Abstract
Epilepsy is one of the most frequent neurological conditions with an estimated prevalence of more than 50 million people worldwide and an annual incidence of two million. Although pharmacotherapy with anti-seizure medication (ASM) is the treatment of choice, ~30% of patients with epilepsy do not respond to ASM and become drug resistant. Focal epilepsy is the most frequent form of epilepsy. In patients with drug-resistant focal epilepsy, epilepsy surgery is a treatment option depending on the localisation of the seizure focus for seizure relief or seizure freedom with consecutive improvement in quality of life. Beside examinations such as scalp video/electroencephalography (EEG) telemetry, structural, and functional magnetic resonance imaging (MRI), which are primary standard tools for the diagnostic work-up and therapy management of epilepsy patients, molecular neuroimaging using different radiopharmaceuticals with single-photon emission computed tomography (SPECT) and positron emission tomography (PET) influences and impacts on therapy decisions. To date, there are no literature-based praxis recommendations for the use of Nuclear Medicine (NM) imaging procedures in epilepsy. The aims of these guidelines are to assist in understanding the role and challenges of radiotracer imaging for epilepsy; to provide practical information for performing different molecular imaging procedures for epilepsy; and to provide an algorithm for selecting the most appropriate imaging procedures in specific clinical situations based on current literature. These guidelines are written and authorized by the European Association of Nuclear Medicine (EANM) to promote optimal epilepsy imaging, especially in the presurgical setting in children, adolescents, and adults with focal epilepsy. They will assist NM healthcare professionals and also specialists such as Neurologists, Neurophysiologists, Neurosurgeons, Psychiatrists, Psychologists, and others involved in epilepsy management in the detection and interpretation of epileptic seizure onset zone (SOZ) for further treatment decision. The information provided should be applied according to local laws and regulations as well as the availability of various radiopharmaceuticals and imaging modalities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Preamble
The EANM is a professional non-profit medical association that facilitates communication worldwide among individuals pursuing clinical and research excellence in NM. The EANM was founded in 1985. These guidelines are intended to assist practitioners in providing appropriate NM care for patients with focal epilepsy. They are flexible rules or requirements of practice and are not intended, nor should they be used, to establish a legal standard of care. The ultimate judgement regarding the propriety of any specific procedure or course of action must be made by medical professionals taking into account the unique circumstances of each case. Thus, there is no implication that an approach differing from the guidelines, standing alone, is below the standard of care. To the contrary, a conscientious practitioner may responsibly adopt a course of action different from that set out in these recommendations when, in the reasonable judgement of the practitioner, such course of action is indicated by the condition of the patient, limitations of available resources, or advances in knowledge or technology subsequent to this publication. The practice of medicine involves not only the science but also the art of dealing with the prevention, diagnosis, alleviation, and treatment of disease. The variety and complexity of human conditions make it impossible to always reach the most appropriate diagnosis or to predict with certainty a particular response to treatment. Therefore, it should be recognized that adherence to these guidelines alone will not ensure an accurate diagnosis or a successful outcome. All that should be expected is that the practitioner will follow a reasonable course of action based on current knowledge, available resources, and the needs of the patient to deliver effective and safe medical care. The sole purpose of these guidelines is to ensure that the practitioner delivers effective and safe medical care by following a reasonable course of action based on current knowledge, available resources, and patient needs.
Purpose
These EANM practice guidelines, written for NM healthcare professionals and other specialists who are involved in epilepsy management, should support the optimal use of NM procedures in epilepsy imaging in children and adults based on current knowledge. The purposes of these recommendations are as follows:
-
To assist in understanding the role and challenges of radiotracer imaging for epilepsy.
-
To provide practical information for performing NM imaging procedures for patients with focal epilepsy.
-
To provide an algorithm for selecting the most appropriate NM imaging procedure in specific clinical situations.
-
To provide information regarding radiotracers that can lead to a better understanding and characterization of epilepsy.
Introduction
Epilepsy is a chronic non-communicable disease of the brain that affects people of all ages and one of the most frequent neurological conditions with an estimated prevalence of more than 50 million people worldwide and an annual incidence of two million [1]. Epilepsies/epileptic syndromes are classified as focal, generalized, combined focal and generalized, or unknown, with focal epilepsy being the most frequent form [2]. A range of aetiologic groups is defined with emphasis on those that have implications for treatment. In patients with focal epilepsy, MRI is the first imaging investigation, which enables the clinician to decide if there is a structural aetiology for the patient’s epilepsy, such as hippocampal sclerosis, tumour, focal cortical dysplasia (FCD), haemorrhage, or other structural lesions. The five additional aetiologic groups are defined as genetic, infectious, metabolic, immune, or unknown. A patient’s epilepsy also may be classified into more than one etiologic category. Moreover, the etiologies are not hierarchical, and the importance given to the patient’s etiological group may depend on the circumstance, as given for instance in patient who has both a structural and a genetic or metabolic aetiology with different potential treatment options [2].
