwers MC, Kerkvliet K, Spithoff K. The AGREE Reporting Checklist: a tool to improve reporting of clinical practice guidelines. BMJ. 2016;352: i1152.
Delgado Bolton RC, Aide N, Colletti PM, Ferrero A, Paez D, Skanjeti A, Giammarile F. EANM guideline on the role of 2-[(18)F]FDG PET/CT in diagnosis, staging, prognostic value, therapy assessment and restaging of ovarian cancer, endorsed by the American College of Nuclear Medicine (ACNM), the Society of Nuclear Medicine and Molecular Imaging (SNMMI) and the International Atomic Energy Agency (IAEA). Eur J Nucl Med Mol Imaging. 2021;48:3286–302.
Salaün PY, Abgral R, Malard O, Querellou-Lefranc S, Quere G, Wartski M, Coriat R, Hindie E, Taieb D, Tabarin A, et al. Good clinical practice recommendations for the use of PET/CT in oncology. Eur J Nucl Med Mol Imaging. 2020;47:28–50.
Caresia Aroztegui AP, García Vicente AM, Alvarez Ruiz S, Delgado Bolton RC, Orcajo Rincon J, Garcia Garzon JR, de Arcocha TM, Garcia-Velloso MJ. 18F-FDG PET/CT in breast cancer: evidence-based recommendations in initial staging. Tumour Biol. 2017;39:1010428317728285.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Henry NL, Cannon-Albright LA. Breast cancer histologic subtypes show excess familial clustering. Cancer. 2019;125:3131–8.
Nascimento R, Otoni K Histological and molecular classification of breast cancer: what do we know? Mastology. 2020;30. https://doi.org/10.29289/25945394202020200024.
Li CI, Anderson BO, Daling JR, Moe RE. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA. 2003;289:1421–4.
Masood S. Breast cancer subtypes: morphologic and biologic characterization. Womens Health (Lond). 2016;12:103–19.
Makki J. Diversity of breast carcinoma: histological subtypes and clinical relevance. Clinical medicine insights Pathology. 2015;8:23–31.
Weigelt B, Geyer FC, Reis-Filho JS. Histological types of breast cancer: how special are they? Mol Oncol. 2010;4:192–208.
Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, Weaver DL, Winchester DJ, Hortobagyi GN. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:290–303.
Groheux D, Espié M, Giacchetti S, Hindié E. Performance of FDG PET/CT in the clinical management of breast cancer. Radiology. 2013;266:388–405.
Riedl CC, Slobod E, Jochelson M, Morrow M, Goldman DA, Gonen M, Weber WA, Ulaner GA. Retrospective analysis of 18F-FDG PET/CT for staging asymptomatic breast cancer patients younger than 40 years. J Nucl Med. 2014;55:1578–83.
Wang R, Zhu Y, Liu X, Liao X, He J, Niu L. The Clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer. 2019;19:1091.
Gerratana L, Fanotto V, Bonotto M, Bolzonello S, Minisini AM, Fasola G, Puglisi F. Pattern of metastasis and outcome in patients with breast cancer. Clin Exp Metas. 2015;32:125–33.
Kast K, Link T, Friedrich K, Petzold A, Niedostatek A, Schoffer O, Werner C, Klug SJ, Werner A, Gatzweiler A, et al. Impact of breast cancer subtypes and patterns of metastasis on outcome. Breast Cancer Res Treat. 2015;150:621–9.
Wenners A, Berlin L, Alkatout I, van Mackelenbergh M, Jonat W, Mundhenke C. Clinical implications of first and multiple locoregional breast cancer recurrences. Arch Gynecol Obstet. 2015;292:165–73.
Kennecke H, Yerushalmi R, Woods R, Cheang MCU, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–7.
Savci-Heijink CD, Halfwerk H, Hooijer GKJ, Horlings HM, Wesseling J, van de Vijver MJ. Retrospective analysis of metastatic behaviour of breast cancer subtypes. Breast Cancer Res Treat. 2015;150:547–57.
Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat. 2009;115:423–8.
Metzger-Filho O, Sun Z, Viale G, Price KN, Crivellari D, Snyder RD, Gelber RD, Castiglione-Gertsch M, Coates AS, Goldhirsch A, Cardoso F. Patterns of recurrence and outcome according to breast cancer subtypes in lymph node–negative disease: results from international breast cancer study group Trials VIII and IX. J Clin Oncol. 2013;31:3083–90.
Paluch-Shimon S, Ben-Baruch N, Wolf I, Zach L, Kopolovic J, Kruglikova A, et al. Hormone receptor expression is associated with a unique pattern of metastatic spread and increased survival among HER2-overexpressing breast cancer patients. Am J Clin Oncol. 2009;32. https://doi.org/10.1002/bjs.6459.
Gil-Rendo A, Martínez-Regueira F, Zornoza G, García-Velloso MJ, Beorlegui C, Rodriguez-Spiteri N. Association between [18F]fluorodeoxyglucose uptake and prognostic parameters in breast cancer. Br J Surg. 2009;96:166–70.
Ueda S, Tsuda H, Asakawa H, Shigekawa T, Fukatsu K, Kondo N, Yamamoto M, Hama Y, Tamura K, Ishida J, et al. Clinicopathological and prognostic relevance of uptake level using 18F-fluorodeoxyglucose positron emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in primary breast cancer. Jpn J Clin Oncol. 2008;38:250–8.
Groheux D, Giacchetti S, Moretti JL, Porcher R, Espié M, Lehmann-Che J, de Roquancourt A, Hamy AS, Cuvier C, Vercellino L, Hindié E. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38:426–35.
Koo HR, Park JS, Kang KW, Cho N, Chang JM, Bae MS, Kim WH, Lee SH, Kim MY, Kim JY, et al. 18F-FDG uptake in breast cancer correlates with immunohistochemically defined subtypes. Eur Radiol. 2014;24:610–8.
Bos R, van Der Hoeven JJ, van Der Wall E, van Der Groep P, van Diest PJ, Comans EF, Joshi U, Semenza GL, Hoekstra OS, Lammertsma AA, Molthoff CF. Biologic correlates of (18)fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol. 2002;20:379–87.
Morris PG, Ulaner GA, Eaton A, Fazio M, Jhaveri K, Patil S, Evangelista L, Park JY, Serna-Tamayo C, Howard J, et al. Standardized uptake value by positron emission tomography/computed tomography as a prognostic variable in metastatic breast cancer. Cancer. 2012;118:5454–62.
Aogi K, Kadoya T, Sugawara Y, Kiyoto S, Shigematsu H, Masumoto N, Okada M. Utility of (18)F FDG-PET/CT for predicting prognosis of luminal-type breast cancer. Breast Cancer Res Treat. 2015;150:209–17.
Jo JE, Kim JY, Lee SH, Kim S, Kang T. Preoperative 18F-FDG PET/CT predicts disease-free survival in patients with primary invasive ductal breast cancer. Acta Radiol. 2015;56:1463–70.
Hogan MP, Goldman DA, Dashevsky B, Riedl CC, Gönen M, Osborne JR, Jochelson M, Hudis C, Morrow M, Ulaner GA. Comparison of 18F-FDG PET/CT for systemic staging of newly diagnosed invasive lobular carcinoma versus invasive ductal carcinoma. J Nucl Med. 2015;56:1674–80.
Groheux D. Breast cancer systemic staging (comparison of computed tomography, bone scan, and 18F-fluorodeoxyglucose PET/computed tomography). PET Clin. 2023;18(4):503–15. https://doi.org/10.1016/j.cpet.2023.04.006.
Schmidt GP, Baur-Melnyk A, Haug A, Heinemann V, Bauerfeind I, Reiser MF, Schoenberg SO. Comprehensive imaging of tumor recurrence in breast cancer patients using whole-body MRI at 1.5 and 3 T compared to FDG-PET-CT. Eur J Radiol. 2008;65:47–58.
Xu GZ, Li CY, Zhao L, He ZY. Comparison of FDG whole-body PET/CT and gadolinium-enhanced whole-body MRI for distant malignancies in patients with malignant tumors: a meta-analysis. Ann Oncol. 2013;24:96–101.
Minamimoto R, Loening A, Jamali M, Barkhodari A, Mosci C, Jackson T, Obara P, Taviani V, Gambhir SS, Vasanawala S, Iagaru A. Prospective comparison of 99mTc-MDP scintigraphy, combined 18F-NaF and 18F-FDG PET/CT, and whole-body MRI in patients with breast and prostate cancer. J Nucl Med. 2015;56:1862–8.
Groheux D, Hindié E, Delord M, Giacchetti S, Hamy AS, de Bazelaire C, de Roquancourt A, Vercellino L, Toubert ME, Merlet P, Espié M. Prognostic impact of (18)FDG-PET-CT findings in clinical stage III and IIB breast cancer. J Natl Cancer Inst. 2012;104:1879–87.