Pharmacotherapy with ASM is the initial treatment of choice for the vast majority of patients with epilepsy. Approximately 30% , however, do not respond to two ASMs and are considered drug resistant [3,4,5]. This is mainly true for focal epilepsies [6]. Similar findings are observed in children and adolescents with approximately 20% of drug resistance with risk for poor long-term cognitive and psychosocial outcomes together with poor quality of life [7, 8]. In patients with drug-resistant focal epilepsy, epilepsy surgery is promising depending on the epilepsy type with approximately 65% of patients becoming seizure free [9,10,11]. If the source of epilepsy is correctly detected and outside of eloquent areas, surgery of epileptogenic zone is overall safe, successful, and cost-effective [12].
Beside scalp video/EEG telemetry, structural MRI, neuropsychological and neuropsychiatric assessment, Wada test, functional MRI, NM imaging procedures, and intracranial EEG electrodes are of additional value depending on the epilepsy type. The development of molecular neuroimaging with interictal [18F]fluorodeoxyglucose ([18F]FDG) positron emission tomography (PET), ictal perfusion SPECT, or ictal subtraction perfusion SPECT (interictal SPECT fused, normalized, and subtracted from ictal SPECT) has influenced and impacted the presurgical management of epilepsy patients, not only for the investigation of non-lesional, but also lesional epilepsy regarding postsurgical seizure outcome over the years. In both adults and children without any visible brain lesion on MRI or in others with multifocal structural cerebral abnormalities such as hemispheric or multi-lobar cortical dysplasia, polymicrogyria, or localized stroke, these functional imaging tools are especially useful to localize the seizure onset for a tailored resection preserving motor, visual, language, or temporal lobe functions [13]. To date, there are no literature-based praxis recommendations for the use of NM imaging procedures in epilepsy. It is the aim of this “guideline” to fill this gap.
Precautions
Imaging pregnant or potentially pregnant adolescents and women with ionizing radiation
The use of radiopharmaceuticals is generally contraindicated in pregnancy. However, if clinically necessary, a decision to perform imaging in cases of known or suspected pregnancy should be based on an analysis of the benefits versus the possible risks to the foetus, as decided by a multidisciplinary team [14, 15]. Otherwise, it should be postponed after pregnancy. Proper institutional and local guidelines should be followed.
Breastfeeding women
In breastfeeding women, it is recommended to consult the International Commission on Radiological Protection (ICRP) Publication 128: Radiation Dose to Patients from Radiopharmaceuticals: A Compendium of Current Information Related to Frequently Used Substances [16]. Although interruption of breastfeeding is not essential in the absence of free pertechnetate in 99mTc-labelled radiopharmaceuticals, a pause of 24 h is recommended in some summaries of product characteristics of 99mTc-labelled radiopharmaceuticals with marketing authorization [17]. There is no information 11C-labelled pharmaceuticals described in these guidelines, including L-([11C]methyl)methionine ([11C]MET), regarding excretion of radioactivity into breast milk. Nevertheless, due to their short physical half-life an interruption is not essential. For [18F]FDG, interruption of breastfeeding is not recommended; for other 18F-labelled compounds described here recommendations concerning the duration of a pause in lactation are not available, but discontinuing breastfeeding with milk discard for 12 h after tracer injection should be considered [16, 18].
Intradepartmental care
A continuous supervision of epilepsy patients, especially prior and during the procedure, is essential to guarantee inter-ictal imaging, a seizure-free examination, and to ensure the safety of these at-risk patients. The healthcare professionals have to be instructed regarding potential ictal signs occurring during the uptake phase of some radiopharmaceuticals and imaging phase. ASM and other medications needed should be available in the division or scanning room. The occurrence of a seizure or status epilepticus needs to be documented and taken into consideration when interpreting the functional images, if relevant.
Imaging of children and adolescents in company of parents or caregivers
Following institutional and local guidelines and to reduce the anxiety of the child and the parents or caregivers, they have to be informed regarding the radiopharmaceutical used and its specific indication as well as procedural details of scanning. Moreover, they have to be instructed to notify the medical staff when an ictal sign occurs prior or during the uptake phase of different tracers.
Imaging of epilepsy patients under anaesthesia
In the case of inability or non-cooperation regarding the requested examination anaesthesia may be necessary in adults as well as paediatric epilepsy patients, even if it has influence on brain metabolism and perfusion [19] and especially if anaesthesia is administered less than 30 min from the injection of the tracer [20]. Beside following the institutional and local guidelines the anaesthesia team has to be informed regarding tracer behaviour, radiation protection, and scanning procedure.
Qualifications and responsibilities of personnel
NM physicians, technologists, nurses, and all other healthcare professionals involved in performing and reporting epilepsy imaging should be qualified according to applicable laws and regulations, and individual responsibilities should be clearly documented.
Useful clinical information for optimal imaging and interpretation
A request for epilepsy imaging must be submitted to a NM physician. It should provide data regarding the clinical history of the patient, with the type of seizure and frequency, dominant hemisphere, current and previous medications, EEG or/and video-telemetry data, and previous MRI imaging, to allow the NM physician responsible to assess the indication for the scan adequately, as well as to interpret the images. The patient is also questioned (if possible) for relevant information that may help interpret the findings (e.g., time of last seizure, current medication). ASM and other required medications are permitted although cautions should be used with certain drugs such as benzodiazepines.
Timing of PET and SPECT imaging, especially for interictal perfusion SPECT and [18F]FDG PET, has to be documented according to the time of last seizure. If clinically possible, high- quality interictal SPECT and [18F]FDG PET imaging in epilepsy patients should be performed at least 24 h after focal aware seizures and 48 h after focal impaired awareness and focal-to-bilateral tonic-clonic seizures [21].
Perfusion SPECT imaging
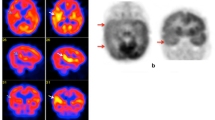
The major originality of SPECT imaging remains its ictal imaging value by measuring the regional cerebral blood flow associated with epileptic seizures [22]. Actually, the radiopharmaceutical administration can be performed during an epileptic discharge, with a brain uptake irreversibly completed in 1 to 2 min [23]. During an ictal scan, the brain regions involved in seizure generation and early propagation demonstrate increased perfusion, with all hyperperfused regions representing an electrically connected epileptic network [23]. By contrast, most epileptic networks are hypoperfused during inter-ictal state. Ictal SPECT presents a sensitivity of 73% and specificity of 75% while inter-ictal SPECT has a much lower localisation value with 50% of sensitivity and 75% of specificity [24]. Moreover, SPECT has higher performances in detection of epileptic networks in temporal lobe epilepsy (TLE) in comparison to extra-temporal lobe epilepsy (ETLE) [25]. Notably, ictal SPECT is useful for lesional epilepsy, suitable in children with focal refractory epilepsies while being a strong predictor of surgical success [26]. With an odds ratio of 0.37, ictal SPECT is also a favourable surgical outcome predictor in non-lesional epilepsy [27]. Subtraction of ictal and inter-ictal SPECT co-registered to MRI (SISCOM) has been shown to improve the sensitivity and specificity of seizure related perfusion networks. SISCOM not only shows regions of hyperperfusion, but also hypoperfusion. These ictal-network related perfusion changes have been well described during focal impaired awareness seizure in mesial temporal lobe epilepsy with hippocampal sclerosis with ictal hyperperfusion of the temporal lobe ipsilateral to the seizure focus, the border of the ipsilateral middle frontal and precentral gyrus, both occipital lobes and small regions the contralateral postcentral gyrus. The frontal lobes, contralateral, the posterior cerebellum and the ipsilateral precuneus showed hypoperfusion [28]. The diagnostic performances of SISCOM are potentially further enhanced when using statistical SPECT processing methods. In this line, studies found that SISCOM localization sensitivity was higher than 90% in temporal lobe seizures [29], but lower in ETLE, with a reported sensitivity of 70% [30]. SISCOM provides useful information for seizure localization in patients with focal cortical dysplasia, even with normal MRI [31]. From a practical clinical value in preoperative evaluation, SISCOM was compared with either MRI, [18F]FDG PET, ictal EEG, or EEG-fMRI or combined modalities. For instance, hyperperfusion patterns in SISCOM images were localized more often than with side-by-side SPECT evaluation (71.0 vs. 47.4%), whereas SISCOM images led to at least a concordant or only slightly lower focus detection rate than [18F]FDG PET, MRI, and EEG modalities alone or combined [32,33,34,35,36,37]. Interestingly, if SISCOM localization is concordant with the surgical resection site on other traditional focus localization techniques, then postoperative outcomes are expected to be favourable [30, 38,39,40,41,42], with a seizure-free odds ratio around 3-times higher in concordant than in non-concordant SISCOM patients [43].
However, spatial resolution of SPECT is limited. Consequently, inter-ictal studies in focal epilepsies are often performed with PET imaging which leads to a better sensitivity in detection of epileptic networks. Albeit studying metabolism and perfusion are different physiological processes [44], they are in most circumstanced coupled. It should nevertheless be reported the recent instrumentation development, particularly Cadmium-Zinc-Telluride (CZT) cameras. These CZT cameras provide more than twofold increase in count sensitivity, as compared with the Anger camera, as well as better image resolution for brain imaging [45].
Technique: ictal, interictal, ictal subtraction SPECT
The reader should refer to the “EANM procedure guidelines for brain perfusion SPECT using 99mTc-labelled radiopharmaceuticals, version 2” for a detailed report of the recommended principles for image interpretation and support from semi-quantification strategies [46]. Here we will provide only additional details referring specifically to SPECT brain imaging in epilepsy.
-
A.
Procedures
-
It should be necessary to differentiate SPECT imaging performed during ictal and interictal phases. During an “ictal” scan, the brain regions involved in seizure generation and early propagation demonstrate increased perfusion, while most epileptic networks are hypoperfused during inter-ictal state.
-
99mTc-radiolabelled tracers such as HexaMethylPropyleneAmine Oxime ([99mTc]Tc-HMPAO) or Ethyl Cystine Dimer ([99mTc]Tc-ECD) are currently used according to the product manufacturing recommendations with an injected activity, in adults, of typically 740 MBq [24].