Cochet A, Dygai-Cochet I, Riedinger JM, Humbert O, Berriolo-Riedinger A, Toubeau M, Guiu S, Coutant C, Coudert B, Fumoleau P, Brunotte F. 18F-FDG PET/CT provides powerful prognostic stratification in the primary staging of large breast cancer when compared with conventional explorations. Eur J Nucl Med Mol Imaging. 2014;41:428–37.
Ko H, Baghdadi Y, Love C, Sparano JA. Clinical utility of 18F-FDG PET/CT in staging localized breast cancer before initiating preoperative systemic therapy. J Natl Compr Canc Netw. 2020;18:1240–6.
Dayes IS, Metser U, Hodgson N, Parpia S, Eisen AF, George R, et al. Impact of (18)F-labeled fluorodeoxyglucose positron emission tomography-computed tomography versus conventional staging in patients with locally advanced breast cancer. J Clin Oncol. 2023;41(23):3909–16. https://doi.org/10.1200/JCO.23.00249.
Han S, Choi JY. Impact of 18F-FDG PET, PET/CT, and PET/MRI on staging and management as an initial staging modality in breast cancer: a systematic review and meta-analysis. Clin Nucl Med. 2021;46:271–82.
Ulaner GA, Castillo R, Goldman DA, Wills J, Riedl CC, Pinker-Domenig K, Jochelson MS, Gönen M. (18)F-FDG-PET/CT for systemic staging of newly diagnosed triple-negative breast cancer. Eur J Nucl Med Mol Imaging. 2016;43:1937–44.
Ulaner GA, Castillo R, Wills J, Gönen M, Goldman DA. (18)F-FDG-PET/CT for systemic staging of patients with newly diagnosed ER-positive and HER2-positive breast cancer. Eur J Nucl Med Mol Imaging. 2017;44:1420–7.
Lebon V, Alberini JL, Pierga JY, Diéras V, Jehanno N, Wartski M. Rate of distant metastases on 18F-FDG PET/CT at initial staging of breast cancer: comparison of women younger and older than 40 years. J Nucl Med. 2017;58:252–7.
Hong S, Li J, Wang S. 18FDG PET-CT for diagnosis of distant metastases in breast cancer patients. A meta-analysis Surg Oncol. 2013;22:139–43.
Sun Z, Yi YL, Liu Y, Xiong JP, He CZ. Comparison of whole-body PET/PET-CT and conventional imaging procedures for distant metastasis staging in patients with breast cancer: a meta-analysis. Eur J Gynaecol Oncol. 2015;36:672–6.
Jung NY, Yoo IR, Kang BJ, Kim SH, Chae BJ, Seo YY. Clinical significance of FDG-PET/CT at the postoperative surveillance in the breast cancer patients. Breast Cancer. 2016;23:141–8.
Cook GJR, Goh V. Molecular imaging of bone metastases and their response to therapy. J Nucl Med. 2020;61:799–806.
Taralli S, Lorusso M, Scolozzi V, Masiello V, Marazzi F, Calcagni ML. Response evaluation with (18)F-FDG PET/CT in metastatic breast cancer patients treated with Palbociclib: first experience in clinical practice. Ann Nucl Med. 2019;33:193–200.
Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, Verzijlbergen FJ, Barrington SF, Pike LC, Weber WA, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54.
Delbeke D, Coleman RE, Guiberteau MJ, Brown ML, Royal HD, Siegel BA, Townsend DW, Berland LL, Parker JA, Hubner K, et al. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. J Nucl Med. 2006;47:885–95.
Dehdashti F, Mortimer JE, Trinkaus K, Naughton MJ, Ellis M, Katzenellenbogen JA, Welch MJ, Siegel BA. PET-based estradiol challenge as a predictive biomarker of response to endocrine therapy in women with estrogen-receptor-positive breast cancer. Breast Cancer Res Treat. 2009;113:509–17.
Mortimer JE, Dehdashti F, Siegel BA, Trinkaus K, Katzenellenbogen JA, Welch MJ. Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol. 2001;19:2797–803.
Dehdashti F, Flanagan FL, Mortimer JE, Katzenellenbogen JA, Welch MJ, Siegel BA. Positron emission tomographic assessment of “metabolic flare” to predict response of metastatic breast cancer to antiestrogen therapy. Eur J Nucl Med. 1999;26:51–6.
Gebhart G, Gámez C, Holmes E, Robles J, Garcia C, Cortés M, de Azambuja E, Fauria K, Van Dooren V, Aktan G, et al. 18F-FDG PET/CT for early prediction of response to neoadjuvant lapatinib, trastuzumab, and their combination in HER2-positive breast cancer: results from Neo-ALTTO. J Nucl Med. 2013;54:1862–8.
Huynh K, Baghdanian AH, Baghdanian AA, Sun DS, Kolli KP, Zagoria RJ. Updated guidelines for intravenous contrast use for CT and MRI. Emerg Radiol. 2020;27:115–26.
Thorwarth D, Beyer T, Boellaard R, de Ruysscher D, Grgic A, Lee JA, Pietrzyk U, Sattler B, Schaefer A, van Elmpt W, et al. Integration of FDG-PET/CT into external beam radiation therapy planning: technical aspects and recommendations on methodological approaches. Nuklearmedizin. 2012;51:140–53.
Groheux D, Mankoff D, Espié M, Hindié E. 18F-FDG PET/CT in the early prediction of pathological response in aggressive subtypes of breast cancer: review of the literature and recommendations for use in clinical trials. Eur J Nucl Med Mol Imaging. 2016;43:983–93.
Kikano EG, Avril S, Marshall H, Jones RS, Montero AJ, Avril N. PET/CT variants and pitfalls in breast cancers. Semin Nucl Med. 2021;51:474–84.
Kumar R, Rani N, Patel C, Basu S, Alavi A. False-negative and false-positive results in FDG-PET and PET/CT in breast cancer. PET Clinics. 2009;4(3):289–98.
Slomka PJ, Pan T, Germano G. Recent advances and future progress in PET instrumentation. Semin Nucl Med. 2016;46:5–19.
Dong A, Wang Y, Lu J, Zuo C. Spectrum of the breast lesions with increased 18F-FDG uptake on PET/CT. Clin Nucl Med. 2016;41:543–57.
Aarstad EM, Nordhaug P, Naghavi-Behzad M, Larsen LB, Gerke O, Hildebrandt MG. Prevalence of focal incidental breast uptake on FDG-PET/CT and risk of malignancy: a systematic review and meta-analysis. European Journal of Hybrid Imaging. 2019;3:16.
Park SA, Lee KM, Choi U, Kim HS, Kim HW, Song JH. Normal physiologic and benign foci with F-18 FDG avidity on PET/CT in patients with breast cancer. Nucl Med Mol Imaging. 2010;44:282–9.
Korn RL, Yost AM, May CC, Kovalsky ER, Orth KM, Layton TA, Drumm D. Unexpected focal hypermetabolic activity in the breast: significance in patients undergoing 18F-FDG PET/CT. AJR Am J Roentgenol. 2006;187:81–5.
Litmanovich D, Gourevich K, Israel O, Gallimidi Z. Unexpected foci of 18F-FDG uptake in the breast detected by PET/CT: incidence and clinical significance. Eur J Nucl Med Mol Imaging. 2009;36:1558–64.
Andersen JD, Zacho HD, Petersen LJ. The frequency and malignancy rate of incidental focal breast lesions identified by 18F-fluorodeoxyglucose positron emission tomography. Nucl Med Commun. 2021;42:93–100.
Benveniste AP, Marom EM, Benveniste MF, Mawlawi OR, Miranda RN, Yang W. Metastases to the breast from extramammary malignancies - PET/CT findings. Eur J Radiol. 2014;83:1106–12.
Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, Pruim J, Price P. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–82.
Pinker K, Riedl CC, Ong L, Jochelson M, Ulaner GA, McArthur H, Dickler M, Gönen M, Weber WA. The impact that number of analyzed metastatic breast cancer lesions has on response assessment by 18F-FDG PET/CT using PERCIST. J Nucl Med. 2016;57:1102–4.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122s–50s.
Lopci E, Hicks RJ, Dimitrakopoulou-Strauss A, Dercle L, Iravani A, Seban RD, et al. Joint EANM/SNMMI/ANZSNM practice guidelines/procedure standards on recommended use of [18F]FDG PET/CT imaging during immunomodulatory treatments in patients with solid tumors version 1.0. Eur J Nucl Med Mol Imaging. 2022;49(7):2323–41. https://doi.org/10.1007/s00259-022-05780-2.
Willemsen A, Vlenterie M, van Herpen CML, van Erp NP, van der Graaf WTA, de Geus-Oei LF, Oyen WJG. Positron emission tomography response criteria in solid tumours criteria for quantitative analysis of [18F]-fluorodeoxyglucose positron emission tomography with integrated computed tomography for treatment response assessment in metastasised solid tumours: all that glitters is not gold. Eur J Cancer. 2016;56:54–8.