-
For ictal SPECT studies, the tracer should be injected as soon as possible after seizure onset, ideally within 20 s (via an intravenous line placed previously). Patients should have continuous video-EEG monitoring [46]. The best results are obtained when injection occurs during focal impaired awareness seizures. It is recommended that prepared syringes be stored in the epilepsy monitoring unit to ensure the quickest possible injection. The use of an automatic injector in ictal SPECT demonstrated significant clinical value by increasing the number of single localizing foci in comparison to scans with a manual injection by decreasing latency time [47, 48].
-
SPECT image acquisition can start 30 to 90 min after tracer injection with acquisition duration of about 20 min. For inter-ictal perfusion SPECT studies, EEG monitoring is recommended, if available, during tracer injection to exclude potential ictal activity especially in the first 2 min of the uptake phase of the radiopharmaceutical. Inter-ictal studies may add useful information to ictal studies. However, these cannot be recommended as a sole diagnostic procedure for focus detection [46].
-
-
B.
Interpretation
-
Interpretation of ictal and inter-ictal perfusion SPECT is based on a visual analysis, which can be improved by the addition of semi-quantitative analyses and co-registration to MRI.
-
As already noted, SISCOM is particularly useful; this has been shown to improve the sensitivity and specificity of seizure localization networks only demonstrating hypoperfusion during inter-ictal scan [49]. Knowledge of the injection timing and injected seizure type is important for a correct interpretation of SISCOM images [50]. The most commonly used thresholds for SISCOM are z=+1.5 and z=+2 [51].
-
Radiation doses
As the ICRP publication 128 reported in the adult population, the absorbed dose per unit of activity (without considering CT attenuation) administered (mGy/MBq) for urinary bladder wall is 5E-02 for [99mTc]Tc-ECD and 2.3E-02 for [99mTc]Tc-HMPAO resulting in an effective dose of 7.7E-03 mSv/MBq for [99mTc]Tc-ECD and 9.3E-03 mSv/MBq for [99mTc]Tc-HMPAO. In children (above 5 years of age) the effective dose raises to 1.5E-02 mSv/MBq for [99mTc]Tc-ECD and 1.7E-02 mSv/MBq for [99mTc]Tc-HMPAO [16].
[18F]FDG brain PET imaging
As a radiolabelled glucose analogue, in resting condition and interictal phase [18F]FDG PET typically shows reduced radiotracer uptake (hypometabolism) in the SOZ and the propagation zones beside a global decrease of cerebral grey matter glucose metabolism of around 10–25% compared with control subjects [52, 53]. This pattern has been addressed to various pathophysiological mechanisms, such as neuronal loss, reduction in synaptic density, and other epileptic-related dysfunctions, including post-ictal vascular changes or post-ictal depression. Factors like epilepsy duration and seizure frequency, ASMs, delay between the last seizures, type of the last seizure (e.g., generalized/unusual seizure) may be also responsible for the reduced uptake of [18F]FDG [54,55,56,57,58,59,60].
Interictal metabolic imaging with [18F]FDG helps the non-invasive detection of epileptogenic focus with higher sensitivity than inter-ictal perfusion SPECT, reflecting seizure-related changes in cerebral functions [44]. In fact, [18F]FDG PET visually demonstrates not only the SOZ, but the whole irritative zone (e.g., the SOZ and subsequent neural networks involved in the generation of inter-ictal paroxysms) [61, 62]. Moreover, [18F]FDG hypometabolism beyond the presumed SOZ is reported with a similar frequency in both TLE and ETLE surgical candidates with significant predictive value for surgical outcome [63].
A step forward in the diagnostic clinical use of [18F]FDG brain PET has been given both by the introduction of digital PET, which provides better image quality, diagnostic confidence and accuracy than analogue PET [64], and from hybrid PET/MRI systems, thanks to their simultaneous multimodal acquisition resulting in a perfect image fit with good surgical outcome [65, 66]. Moreover concordance of PET/MRI and magnetoencephalography (MEG) showed improvement of the presurgical localization of the epileptogenic focus [67]. In non-PET/MRI systems, the co-registration between PET/CT and MRI can be performed using dedicated software packages [68].
Over the years, [18F]FDG PET has become part of the systematic pre-surgical assessment of drug-resistant epilepsies. In decision making studies it showed to be useful for surgery selection in up to 47% and most beneficial in TLE (58–69%) compared to ETLE (23–44%) [69, 70]. It can also contribute to guide in the search for subtle cortical dysplasia or the placement of intracranial electrode [71], as well as to the assessment of patients with multifocal abnormalities to determine which is most likely to cause the seizure as the epileptogenic focus [72]. Moreover, [18F]FDG PET helps to re-interpret the negative MRI, detecting lesions missed at the first look, for example cases of focal cortical dysplasia in adults or immature myelination and poor grey to white matter differentiation in infants [73, 74]. It was also shown to be a valuable non-invasive method to guide intracranial electrode placement, and can also reduce the number of patients requiring invasive EEG [75]. Finally, [18F]FDG PET can be useful for the evaluation of the functional status of the rest of the cortex, which could serve as a predictor of the cognitive outcome after resection [76].
Lesional and non-lesional temporal lobe epilepsy in adults
The role of [18F]FDG PET in the assessment of TLE is of paramount importance in neuro-imaging. This has a reported pooled sensitivity and specificity of 79–95% in detection of the epileptic brain region in cases of TLE [63, 77]. Different groups of electro-clinical patterns, accordingly to distinct patterns of TLE, showed good accordance with inter-ictal [18F]FDG PET hypometabolisms at group level [61]. Further concordance with stereoelectroencephalography (SEEG) findings and inter-ictal [18F]FDG PET hypometabolism were also reported at the group and individual level [78]. It is however worth to underline again that temporal lobe hypometabolic regions often extend beyond the presumed epileptogenic zone, likely reflecting the degree of cerebral dysfunction that may be due to loss of synaptic inputs related to seizures. A gradient of [18F]FDG hypometabolism is here described between non-involved zone and propagation, epileptogenic, and lesional zones with nevertheless good performance in defining the epileptic zone [62]. These findings are also consistent with the current notion of epileptogenic and propagation networks [79] and allow to consider [18F]FDG metabolism as a network rather than a combination of regional metabolic measurements impacting on surgical outcome [80] with also surgical failure characterized by relatively high extratemporal hypometabolism on both sides [81,82,83].
In general, it is not infrequent to see other regions of diffuse glucose hypometabolism in epileptic patients, the significance of which is not always simple to determine. As known these patients, due to seizure and generally abnormal electric (consequent) metabolic activations of certain areas, may have interictally different neurological conditions. In patients with unilateral TLE, prefrontal asymmetric interictal hypometabolism is described to be associated with mild cognitive impairment [84], similarly, the presence of bitemporal glucose hypometabolism reflecting memory deficit with higher risk of postoperative memory decline [85]. Moreover, the left temporal pole seems also to be involved in lexical and semantic retrieval of knowledge of famous persons [86], the left temporo-occipital areas with potential deficit on word findings [87]. Hypometabolism associated with the ipsilateral insular cortex may correlate with emotional or somesthetic symptoms [88]. Evidence of contralateral thalamic hypometabolism is described to be associated with poorer surgical outcome compared to ipsilateral thalamic hypometabolism usual seen in mesial temporal lobe epilepsy [89]. More recently, it has been shown that cognitive impairment correlates with extratemporal hypometabolism, involving the mesial frontoparietal networks implicated in the default mode network and suggesting a disconnection with the affected hippocampus [90].
Lesional and non-lesional extra-temporal lobe epilepsy in adults
In ETLE, [18F]FDG PET performs with a lesser (as compared to TLE) sensitivity of approximately 55% in localizing the epileptogenic zone in retrospective studies [91]. One prospective trial, however, reported a sensitivity and specificity of 80% and 95% in a presurgical setting [63]. When focal hypometabolism is present, this means a significant positive indicator of postoperative seizure freedom when used as a means of secondary diagnostic imaging. Especially the combination of visual together with quantification tools provides additional prognostic outcome [27, 63, 92]. Sometimes [18F]FDG pattern in patients with ETLE can be misinterpreted because of also prominent hypometabolism in the temporal lobe [91]. Other factors which may be associated with a poorer post-surgical prognosis is hypometabolism remote from the epileptogenic zone [63, 93]. In frontal lobe epilepsy the sensitivity of [18F]FDG PET is reported to be higher (73%) in patients with structural lesions compared to patients without structural lesions on MRI (36%) [94]. In parietal epilepsy no differences were seen in PET focus localization frequency in seizure free compared to non-seizure free patients after surgery, although MRI abnormality and concordance of different diagnostic modalities such as PET, ictal SPECT, and ictal EEG were associated with high seizure-free rate [95]. Moreover, functional neuroimaging may also aid to confirm and complementary an occipital epileptogenic focus, with normal MRI with better performance of PET compared to SPECT to define the epileptogenic zones [96, 97]. Finally, co-registering PET images with MRI or using PET/MRI serves as additional tool to enhance the results for the detection of lesions on MRI in ETLE patients providing additional information on good prognostic surgical outcome or increased confidence in the original clinical readings [74, 98,99,100,101].
Although [18F]FDG hypometabolism is the most seen imaging pattern in interictal PET, hypermetabolic cortical or subcortical foci can sometimes be observed. Grey matter heterotopic tissue, a neuronal migration disorder as malformation of cortical development (MCD) may be present as hypermetabolism surrounded by white matter with only low [18F]FDG uptake [102, 103]. Others discuss hypermetabolic brain abnormalities as pathological neuronal hyperactivity predisposed to epileptic discharge generation or as a network of activated inhibitory circuits preventing the spread of epileptic discharge originating from a localized epileptogenic zone [101, 104,105,106].