Bernsdorf M, Berthelsen AK, Wielenga VT, Kroman N, Teilum D, Binderup T, Tange UB, Andersson M, Kjær A, Loft A, Graff J. Preoperative PET/CT in early-stage breast cancer. Ann Oncol. 2012;23:2277–82.
Chen L, Yang Q, Bao J, Liu D, Huang X, Wang J. Direct comparison of PET/CT and MRI to predict the pathological response to neoadjuvant chemotherapy in breast cancer: a meta-analysis. Sci Rep. 2017;7:8479.
Park S, Yoon JK, Jin Lee S, Kang SY, Yim H, An YS. Prognostic utility of FDG PET/CT and bone scintigraphy in breast cancer patients with bone-only metastasis. Medicine (Baltimore). 2017;96: e8985.
Groheux D, Martineau A, Teixeira L, Espié M, de Cremoux P, Bertheau P, Merlet P, Lemarignier C. (18)FDG-PET/CT for predicting the outcome in ER+/HER2- breast cancer patients: comparison of clinicopathological parameters and PET image-derived indices including tumor texture analysis. Breast Cancer Res. 2017;19:3.
Evangelista L, Urso L, Caracciolo M, Stracuzzi F, Panareo S, Cistaro A, et al. FDG PET/CT volume-based quantitative data and survival analysis in breast cancer patients: a systematic review of the literature. Curr Med Imaging. 2023;19(8):807–16. https://doi.org/10.2174/1573405618666220329094423.
Groheux D, Sanna A, Majdoub M, de Cremoux P, Giacchetti S, Teixeira L, Espié M, Merlet P, de Roquancourt A, Visvikis D, et al. Baseline tumor 18F-FDG uptake and modifications after 2 cycles of neoadjuvant chemotherapy are prognostic of outcome in ER+/HER2- breast cancer. J Nucl Med. 2015;56:824–31.
Diao W, Tian F, Jia Z. The prognostic value of SUV(max) measuring on primary lesion and ALN by (18)F-FDG PET or PET/CT in patients with breast cancer. Eur J Radiol. 2018;105:1–7.
Garcia-Vicente AM, Pérez-Beteta J, Amo-Salas M, Molina D, Jimenez-Londoño GA, Soriano-Castrejón AM, Pena Pardo FJ, Martínez-González A. Predictive and prognostic potential of volume-based metabolic variables obtained by a baseline (18)F-FDG PET/CT in breast cancer with neoadjuvant chemotherapy indication. Rev Esp Med Nucl Imagen Mol (Engl Ed). 2018;37:73–9.
Kadoya T, Aogi K, Kiyoto S, Masumoto N, Sugawara Y, Okada M. Role of maximum standardized uptake value in fluorodeoxyglucose positron emission tomography/computed tomography predicts malignancy grade and prognosis of operable breast cancer: a multi-institute study. Breast Cancer Res Treat. 2013;141:269–75.
Pak K, Seok JW, Kim HY, Nguyen TL, Kim K, Kim SJ, Kim IJ, Hopper J. Prognostic value of metabolic tumor volume and total lesion glycolysis in breast cancer: a meta-analysis. Nucl Med Commun. 2020;41:824–9.
Aide N, Lasnon C, Veit-Haibach P, Sera T, Sattler B, Boellaard R. EANM/EARL harmonization strategies in PET quantification: from daily practice to multicentre oncological studies. Eur J Nucl Med Mol Imaging. 2017;44:17–31.
Complete accreditation information: nuclear medicine and PET (Revised 1–24–2024): Accreditation Support (acr.org). https://accreditationsupport.acr.org/support/solutions/articles/11000063279-complete-accreditation-information-nuclear-medicine-and-pet-revised-12-16-2022-.
The IAC standards and guidelines for nuclear/PET accreditation. https://intersocietal.org/wp-content/uploads/2023/07/IACNuclearPETStandards2023B.pdf.
Giuliano AE, Edge SB, Hortobagyi GN. Eighth edition of the AJCC cancer staging manual: breast cancer. Ann Surg Oncol. 2018;25:1783–5.
Cooper KL, Harnan S, Meng Y, Ward SE, Fitzgerald P, Papaioannou D, Wyld L, Ingram C, Wilkinson ID, Lorenz E. Positron emission tomography (PET) for assessment of axillary lymph node status in early breast cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2011;37:187–98.
Mori M, Fujioka T, Kubota K, Katsuta L, Yashima Y, Nomura K, et al. Relationship between prognostic stage in breast cancer and fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography. J Clin Med. 2021;10(14):3173. https://doi.org/10.3390/jcm10143173.
Pritchard KI, Julian JA, Holloway CM, McCready D, Gulenchyn KY, George R, Hodgson N, Lovrics P, Perera F, Elavathil L, et al. Prospective study of 2-[18F]fluorodeoxyglucose positron emission tomography in the assessment of regional nodal spread of disease in patients with breast cancer: an Ontario clinical oncology group study. J Clin Oncol. 2012;30:1274–9.
Mankoff DA, Specht JM, Eubank WB, Kessler L. [18F]fluorodeoxyglucose positron emission tomography-computed tomography in breast cancer: when... and when not? J Clin Oncol. 2012;30:1252–4.
Srour MK, Lee M, Walcott-Sapp S, Luu M, Chung A, Giuliano AE, Amersi F. Overuse of preoperative staging of patients undergoing neoadjuvant chemotherapy for breast cancer. Ann Surg Oncol. 2019;26:3289–94.
Groheux D, Hindie E. Breast cancer: initial workup and staging with FDG PET/CT. ClinTransl Imaging. 2021;9(3):221–31. https://doi.org/10.1007/s40336-021-00426-z.
Hyland CJ, Varghese F, Yau C, Beckwith H, Khoury K, Varnado W, Hirst GL, Flavell RR, Chien AJ, Yee D, et al. Use of 18F-FDG PET/CT as an initial staging procedure for stage II-III breast cancer: a multicenter value analysis. J Natl Compr Canc Netw. 2020;18:1510–7.
Koolen BB, Vrancken Peeters MJ, Aukema TS, Vogel WV, Oldenburg HS, van der Hage JA, Hoefnagel CA, Stokkel MP, Loo CE, Rodenhuis S, et al. 18F-FDG PET/CT as a staging procedure in primary stage II and III breast cancer: comparison with conventional imaging techniques. Breast Cancer Res Treat. 2012;131:117–26.
Ming Y, Wu N, Qian T, Li X, Wan DQ, Li C, Li Y, Wu Z, Wang X, Liu J, Wu N. Progress and future trends in PET/CT and PET/MRI molecular imaging approaches for breast cancer. Front Oncol. 2020;10:1301.
Groheux D, Giacchetti S, Espié M, Vercellino L, Hamy AS, Delord M, Berenger N, Toubert ME, Misset JL, Hindié E. The yield of 18F-FDG PET/CT in patients with clinical stage IIA, IIB, or IIIA breast cancer: a prospective study. J Nucl Med. 2011;52:1526–34.
NCCN clinical practice guidelines in oncology. Breast Cancer. Version 4. 2023. Available at: https://www.nccn.org/professionals/physician_gls/pdf/brease.pdf. Accessed 10 Jul 2023
Fuster D, Duch J, Paredes P, Velasco M, Muñoz M, Santamaría G, Fontanillas M, Pons F. Preoperative staging of large primary breast cancer with [18F]fluorodeoxyglucose positron emission tomography/computed tomography compared with conventional imaging procedures. J Clin Oncol. 2008;26:4746–51.
Koolen BB, Valdés Olmos RA, Elkhuizen PH, Vogel WV, Vrancken Peeters MJ, Rodenhuis S, Rutgers EJ. Locoregional lymph node involvement on 18F-FDG PET/CT in breast cancer patients scheduled for neoadjuvant chemotherapy. Breast Cancer Res Treat. 2012;135:231–40.
Seo MJ, Lee JJ, Kim HO, Chae SY, Park SH, Ryu JS, Ahn SH, Lee JW, Son BH, Gong GY, Moon DH. Detection of internal mammary lymph node metastasis with (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with stage III breast cancer. Eur J Nucl Med Mol Imaging. 2014;41:438–45.
Bellevre D, Blanc Fournier C, Switsers O, Dugué AE, Levy C, Allouache D, Desmonts C, Crouet H, Guilloit JM, Grellard JM, Aide N. staging the axilla in breast cancer patients with 18F-FDG PET: how small are the metastases that we can detect with new generation clinical PET systems? Eur J Nucl Med Mol Imaging. 2014;41:1103–12.
Segaert I, Mottaghy F, Ceyssens S, De Wever W, Stroobants S, Van Ongeval C, Van Limbergen E, Wildiers H, Paridaens R, Vergote I, et al. Additional value of PET-CT in staging of clinical stage IIB and III breast cancer. Breast J. 2010;16:617–24.