Paediatric focal epilepsy and syndromes
In children and adolescents, the aetiology of focal epilepsy is multifactorial and includes other entities such as tumours (especially low grade epilepsy associated brain tumours—LEAT), birth-related lesions (stroke or haemorrhages), or MCD rather than mesial temporal sclerosis [107]. A number of MCDs are caused by an underlying genetic defect, seen as an isolated disorder, or associated with a wide variety of neurological symptoms such as developmental delay and/or motor abnormalities and extra-neurological features [108, 109]. Based on their MCD phenotype they could variably present as a focal irregularity of cortical morphology and thickness as observed in FCD or as an excessive number of abnormally small cerebral gyri with cortical overfolding as seen in polymicrogyria (PMG) or as hemimegalencephaly (a congenital unilateral disorder with cortical malformation). [18F]FDG PET may provide important information together with structural and other functional imaging techniques beside EEG regarding the localization of epileptogenic foci and its extension for treatment planning and prediction of postsurgical seizure outcome, avoiding incomplete resection [110]. The lesions will typically exhibit hypometabolism with some exceptions as discussed elsewhere in the text. The additional value of [18F]FDG PET in paediatric epilepsy cases is the possibility of defining the lesion with higher sensitivity (and usually larger extent) as compared to MRI, or localizing lesions in patients with negative or doubtful findings on MRI, especially in patients with FCD type 2 [99, 111,112,113]. Moreover, in situations of multilobar drug-resistant foci of epilepsy, a well-defined surgical approach such as the posterior disconnection or functional hemispherotomy (HE) is possible [114,115,116]. To achieve a good post-surgical epileptogenic outcome in these patients with potential of improvement of mental and physical development, the epileptogenic lesion should not extend beyond the confines of the disconnected cerebral volume. Subtle MRI abnormalities more widespread than the clear-cut lesion or MRI abnormalities in the contralateral remaining hemisphere may influence the surgical outcome [116, 117]. [18F]FDG, in the setting of bilateral focal metabolic abnormalities, has been therefore recognized as an important additional imaging tool [118, 119] and moreover described as an independent predictor of seizure recurrence after HE [117].
For epilepsy syndromes (ES) [18F]FDG PET also showed to be of interest especially in the setting of pre-surgical evaluation and postsurgical outcome estimation. ES are described as “characteristic clusters of clinical and electroencephalographic features, often supported by specific etiological findings (structural, genetic, metabolic, immune, and infectious)” and have been recently defined by the International League Against Epilepsy (ILAE) Task Force on Nosology and Definitions by dividing syndromes into typical age at onset, and based on seizure and epilepsy type in association with developmental and or epileptic encephalopathy or progressive neurological deterioration [120].
Tuberous Sclerosis (TSC), an autosomal-dominant multiorgan disorder caused by TSC1 gene (hamartin; chromosome 9q34) or TSC2 gene (tuberin; chromosome 16p13.3) mutations, is characterized by the development of hamartomatous lesions in various organs, including the brain. Epilepsy is drug resistant in 50–80% of these patients with poor cognitive outcome [121]. Early epilepsy surgery shows some benefit with resecting the main epileptogenic tuber [122]. Beside perfusion SPECT imaging [123, 124] [18F]FDG PET can also play a valuable role by non-invasively lateralizing and localizing the main epileptogenic tuber as area of glucose hypometabolism [125, 126], often more extended than observed with MRI indicating dysplastic cortex [127].
Sturge-Weber Syndrome is a rare sporadic neurocutaneous syndrome with seizure development present in up to 90% of these children. In these cases [18F]FDG usually shows larger area of abnormal cortex as compared to MRI. Although the involved cortex is hypometabolic interictally, the presence of cortical hypermetabolism in young children may be an imaging marker of subsequent severe epilepsy, requiring early surgical intervention [128,129,130].
Lennox-Gastaut Syndrome is characterized by a profoundly impairing developmental and epileptic encephalopathy with poor responsiveness to antiepileptic medication. Few publications are available regarding the utility of [18F]FDG PET in this disorder, with description of different tracer uptake patterns [131] and possible value in surgical treatment planning in patients with unilateral focal hypometabolism based on good concordance of PET and ictal EEG findings [132].
Hemimegalencephaly is a severe congenital malformation with unilateral enlarged and defectively developed hemisphere in the children affected by intractable seizures. Early hemisphere disconnection may lead to seizure control and adequate cognitive development. Therefore, the evaluation of the remaining contralateral hemisphere is important. [18F]FDG PET showed to be useful for assessing the functional integrity of the contralateral hemisphere and supporting in prediction of cognitive outcome [133].
Rasmussen Encephalitis is a form of chronic focal encephalitis associated with inflammation and progressive atrophy of a single hemisphere. During the early stages of the disease, [18F]FDG but also perfusion SPECT imaging can detect functional abnormalities as both hyper- and hypometabolism depending on the seizure status during the examination and may be helpful in identification of inconclusive MRI findings of the affected side [134,135,136].The most useful aspect of [18F]FDG PET in this setting is probably the exclusion of a bi-hemispheric involvement [137].
In general, brain [18F]FDG PET images of paediatric epilepsy patients are—as those of adult patients—mostly assessed visually. As the normal pattern of cerebral glucose consumption shows a strong age dependency in children/adolescents [112,113,114,115], such an approach, however, may be particularly subjective and user dependent. Therefore, a comparison of the respective experiences across different centres is difficult and may be a reason for the variable performance of [18F]FDG PET reported in the paediatric epilepsy literature. Software-based quantifying systems may be useful for better definition of the epileptic zone in paediatric patients with focal epilepsy. Such systems have so far been tested, however, only in children older than 6–8 years. Semi-quantitative data should, as such, be interpreted carefully, considered abnormal if significantly outside the range of normal data obtained from age-matched controls and only in combination with the visual inspection, as also recommended by others [138].