García Vicente AM, Soriano Castrejón A, Cruz Mora M, Ortega Ruiperez C, Espinosa Aunión R, León Martín A, González Ageitos A, Van Gómez LO. Dual time point 2-deoxy-2-[18F]fluoro-D-glucose PET/CT: nodal staging in locally advanced breast cancer. Rev Esp Med Nucl Imagen Mol. 2014;33:1–5.
Manohar K, Mittal BR, Bhoil A, Bhattacharya A, Singh G. Role of 18F-FDG PET/CT in identifying distant metastatic disease missed by conventional imaging in patients with locally advanced breast cancer. Nucl Med Commun. 2013;34:557–61.
Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, Zackrisson S, Cardoso F. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v8-30.
Carkaci S, Adrada BE, Rohren E, Wei W, Quraishi MA, Mawlawi O, Buchholz TA, Yang W. Semiquantitative analysis of maximum standardized uptake values of regional lymph nodes in inflammatory breast cancer: is there a reliable threshold for differentiating benign from malignant? Acad Radiol. 2012;19:535–41.
Yang WT, Le-Petross HT, Macapinlac H, Carkaci S, Gonzalez-Angulo AM, Dawood S, Resetkova E, Hortobagyi GN, Cristofanilli M. Inflammatory breast cancer: PET/CT, MRI, mammography, and sonography findings. Breast Cancer Res Treat. 2008;109:417–26.
Alberini JL, Lerebours F, Wartski M, Fourme E, Le Stanc E, Gontier E, Madar O, Cherel P, Pecking AP. 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) imaging in the staging and prognosis of inflammatory breast cancer. Cancer. 2009;115:5038–47.
Carkaci S, Macapinlac HA, Cristofanilli M, Mawlawi O, Rohren E, Gonzalez Angulo AM, Dawood S, Resetkova E, Le-Petross HT, Yang WT. Retrospective study of 18F-FDG PET/CT in the diagnosis of inflammatory breast cancer: preliminary data. J Nucl Med. 2009;50:231–8.
Groheux D, Giacchetti S, Delord M, Hindié E, Vercellino L, Cuvier C, Toubert ME, Merlet P, Hennequin C, Espié M. 18F-FDG PET/CT in staging patients with locally advanced or inflammatory breast cancer: comparison to conventional staging. J Nucl Med. 2013;54:5–11.
Niikura N, Costelloe CM, Madewell JE, Hayashi N, Yu TK, Liu J, Palla SL, Tokuda Y, Theriault RL, Hortobagyi GN, Ueno NT. FDG-PET/CT compared with conventional imaging in the detection of distant metastases of primary breast cancer. Oncologist. 2011;16:1111–9.
Walker GV, Niikura N, Yang W, Rohren E, Valero V, Woodward WA, Alvarez RH, Lucci A Jr, Ueno NT, Buchholz TA. Pretreatment staging positron emission tomography/computed tomography in patients with inflammatory breast cancer influences radiation treatment field designs. Int J Radiat Oncol Biol Phys. 2012;83:1381–6.
Champion L, Lerebours F, Cherel P, Edeline V, Giraudet A-L, Wartski M, Bellet D, Alberini J-L. 18F-FDG PET/CT imaging versus dynamic contrast-enhanced CT for staging and prognosis of inflammatory breast cancer. Eur J Nucl Med Mol Imaging. 2013;40:1206–13.
Vogsen M, Jensen JD, Christensen IY, Gerke O, Jylling AMB, Larsen LB, Braad PE, Søe KL, Bille C, Ewertz M, Hildebrandt MG. FDG-PET/CT in high-risk primary breast cancer-a prospective study of stage migration and clinical impact. Breast Cancer Res Treat. 2021;185:145–53.
Ng SP, David S, Alamgeer M, Ganju V. Impact of pretreatment combined (18)F-fluorodeoxyglucose positron emission tomography/computed tomography staging on radiation therapy treatment decisions in locally advanced breast cancer. Int J Radiat Oncol Biol Phys. 2015;93:111–7.
Koolen BB, Valdés Olmos RA, Vogel WV, Vrancken Peeters MJ, Rodenhuis S, Rutgers EJ, Elkhuizen PH. Pre-chemotherapy 18F-FDG PET/CT upstages nodal stage in stage II-III breast cancer patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. 2013;141:249–54.
Lee YS, Kim KJ, Ahn SD, Choi EK, Kim JH, Lee SW, Song SY, Yoon SM, Kim YS, Park JH, et al. The application of PET-CT to post-mastectomy regional radiation therapy using a deformable image registration. Radiat Oncol. 2013;8:104.
Kowalski ES, Feigenberg SJ, Cohen J, Fellows Z, Vadnais P, Rice S, Mishra MV, Molitoris JK, Nichols EM, Snider JW 3rd. Optimal target delineation and treatment techniques in the era of conformal photon and proton breast and regional nodal irradiation. Pract Radiat Oncol. 2020;10:174–82.
Davidson T, Ben-David M, Galper S, Haskin T, Howes M, Scaife R, Kanana N, Amit U, Weizman N, Chikman B, et al. Use of (18)F-FDG PET-CT imaging to determine internal mammary lymph node location for radiation therapy treatment planning in breast cancer patients. Pract Radiat Oncol. 2017;7:373–81.
van Uden DJP, Prins MW, Siesling S, de Wilt JHW, Blanken-Peeters C, Aarntzen E. [18F]FDG PET/CT in the staging of inflammatory breast cancer: a systematic review. Crit Rev Oncol Hematol. 2020;151: 102943.
Ueno NT, Fernandez JRE, Cristofanilli M, Overmoyer B, Rea D, Berdichevski F, El-Shinawi M, Bellon J, Le-Petross HT, Lucci A, et al. International consensus on the clinical management of inflammatory breast cancer from the Morgan Welch Inflammatory Breast Cancer Research Program 10th anniversary conference. J Cancer. 2018;9:1437–47.
Jacene HA, DiPiro PJ, Bellon J, Hu J, Cheng SC, Warren L, Schlosnagle E, Nakhlis F, Rosenbluth JM, Yeh E, Overmoyer B. Discrepancy between FDG-PET/CT and contrast-enhanced CT in the staging of patients with inflammatory breast cancer: implications for treatment planning. Breast Cancer Res Treat. 2020;181:383–90.
Choi YJ, Shin YD, Kang YH, Lee MS, Lee MK, Cho BS, Kang YJ, Park JS. The effects of preoperative (18)F-FDG PET/CT in breast cancer patients in comparison to the conventional imaging study. J Breast Cancer. 2012;15:441–8.
van Es SC, Velleman T, Elias SG, Bensch F, Brouwers AH, Glaudemans A, Kwee TC, Iersel MW, Maduro JH, Oosting SF, et al. Assessment of bone lesions with (18)F-FDG PET compared with (99m)Tc bone scintigraphy leads to clinically relevant differences in metastatic breast cancer management. J Nucl Med. 2021;62:177–83.
Morris PG, Lynch C, Feeney JN, Patil S, Howard J, Larson SM, Dickler M, Hudis CA, Jochelson M, McArthur HL. Integrated positron emission tomography/computed tomography may render bone scintigraphy unnecessary to investigate suspected metastatic breast cancer. J Clin Oncol. 2010;28:3154–9.
Cook GJ, Houston S, Rubens R, Maisey MN, Fogelman I. Detection of bone metastases in breast cancer by 18FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. J Clin Oncol. 1998;16:3375–9.
Capitanio S, Bongioanni F, Piccardo A, Campus C, Gonella R, Tixi L, Naseri M, Pennone M, Altrinetti V, Buschiazzo A, et al. Comparisons between glucose analogue 2-deoxy-2-((18)F)fluoro-D-glucose and (18)F-sodium fluoride positron emission tomography/computed tomography in breast cancer patients with bone lesions. World J Radiol. 2016;8:200–9.
Coudert B, Pierga JY, Mouret-Reynier MA, Kerrou K, Ferrero JM, Petit T, Kerbrat P, Dupré PF, Bachelot T, Gabelle P, et al. Use of [(18)F]-FDG PET to predict response to neoadjuvant trastuzumab and docetaxel in patients with HER2-positive breast cancer, and addition of bevacizumab to neoadjuvant trastuzumab and docetaxel in [(18)F]-FDG PET-predicted non-responders (AVATAXHER): an open-label, randomised phase 2 trial. Lancet Oncol. 2014;15:1493–502.
Pérez-García JM, Gebhart G, Ruiz Borrego M, Stradella A, Bermejo B, Schmid P, Marmé F, Escrivá-de-Romani S, Calvo L, Ribelles N, et al. Chemotherapy de-escalation using an (18)F-FDG-PET-based pathological response-adapted strategy in patients with HER2-positive early breast cancer (PHERGain): a multicentre, randomised, open-label, non-comparative, phase 2 trial. Lancet Oncol. 2021;22:858–71.