Principles and techniques for image interpretation
The reader should refer to the recently published “EANM Procedure Guidelines for Brain PET Imaging using [18F]FDG, version 3” for a detailed report of the recommended principles for image interpretation and support from semi-quantification strategies [20]. Here we will provide only additional details referring specifically to [18F]FDG brain imaging in epilepsy.
-
A.
Visual interpretation
-
The images should be critically examined prior to interpretation for presence of movement artefacts, the risk of which is higher in the epileptic population as seizures might occur during the acquisition.
-
Similarly, alignment of the images used for attenuation correction (CT for PET/CT, or µ-maps or specific sequences for PET/MRI) with the emission images should be systematically checked as mismatches can lead to erroneous interpretation. Furthermore, especially in very young children (therefore with possible incomplete myelinization) MRI derived µ-maps should be checked for possible biases related to erroneous segmentation.
-
Attenuation artefacts might occur in the presence of electrodes for EEG monitoring and might have an impact on quantification that should be taken into account interpreting the images [139].
-
The visual analysis should systematically evaluate all brain regions, using asymmetry as the main criterion for abnormality. This visual analysis can be refined using an anatomy-corrected asymmetry index image [140]. One should be careful not to miss bilateral and symmetric hypometabolism [53].
-
The visual analysis should also systematically evaluate the whole brain cortex after fusion of [18F]FDG images with a recent MRI (or CT in patients with contraindications for MRI) scan, preferably obtained near contemporary especially in children under 6 years old. The added value of PET and MRI image coregistration has been consistently confirmed across different patient series [74, 99, 141] and could potentially reduce confounding factors (e.g., fissures, CSF spaces, or cortical damages).
-
PET/MRI systems provide images acquired in the same reference space and thus automatically fused without the need of specific software for image registration (however, alignment of attenuation and emission data still needs to be checked especially if both have not been acquired at the same time). PET/MRI systems have been specifically employed in epilepsy, showing the ability of this tool to provide relevant diagnostic information across different modalities, including also electroencephalography [65, 101, 142,143,144,145,146].
-
-
B.
Semi-quantification and automated analysis
-
The added value of automated tools for the interpretation of images has been reported in the literature [68, 94, 147, 148]. The majority of tools have been validated and used for the evaluation of neurodegenerative patterns, and reference populations included or publicly available are usually composed of older individuals [20]. One of the main challenges is thus the use of a reference database and a pipeline adapted to the age of the subject investigated, mainly for children and adolescent patients: the limitation associated with a limited matching should thus be taken into account [149,150,151]. In addition to age, PET data acquisition and reconstruction differences should be considered when making comparisons against a normal database.
-
The results of software for semi-quantification and voxel-wise analysis should be used to guide a “second-look” visual analysis to detect subtle asymmetries or focal reductions.
-
PET data post-processing taking into account partial volume effects might be useful in this indication, but has not been extensively validated so far [152, 153].
-
A number of automated methods, including deep-learning and artificial intelligence approaches, have been more recently developed and reported for detection of abnormalities in epilepsy and represent promising developments; however, none has been clinically validated yet [153,154,155,156].
-
-
C.
Final report
-
For the final report see also the EANM Procedure Guidelines for Brain PET Imaging using [18F]FDG, version 3. [20] Moreover, it is important to combine the visual and if available semi-quantitative analysis, pointing out the presence of abnormalities and, in case of hypo- and/or hypermetabolism, their location and extent, as this information has been shown to carry not only diagnostic but also prognostic value for the surgical treatment of both TLE and ETLE [63].
-
Radiation doses
Based on activities administered between 125 and 250 MBq and based on the EANM dosage card v.01.02.20 in children as recommended by the EANM Procedure Guidelines for Brain PET Imaging using [18F]FDG, version 3, an effective dose of 0.019 mSv/MBq in adults and 0.056 mSv/MBq in children can be assumed [20, 157]. With the possibility of lowering activities, probably by a factor of 2 or more, with the use of high sensitivity digital systems (TOF < 400ps) and/or long axial field of view systems, the effective dose will be reduced via reduced tracer dose administration, but limited data are available at present to give clear recommendations [20].
Other tracers for epilepsy imaging
In this section, additional radioligands are presented, which are complementary, clinically relevant in the pre-surgical work-up of epilepsy or may contribute to a more detailed characterization and understanding of this disease.