Wang Y, Zhang C, Liu J, Huang G. Is 18F-FDG PET accurate to predict neoadjuvant therapy response in breast cancer? A meta-analysis Breast Cancer Res Treat. 2012;131:357–69.
Cheng X, Li Y, Liu B, Xu Z, Bao L, Wang J. 18F-FDG PET/CT and PET for evaluation of pathological response to neoadjuvant chemotherapy in breast cancer: a meta-analysis. Acta Radiol. 2012;53:615–27.
Mghanga FP, Lan X, Bakari KH, Li C, Zhang Y. Fluorine-18 fluorodeoxyglucose positron emission tomography-computed tomography in monitoring the response of breast cancer to neoadjuvant chemotherapy: a meta-analysis. Clin Breast Cancer. 2013;13:271–9.
Tian F, Shen G, Deng Y, Diao W, Jia Z. The accuracy of (18)F-FDG PET/CT in predicting the pathological response to neoadjuvant chemotherapy in patients with breast cancer: a meta-analysis and systematic review. Eur Radiol. 2017;27:4786–96.
Liu Q, Wang C, Li P, Liu J, Huang G, Song S. The role of (18)F-FDG PET/CT and MRI in assessing pathological complete response to neoadjuvant chemotherapy in patients with breast cancer: a systematic review and meta-analysis. Biomed Res Int. 2016;2016:3746232.
Han S, Choi JY. Prognostic value of (18)F-FDG PET and PET/CT for assessment of treatment response to neoadjuvant chemotherapy in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2020;22:119.
Groheux D, Biard L, Giacchetti S, Teixeira L, Hindié E, Cuvier C, Vercellino L, Merlet P, de Roquancourt A, de Cremoux P, et al. 18F-FDG PET/CT for the early evaluation of response to neoadjuvant treatment in triple-negative breast cancer: influence of the chemotherapy regimen. J Nucl Med. 2016;57:536–43.
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–72.
Humbert O, Riedinger JM, Vrigneaud JM, Kanoun S, Dygai-Cochet I, Berriolo-Riedinger A, Toubeau M, Depardon E, Lassere M, Tisserand S, et al. 18F-FDG PET-derived tumor blood flow changes after 1 cycle of neoadjuvant chemotherapy predicts outcome in triple-negative breast cancer. J Nucl Med. 2016;57:1707–12.
Humbert O, Lasserre M, Bertaut A, Fumoleau P, Coutant C, Brunotte F, Cochet A. Breast cancer blood flow and metabolism on dual-acquisition (18)F-FDG PET: correlation with tumor phenotype and neoadjuvant chemotherapy response. J Nucl Med. 2018;59:1035–41.
Humbert O, Berriolo-Riedinger A, Riedinger JM, Coudert B, Arnould L, Cochet A, Loustalot C, Fumoleau P, Brunotte F. Changes in 18F-FDG tumor metabolism after a first course of neoadjuvant chemotherapy in breast cancer: influence of tumor subtypes. Ann Oncol. 2012;23:2572–7.
Groheux D, Hindié E, Giacchetti S, Delord M, Hamy AS, de Roquancourt A, Vercellino L, Berenger N, Marty M, Espié M. Triple-negative breast cancer: early assessment with 18F-FDG PET/CT during neoadjuvant chemotherapy identifies patients who are unlikely to achieve a pathologic complete response and are at a high risk of early relapse. J Nucl Med. 2012;53:249–54.
Zucchini G, Quercia S, Zamagni C, Santini D, Taffurelli M, Fanti S, Martoni AA. Potential utility of early metabolic response by 18F-2-fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography in a selected group of breast cancer patients receiving preoperative chemotherapy. Eur J Cancer. 2013;49:1539–45.
Groheux D, Hindié E, Giacchetti S, Hamy AS, Berger F, Merlet P, de Roquancourt A, de Cremoux P, Marty M, Hatt M, Espié M. Early assessment with 18F-fluorodeoxyglucose positron emission tomography/computed tomography can help predict the outcome of neoadjuvant chemotherapy in triple negative breast cancer. Eur J Cancer. 2014;50:1864–71.
Humbert O, Riedinger JM, Charon-Barra C, Berriolo-Riedinger A, Desmoulins I, Lorgis V, Kanoun S, Coutant C, Fumoleau P, Cochet A, Brunotte F. Identification of biomarkers including 18FDG-PET/CT for early prediction of response to neoadjuvant chemotherapy in triple-negative breast cancer. Clin Cancer Res. 2015;21:5460–8.
Connolly RM, Leal JP, Solnes L, Huang CY, Carpenter A, Gaffney K, Abramson V, Carey LA, Liu MC, Rimawi M, et al. Updated results of TBCRC026: phase II trial correlating standardized uptake value with pathological complete response to pertuzumab and trastuzumab in breast cancer. J Clin Oncol. 2021;39:2247–56.
Coudert B, Pierga JY, Mouret-Reynier MA, Kerrou K, Ferrero JM, Petit T, Du FL, Dupré PF, Bachelot T, Gabelle P, et al. Long-term outcomes in patients with PET-predicted poor-responsive HER2-positive breast cancer treated with neoadjuvant bevacizumab added to trastuzumab and docetaxel: 5-year follow-up of the randomised Avataxher study. EClinicalMedicine. 2020;28: 100566.
Pérez-García JM, Gebhart G, Borrego MR, Schmid P, Marmé F, Prat A, Dalenc F, Kerrou K, Colleoni M, Braga S, et al. Trastuzumab and pertuzumab without chemotherapy in early-stage HER2+ breast cancer: a plain language summary of the PHERGain study. Future Oncol. 2022;18:3677–88.
Semiglazov VF, Semiglazov VV, Dashyan GA, Ziltsova EK, Ivanov VG, Bozhok AA, Melnikova OA, Paltuev RM, Kletzel A, Berstein LM. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer. 2007;110:244–54.
von Minckwitz G, Kümmel S, Vogel P, Hanusch C, Eidtmann H, Hilfrich J, Gerber B, Huober J, Costa SD, Jackisch C, et al. Intensified neoadjuvant chemotherapy in early-responding breast cancer: phase III randomized GeparTrio study. J Natl Cancer Inst. 2008;100:552–62.
Dose-Schwarz J, Tiling R, Avril-Sassen S, Mahner S, Lebeau A, Weber C, Schwaiger M, Jänicke F, Untch M, Avril N. Assessment of residual tumour by FDG-PET: conventional imaging and clinical examination following primary chemotherapy of large and locally advanced breast cancer. Br J Cancer. 2010;102:35–41.
Burcombe RJ, Makris A, Pittam M, Lowe J, Emmott J, Wong WL. Evaluation of good clinical response to neoadjuvant chemotherapy in primary breast cancer using [18F]-fluorodeoxyglucose positron emission tomography. Eur J Cancer. 2002;38:375–9.
Kitajima K, Nakatani K, Yamaguchi K, Nakajo M, Tani A, Ishibashi M, Hosoya K, Morita T, Kinoshita T, Kaida H, Miyoshi Y. Response to neoadjuvant chemotherapy for breast cancer judged by PERCIST - multicenter study in Japan. Eur J Nucl Med Mol Imaging. 2018;45:1661–71.
Choi EK, Yoo IR, Kim SH, Park SY. O JH, Kang BJ: The value of pre- and post-neoadjuvant chemotherapy F-18 FDG PET/CT scans in breast cancer: comparison with MRI. Acta Radiol. 2018;59:41–9.
Schipper RJ, Moossdorff M, Beets-Tan RGH, Smidt ML, Lobbes MBI. Noninvasive nodal restaging in clinically node positive breast cancer patients after neoadjuvant systemic therapy: a systematic review. Eur J Radiol. 2015;84:41–7.
Samiei S, de Mooij CM, Lobbes MBI, Keymeulen K, van Nijnatten TJA, Smidt ML. Diagnostic performance of noninvasive imaging for assessment of axillary response after neoadjuvant systemic therapy in clinically node-positive breast cancer: a systematic review and meta-analysis. Ann Surg. 2021;273:694–700.
Riedl CC, Pinker K, Ulaner GA, Ong LT, Baltzer P, Jochelson MS, McArthur HL, Gönen M, Dickler M, Weber WA. Comparison of FDG-PET/CT and contrast-enhanced CT for monitoring therapy response in patients with metastatic breast cancer. Eur J Nucl Med Mol Imaging. 2017;44:1428–37.
Helland F, Henriksen MH, Gerke O, Vogsen M, Høilund-Carlsen PF, Hildebrandt MG. FDG-PET/CT versus contrast-enhanced CT for response evaluation in metastatic breast cancer: a systematic review. Diagnostics (Basel). 2019;9(3):106. https://doi.org/10.3390/diagnostics9030106.
Vogsen M, Harbo F, Jakobsen NM, Nissen HJ, Dahlsgaard-Wallenius SE, Gerke O, Jensen JD, Asmussen JT, Jylling AMB, Braad PE, et al. Response monitoring in metastatic breast cancer: a prospective study comparing (18)F-FDG PET/CT with conventional CT. J Nucl Med. 2023;64:355–61.
Vogsen M, Naghavi-Behzad M, Harbo FG, Jakobsen NM, Gerke O, Asmussen JT, Nissen HJ, Dahlsgaard-Wallenius SE, Braad P-E, Jensen JD, et al. 2-[18F]FDG-PET/CT is a better predictor of survival than conventional CT: a prospective study of response monitoring in metastatic breast cancer. Sci Rep. 2023;13:5552.
Hildebrandt MG, Naghavi-Behzad M, Vogsen M. A role of FDG-PET/CT for response evaluation in metastatic breast cancer? Semin Nucl Med. 2022;52:520–30.
Naghavi-Behzad M, Vogsen M, Vester RM, Olsen MMB, Oltmann H, Braad PE, Asmussen JT, Gerke O, Vach W, Kidholm K, et al. Response monitoring in metastatic breast cancer: a comparison of survival times between FDG-PET/CT and CE-CT. Br J Cancer. 2022;126:1271–9.
Groheux D. Role of fludeoxyglucose in breast cancer: treatment response. PET Clin. 2018;13:395–414.
Du Y, Cullum I, Illidge TM, Ell PJ. Fusion of metabolic function and morphology: sequential [18F]fluorodeoxyglucose positron-emission tomography/computed tomography studies yield new insights into the natural history of bone metastases in breast cancer. J Clin Oncol. 2007;25:3440–7.
Tateishi U, Gamez C, Dawood S, Yeung HW, Cristofanilli M, Macapinlac HA. Bone metastases in patients with metastatic breast cancer: morphologic and metabolic monitoring of response to systemic therapy with integrated PET/CT. Radiology. 2008;247:189–96.
Azad GK, Taylor BP, Green A, Sandri I, Swampillai A, Harries M, Kristeleit H, Mansi J, Goh V, Cook GJR. Prediction of therapy response in bone-predominant metastatic breast cancer: comparison of [(18)F] fluorodeoxyglucose and [(18)F]-fluoride PET/CT with whole-body MRI with diffusion-weighted imaging. Eur J Nucl Med Mol Imaging. 2019;46:821–30.
Makhlin I, Korhonen KE, Martin ML, Gillman J, Schubert E, Pantel AR, Mankoff DA, Clark AS. (18)F-FDG PET/CT for the evaluation of therapy response in hormone receptor-positive bone-dominant metastatic breast cancer. Radiol Imaging Cancer. 2022;4: e220032.
Hildebrandt MG, Gerke O, Baun C, Falch K, Hansen JA, Farahani ZA, Petersen H, Larsen LB, Duvnjak S, Buskevica I, et al. [18F]Fluorodeoxyglucose (FDG)-positron emission tomography (PET)/computed tomography (CT) in suspected recurrent breast cancer: a prospective comparative study of dual-time-point FDG-PET/CT, contrast-enhanced CT, and bone scintigraphy. J Clin Oncol. 2016;34:1889–97.
Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii11-19.
Piva R, Ticconi F, Ceriani V, Scalorbi F, Fiz F, Capitanio S, Bauckneht M, Cittadini G, Sambuceti G, Morbelli S. Comparative diagnostic accuracy of 18F-FDG PET/CT for breast cancer recurrence. Breast Cancer (Dove Med Press). 2017;9:461–71.
Vogsen M, Jensen JD, Gerke O, Jylling AMB, Asmussen JT, Christensen IY, Braad PE, Thye-Rønn P, Søe KL, Ewertz M, Hildebrandt MG. Benefits and harms of implementing [(18)F]FDG-PET/CT for diagnosing recurrent breast cancer: a prospective clinical study. EJNMMI Res. 2021;11:93.
Hansen JA, Naghavi-Behzad M, Gerke O, Baun C, Falch K, Duvnjak S, Alavi A, Høilund-Carlsen PF, Hildebrandt MG. Diagnosis of bone metastases in breast cancer: lesion-based sensitivity of dual-time-point FDG-PET/CT compared to low-dose CT and bone scintigraphy. PLoS ONE. 2021;16: e0260066.
Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F, Barrios CH, Bergh J, Bhattacharyya GS, Biganzoli L, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020;31:1623–49.
Lee CI, Gold LS, Nelson HD, Chou R, Ramsey SD, Sullivan SD. Comparative effectiveness of imaging modalities to determine metastatic breast cancer treatment response. Breast. 2015;24:3–11.
Cochet A, David S, Moodie K, Drummond E, Dutu G, MacManus M, Chua B, Hicks RJ. The utility of 18 F-FDG PET/CT for suspected recurrent breast cancer: impact and prognostic stratification. Cancer Imaging. 2014;14:13.
Pak K, Yoon HJ, Lim W, Kim HY. Impact of 18F-FDG PET on the management of recurrent breast cancer: a meta-analysis. Clinical and Translational Imaging. 2021;9(3):255–63. https://doi.org/10.1007/s40336-021-00424-1.
Xiao Y, Wang L, Jiang X, She W, He L, Hu G. Diagnostic efficacy of 18F-FDG-PET or PET/CT in breast cancer with suspected recurrence: a systematic review and meta-analysis. Nucl Med Commun. 2016;37:1180–8.
Evangelista L, Cervino AR, Ghiotto C, Al-Nahhas A, Rubello D, Muzzio PC. Tumor marker-guided PET in breast cancer patients-a recipe for a perfect wedding: a systematic literature review and meta-analysis. Clin Nucl Med. 2012;37:467–74.
Filippi V, Malamitsi J, Vlachou F, Laspas F, Georgiou E, Prassopoulos V, Andreou J. The impact of FDG-PET/CT on the management of breast cancer patients with elevated tumor markers and negative or equivocal conventional imaging modalities. Nucl Med Commun. 2011;32:85–90.
Corso G, Gilardi L, Girardi A, De Scalzi AM, Pagani G, Rossi EMC, Montagna G, Veronesi P, Pagan E, Bagnardi V, Grana CM. How useful are tumor markers in detecting metastases with FDG-PET/CT during breast cancer surveillance? Oncology. 2020;98:714–8.
Groheux D, Giacchetti S, Delord M, de Roquancourt A, Merlet P, Hamy AS, Espié M, Hindié E. Prognostic impact of 18F-FDG PET/CT staging and of pathological response to neoadjuvant chemotherapy in triple-negative breast cancer. Eur J Nucl Med Mol Imaging. 2015;42:377–85.
Groheux D. FDG-PET/CT for primary staging and detection of recurrence of breast cancer. Semin Nucl Med. 2022;52:508–19.
Fei B, Schuster DM. PET molecular imaging-directed biopsy: a review. AJR Am J Roentgenol. 2017;209:255–69.
Ulaner GA, Mankoff DA, Clark AS, Fowler AM, Linden HM, Peterson LM, Dehdashti F, Kurland BF, Mortimer J, Mouabbi J, et al. Summary: appropriate use criteria for estrogen receptor-targeted pet imaging with 16α-(18)F-fluoro-17β-fluoroestradiol. J Nucl Med. 2023;64:351–4.
Peterson LM, O’Sullivan J, Wu QV, Novakova-Jiresova A, Jenkins I, Lee JH, Shields A, Montgomery S, Linden HM, Gralow J, et al. Prospective study of serial (18)F-FDG PET and (18)F-fluoride PET to predict time to skeletal-related events, time to progression, and survival in patients with bone-dominant metastatic breast cancer. J Nucl Med. 2018;59:1823–30.
Avril N, Rosé CA, Schelling M, Dose J, Kuhn W, Bense S, Weber W, Ziegler S, Graeff H, Schwaiger M. Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: use and limitations. J Clin Oncol. 2000;18:3495–502.
Dashevsky BZ, Goldman DA, Parsons M, Gönen M, Corben AD, Jochelson MS, Hudis CA, Morrow M, Ulaner GA. Appearance of untreated bone metastases from breast cancer on FDG PET/CT: importance of histologic subtype. Eur J Nucl Med Mol Imaging. 2015;42:1666–73.
Tade FI, Cohen MA, Styblo TM, Odewole OA, Holbrook AI, Newell MS, Savir-Baruch B, Li X, Goodman MM, Nye JA, Schuster DM. Anti-3-18F-FACBC (18F-fluciclovine) PET/CT of breast cancer: an exploratory study. J Nucl Med. 2016;57:1357–63.
Ulaner GA, Goldman DA, Gönen M, Pham H, Castillo R, Lyashchenko SK, Lewis JS, Dang C. Initial results of a prospective clinical trial of 18F-fluciclovine PET/CT in newly diagnosed invasive ductal and invasive lobular breast cancers. J Nucl Med. 2016;57:1350–6.
Ulaner GA, Goldman DA, Corben A, Lyashchenko SK, Gönen M, Lewis JS, Dickler M. Prospective Clinical trial of (18)F-fluciclovine PET/CT for determining the response to neoadjuvant therapy in invasive ductal and invasive lobular breast cancers. J Nucl Med. 2017;58:1037–42.
Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, Adeberg S, Rathke H, Röhrich M, Winter H, et al. (68)Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med. 2019;60:801–5.
Backhaus P, Burg MC, Roll W, Büther F, Breyholz HJ, Weigel S, Heindel W, Pixberg M, Barth P, Tio J, Schäfers M. Simultaneous FAPI PET/MRI targeting the fibroblast-activation protein for breast cancer. Radiology. 2022;302:39–47.
Elboga U, Sahin E, Kus T, Cayirli YB, Aktas G, Uzun E, Cinkir HY, Teker F, Sever ON, Aytekin A, et al. Superiority of (68)Ga-FAPI PET/CT scan in detecting additional lesions compared to (18)FDG PET/CT scan in breast cancer. Ann Nucl Med. 2021;35:1321–31.
Mona CE, Benz MR, Hikmat F, Grogan TR, Lückerath K, Razmaria A, et al. Correlation of (68)Ga-FAPi-46 PET biodistribution with FAP expression by immunohistochemistry in patients with solid cancers: a prospective translational exploratory study. J Nucl Med. 2022;63(7):1021–6. https://doi.org/10.2967/jnumed.121.262426.
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92.
Niikura N, Liu J, Hayashi N, Mittendorf EA, Gong Y, Palla SL, Tokuda Y, Gonzalez-Angulo AM, Hortobagyi GN, Ueno NT. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J Clin Oncol. 2012;30:593–9.
Priedigkeit N, Hartmaier RJ, Chen Y, Vareslija D, Basudan A, Watters RJ, Thomas R, Leone JP, Lucas PC, Bhargava R, et al. Intrinsic subtype switching and acquired ERBB2/HER2 amplifications and mutations in breast cancer brain metastases. JAMA Oncol. 2017;3:666–71.
Dijkers EC, Oude Munnink TH, Kosterink JG, Brouwers AH, Jager PL, de Jong JR, van Dongen GA, Schröder CP, Lub-de Hooge MN, de Vries EG. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther. 2010;87:586–92.
Gebhart G, Lamberts LE, Wimana Z, Garcia C, Emonts P, Ameye L, Stroobants S, Huizing M, Aftimos P, Tol J, et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR trial. Ann Oncol. 2016;27:619–24.
Ulaner GA, Hyman DM, Ross DS, Corben A, Chandarlapaty S, Goldfarb S, McArthur H, Erinjeri JP, Solomon SB, Kolb H, et al. Detection of HER2-positive metastases in patients with HER2-negative primary breast cancer using 89Zr-trastuzumab PET/CT. J Nucl Med. 2016;57:1523–8.
Dehdashti F, Wu N, Bose R, Naughton MJ, Ma CX, Marquez-Nostra BV, Diebolder P, Mpoy C, Rogers BE, Lapi SE, et al. Evaluation of [(89)Zr]trastuzumab-PET/CT in differentiating HER2-positive from HER2-negative breast cancer. Breast Cancer Res Treat. 2018;169:523–30.
Bensch F, Brouwers AH, Lub-de Hooge MN, de Jong JR, van der Vegt B, Sleijfer S, de Vries EGE, Schröder CP. (89)Zr-trastuzumab PET supports clinical decision making in breast cancer patients, when HER2 status cannot be determined by standard work up. Eur J Nucl Med Mol Imaging. 2018;45:2300–6.
Ulaner GA, Hyman DM, Lyashchenko SK, Lewis JS, Carrasquillo JA. 89Zr-trastuzumab PET/CT for detection of human epidermal growth factor receptor 2-positive metastases in patients with human epidermal growth factor receptor 2-negative primary breast cancer. Clin Nucl Med. 2017;42:912–7.
Mortimer JE, Bading JR, Frankel PH, Caroll M, Yuan Y, Park J, et al. Use of 64Cu-DOTA-trastuzumab PET to predict response and outcome of patients receiving trastuzumab emtansine (T-DM1) for metastatic breast cancer: a pilot study. J Nucl Med. 2022;63(8):1145–8. https://doi.org/10.2967/jnumed.121.262940.
Ulaner GA, Lyashchenko SK, Riedl C, Ruan S, Zanzonico PB, Lake D, Jhaveri K, Zeglis B, Lewis JS, O’Donoghue JA. First-in-human human epidermal growth factor receptor 2-targeted imaging using (89)Zr-pertuzumab PET/CT: dosimetry and clinical application in patients with breast cancer. J Nucl Med. 2018;59:900–6.
Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, Tsurutani J, Ueno NT, Prat A, Chae YS, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387:9–20.
Tarantino P, Hamilton E, Tolaney SM, Cortes J, Morganti S, Ferraro E, Marra A, Viale G, Trapani D, Cardoso F, et al. HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol. 2020;38:1951–62.
Pesapane F, Downey K, Rotili A, Cassano E, Koh DM. Imaging diagnosis of metastatic breast cancer. Insights Imaging. 2020;11:79.
Hadjipanteli A, Doolan P, Kyriacou E, Constantinidou A. Breast cancer brain metastasis: the potential role of MRI beyond current clinical applications. Cancer Manag Res. 2020;12:9953–64.
Stecco A, Trisoglio A, Soligo E, Berardo S, Sukhovei L, Carriero A. Whole-body MRI with diffusion-weighted imaging in bone metastases: a narrative review. Diagnostics (Basel). 2018Jul 9;8(3):45. https://doi.org/10.3390/diagnostics8030045.
Catana C. Attenuation correction for human PET/MRI studies. Phys Med Biol. 2020;65:23tr02.
Ehman EC, Johnson GB, Villanueva-Meyer JE, Cha S, Leynes AP, Larson PEZ, Hope TA. PET/MRI: where might it replace PET/CT? J Magn Reson Imaging. 2017;46:1247–62.
Pace L, Nicolai E, Luongo A, Aiello M, Catalano OA, Soricelli A, Salvatore M. Comparison of whole-body PET/CT and PET/MRI in breast cancer patients: lesion detection and quantitation of 18F-deoxyglucose uptake in lesions and in normal organ tissues. Eur J Radiol. 2014;83:289–96.
Pujara AC, Raad RA, Ponzo F, Wassong C, Babb JS, Moy L, Melsaether AN. Standardized uptake values from PET/MRI in metastatic breast cancer: an organ-based comparison with PET/CT. Breast J. 2016;22:264–73.
Grueneisen J, Nagarajah J, Buchbender C, Hoffmann O, Schaarschmidt BM, Poeppel T, Forsting M, Quick HH, Umutlu L, Kinner S. Positron emission tomography/magnetic resonance imaging for local tumor staging in patients with primary breast cancer: a comparison with positron emission tomography/computed tomography and magnetic resonance imaging. Invest Radiol. 2015;50:505–13.
Goorts B, Vöö S, van Nijnatten TJA, Kooreman LFS, de Boer M, Keymeulen K, Aarnoutse R, Wildberger JE, Mottaghy FM, Lobbes MBI, Smidt ML. Hybrid (18)F-FDG PET/MRI might improve locoregional staging of breast cancer patients prior to neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2017;44:1796–805.
Taneja S, Jena A, Goel R, Sarin R, Kaul S. Simultaneous whole-body 18F-FDG PET-MRI in primary staging of breast cancer: a pilot study. Eur J Radiol. 2014;83:2231–9.
Bruckmann NM, Sawicki LM, Kirchner J, Martin O, Umutlu L, Herrmann K, Fendler W, Bittner AK, Hoffmann O, Mohrmann S, et al. Prospective evaluation of whole-body MRI and (18)F-FDG PET/MRI in N and M staging of primary breast cancer patients. Eur J Nucl Med Mol Imaging. 2020;47:2816–25.
Morawitz J, Bruckmann NM, Dietzel F, Ullrich T, Bittner AK, Hoffmann O, Ruckhäberle E, Mohrmann S, Häberle L, Ingenwerth M, et al. Comparison of nodal staging between CT, MRI, and [(18)F]-FDG PET/MRI in patients with newly diagnosed breast cancer. Eur J Nucl Med Mol Imaging. 2022;49:992–1001.
de Mooij CM, Sunen I, Mitea C, Lalji UC, Vanwetswinkel S, Smidt ML, van Nijnatten TJA. Diagnostic performance of PET/computed tomography versus PET/MRI and diffusion-weighted imaging in the N- and M-staging of breast cancer patients. Nucl Med Commun. 2020;41:995–1004.
Botsikas D, Bagetakos I, Picarra M. Da Cunha Afonso Barisits AC, Boudabbous S, Montet X, Lam GT, Mainta I, Kalovidouri A, Becker M: What is the diagnostic performance of 18-FDG-PET/MR compared to PET/CT for the N- and M- staging of breast cancer? Eur Radiol. 2019;29:1787–98.
Catalano OA, Nicolai E, Rosen BR, Luongo A, Catalano M, Iannace C, Guimaraes A, Vangel MG, Mahmood U, Soricelli A, Salvatore M. Comparison of CE-FDG-PET/CT with CE-FDG-PET/MR in the evaluation of osseous metastases in breast cancer patients. Br J Cancer. 2015;112:1452–60.
Lin CY, Lin CL, Kao CH. Staging/restaging performance of F18-fluorodeoxyglucose positron emission tomography/magnetic resonance imaging in breast cancer: a review and meta-analysis. Eur J Radiol. 2018;107:158–65.
Incoronato M, Grimaldi AM, Cavaliere C, Inglese M, Mirabelli P, Monti S, Ferbo U, Nicolai E, Soricelli A, Catalano OA, et al. Relationship between functional imaging and immunohistochemical markers and prediction of breast cancer subtype: a PET/MRI study. Eur J Nucl Med Mol Imaging. 2018;45:1680–93.
Catalano OA, Horn GL, Signore A, Iannace C, Lepore M, Vangel M, Luongo A, Catalano M, Lehman C, Salvatore M, et al. PET/MR in invasive ductal breast cancer: correlation between imaging markers and histological phenotype. Br J Cancer. 2017;116:893–902.
Mutic S, Dempsey JF. The ViewRay system: magnetic resonance-guided and controlled radiotherapy. Semin Radiat Oncol. 2014;24:196–9.
Winkel D, Bol GH, Kroon PS, van Asselen B, Hackett SS, Werensteijn-Honingh AM, Intven MPW, Eppinga WSC, Tijssen RHN, Kerkmeijer LGW, et al. Adaptive radiotherapy: The Elekta Unity MR-linac concept. Clin Transl Radiat Oncol. 2019;18:54–9.
Byrne M, Archibald-Heeren B, Hu Y, Teh A, Beserminji R, Cai E, Liu G, Yates A, Rijken J, Collett N, Aland T. Varian ethos online adaptive radiotherapy for prostate cancer: Early results of contouring accuracy, treatment plan quality, and treatment time. J Appl Clin Med Phys. 2022;23: e13479.
Moazzezi M, Rose B, Kisling K, Moore KL, Ray X. Prospects for daily online adaptive radiotherapy via ethos for prostate cancer patients without nodal involvement using unedited CBCT auto-segmentation. J Appl Clin Med Phys. 2021;22:82–93.
Teixeira SC, Rebolleda JF, Koolen BB, Wesseling J, Jurado RS, Stokkel MP. Del Puig Cózar Santiago M, van der Noort V, Rutgers EJ, Valdés Olmos RA: Evaluation of a hanging-breast PET system for primary tumor visualization in patients with stage I-III breast cancer: comparison with standard PET/CT. AJR Am J Roentgenol. 2016;206:1307–14.
Koolen BB, Aukema TS, González Martínez AJ, Vogel WV, Caballero Ontanaya L, Vrancken Peeters MJ, Vroonland CJ, Rutgers EJ, Benlloch Baviera JM, Valdés Olmos RA. First clinical experience with a dedicated PET for hanging breast molecular imaging. Q J Nucl Med Mol Imaging. 2013;57:92–100.
deSouza NM, Liu Y, Chiti A, Oprea-Lager D, Gebhart G, Van Beers BE, Herrmann K, Lecouvet FE. Strategies and technical challenges for imaging oligometastatic disease: recommendations from the European Organisation for Research and Treatment of Cancer imaging group. Eur J Cancer. 2018;91:153–63.
Trovo M, Furlan C, Polesel J, Fiorica F, Arcangeli S, Giaj-Levra N, Alongi F, Del Conte A, Militello L, Muraro E, et al. Radical radiation therapy for oligometastatic breast cancer: results of a prospective phase II trial. Radiother Oncol. 2018;126:177–80.
Greco C, Pares O, Pimentel N, Louro V, Morales J, Nunes B, Antunes I, Vasconcelos AL, Kociolek J, Castanheira J, et al. Positron emission tomography-derived metrics predict the probability of local relapse after oligometastasis-directed ablative radiation therapy. Adv Radiat Oncol. 2022;7: 100864.
Solanki AA, Weichselbaum RR, Appelbaum D, Farrey K, Yenice KM, Chmura SJ, Salama JK. The utility of FDG-PET for assessing outcomes in oligometastatic cancer patients treated with stereotactic body radiotherapy: a cohort study. Radiat Oncol. 2012;7:216.
Saeed H, Kirby A. Local therapies for managing oligometastatic breast cancer: a review. Ann Breast Surg. 2021;6. https://doi.org/10.21037/abs-20-145.
Soussan M, Orlhac F, Boubaya M, Zelek L, Ziol M, Eder V, Buvat I. Relationship between tumor heterogeneity measured on FDG-PET/CT and pathological prognostic factors in invasive breast cancer. PLoS ONE. 2014;9: e94017.
Son SH, Kim DH, Hong CM, Kim CY, Jeong SY, Lee SW, Lee J, Ahn BC. Prognostic implication of intratumoral metabolic heterogeneity in invasive ductal carcinoma of the breast. BMC Cancer. 2014;14:585.
Sollini M, Cozzi L, Ninatti G, Antunovic L, Cavinato L, Chiti A, Kirienko M. PET/CT radiomics in breast cancer: mind the step. Methods. 2021;188:122–32.
Zwanenburg A, Vallières M, Abdalah MA, Aerts H, Andrearczyk V, Apte A, Ashrafinia S, Bakas S, Beukinga RJ, Boellaard R, et al. The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology. 2020;295:328–38.
Acknowledgements
This guideline was brought to the attention of the EANM Oncology and Theranostics Committee. The comments and suggestions from the SNMMI, EANM Committees, EANM National Societies, and from the other societies that endorsed the documents were highly appreciated and have been included in this guideline.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
Gary Cook: research grant from Breast Cancer Now and he was a previous member of their scientific advisory board.
Gary Ulaner: Consultant/Advisory Board/Research Funding – GE Healthcare, Lantheus, ImaginAb, Point Biopharma
Heather Jacene: Blue Earth Diagnostics, honoraria and research support; Consulting, advanced accelerator applications, spectrum dynamics, royalties: Cambridge University Press; all are not related to the work presented in this manuscript.
Philip Poortmans: medical advisor of Sordina IORT Technologies S.p.A., not related to the work presented in this manuscript.
Ritse Mann: research grants from/with Beckton and Dickinson, Siemens, Bayer Healthcare, Screenpoint medical, Koning, and PA Imaging, and is a medical advisor to Screenpoint, Bayer, Guerbet, and BD. All are unrelated to the work in this manuscript.
Fatima Cardoso: Personal financial interest in form of consultancy role for: Amgen, Astellas/Medivation, AstraZeneca, Celgene, Daiichi-Sankyo, Eisai, GE Oncology, Genentech, Gilead, GlaxoSmithKline, Iqvia, Macrogenics, Medscape, Merck-Sharp, Merus BV, Mylan, Mundipharma, Novartis, Pfizer, Pierre-Fabre, prIME Oncology, Roche, Sanofi, Samsung Bioepis, Seagen, Teva, Touchime.
Institutional financial support for clinical trials from: Amgen, Astra-Zeneca, Boehringer-Ingelheim, Bristol-Myers-Squibb, Daiichi-Sankyo, Eisai, Fresenius GmbH, Genentech, Gilead, GlaxoSmithKline, Ipsen, Incyte, Nektar Therapeutics, Nerviano, Novartis, Macrogenics, Medigene, MedImmune, Merck, Millenium, Pfizer, Pierre-Fabre, Roche, Sanofi-Aventis, Sonus, Tesaro, Tigris, Wilex, Wyeth.
All the other authors declare no conflict of interest.
Endorsements
This guideline has been reviewed and endorsed by the following societies: American College of Radiology (ACR), European Society of Surgical Oncology (ESSO), European Society for Radiotherapy and Oncology (ESTRO), European Society of Breast Imaging/ European Society of Radiology (EUSOBI/ESR), and European Society of Breast Cancer Specialists (EUSOMA).
Liability statement
This guideline summarizes the views of the EANM Oncology and Theranostics Committee and SNMMI. It reflects recommendations for which the EANM and SNMMI cannot be held responsible. The recommendations should be taken into the context of good practice of the different disciplines (mainly Nuclear Medicine, Breast Radiology, Radiation Oncology, Medical Oncology, and Breast Surgery) and do not substitute for national and international legal or regulatory provisions.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Elizabeth H. Dibble and Lioe-Fee de Geus-Oei are the co-chair and chair authors, respectively.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vaz, S.C., Woll, J.P.P., Cardoso, F. et al. Joint EANM-SNMMI guideline on the role of 2-[18F]FDG PET/CT in no special type breast cancer. Eur J Nucl Med Mol Imaging 51, 2706–2732 (2024). https://doi.org/10.1007/s00259-024-06696-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-024-06696-9