[11C]/[18F]flumazenil
Gamma-aminobutyric acid (GABA) is the most important inhibitory neurotransmitter in the central nervous system. Flumazenil blocks the benzodiazepine sites on GABA-A receptors and thus antagonizes the action benzodiazepines have on the central nervous system. Flumazenil was introduced in 1987, and over time it was used as an antidote for the treatment of benzodiazepine overdoses. In epileptogenic regions a reduced level of benzodiazepine receptors was found by some authors [158], others reported false lateralization [159]. The PET radioligand [11C]flumazenil ([11C]FMZ) is selective for the GABA-A receptor subunits α1–3 and α5 [160]. [11C]FMZ binding is reduced in the hippocampi and other temporal lobe regions of individuals with refractory TLE even in the presence of a normal MRI [161] and in cryptogenic frontal epilepsy [159]. Similarly [11C]FMZ-PET has been used for detection of neocortical extratemporal epilepsy [162]. PET acquisition is usually performed 20 to 40 min after the injection of 370–555 MBq of [11C]FMZ. However, full quantification with an arterial input function leads to higher detection rates of the known epileptogenic lesion hippocampal sclerosis compared to summed static images [163]. Treatment with benzodiazepines, γ-vinyl-GABA and tiagabine should be disrupted one month before PET acquisition. In a recent meta-analysis, [11C]FMZ displayed an overall sensitivity of 62% (95% CI: 49–73%) and specificity of 73% (95% CI: 59–84%) for the localization of the epileptogenic zone and to provide evidence for practitioners’ clinical decision-making [77]. The area of decreased [11C]FMZ binding is often smaller than that of [18F]FDG hypometabolism (48%) or larger than that of the MRI abnormality (28%) [159]. Moreover, [11C]FMZ binding is positively correlated with interictal interval, and negatively correlated with seizure frequency [164, 165]. Nevertheless, the added value of FMZ as compared to [18F]FDG-PET has not been fully demonstrated with also false lateralization of epileptic focus reported by FMZ-PET in a study using quantification with the partial saturation method [166]. Moreover, the use of [11C]FMZ in clinical practice has been limited by its short half-life and necessitating an on-site cyclotron for production. 18F-labelled FMZ tracers might be the alternative of choice for patients with refractory epilepsy, but are not routinely in clinical use [167]. There are some compounds available, including 5-(2′-[18F]fluoroethyl) flumazenil ([18F]FEFMZ), 3-(2′-[18F]fluoroflumazenil ([18F]FFMZ), 5-(2′-[18F]fluoroethyl)flumazenil ([18F]FEF), and [18F]flumazenil ([18F]FMZ). Compared to [11C]FMZ, [18F]FEFMZ has lower receptor affinity and higher nonspecific binding due to faster metabolism and faster kinetics. [18F]FFMZ also has faster kinetics than [11C]FMZ [167]. Static images can be obtained as early as 10 to 15 min of the injection 197 to 370 MBq of [18F]FMZ. Binding of FMZ is, however, more indicative of the expression of α1 rather than α5 subunits of the GABA-A receptor with potential impact of benzodiazepine receptor binding medications [168]. New tracers like [11C]Ro15-4513 have approximately nanomolar affinity for GABAA receptors, with approximately 10–15 times higher affinity for those receptors containing α5 than for those that do not. Clinical validity has not been demonstrated for [11C]Ro15-4513. However, a recent study in MRI-negative TLE produced, from a single injection, two parametric maps (one each for α1 and α5) via bandpass analysis showing bilaterally increased α5 binding, likely linked to poor memory function, and ipsilaterally decreased α1 binding as with flumazenil radiopharmaceuticals [169].
[11C]methyl-L-tryptophan
Alpha-[11C]methyl-L-tryptophan ([11C]AMT) PET shows increased tracer uptake interictally in epileptogenic tubers only of patients with tuberous sclerosis (TSC1 and TSC2) and in dysplastic cortex with an excellent agreement in seizure focus lateralization between ictal scalp EEG and [11C]AMT. It is also found to be more localizing in patients with non-lateralized ictal EEG for a successful surgical intervention. For PET imaging, fasting for 6 h to obtain stable plasma tryptophan and large neutral amino acid levels is advised with image acquisition 25 min after tracer injection (3,7 MBg/kg) under sedation, if needed. To achieve a seizure-free outcome after surgery with an accuracy of ~83% for the detection of epileptogenic tubers a cut-off threshold uptake ratio relative to normal-appearing cortex of 1.02 is recommended, evaluated based on image fusion with MRI for quantitative [11C]AMT PET assessment [170,171,172]. The tracer uptake appears to be due to the activation of the kynurenine pathway, leading to the production of quinolinic acid, a proconvulsant [173]. With a short physical half-life of 11C, the radiation exposure is low and comparable to other 11C-labelled amino-acid tracers. Because facilities with an on-site cyclotron are needed, [11C]AMT is only used in few NM units.
Radiolabelled amino-acid compounds
Published data within radiolabelled amino acid (AA) compounds have primary been using [11C]MET, O-(2-[18F]fluoroethyl)-L-tyrosine ([18F]FET) and also 3,4-dihydroxy-6-[18F]fluoro-L-phenylalanine ([18F]FDOPA). The performance, interpretation, and reporting of PET scans follow the principles for glioma as outlined in recently published procedure guidelines by the clinical and nuclear imaging societies [174, 175]. The increased AA uptake in glioma is caused by an upregulation of the L-aminoacid transporter (LAT). The primary clinical use is in the surgical workup of medically refractory epilepsy to distinguish slowly progressing ambiguous and focal MRI lesions that may be caused by ganglioglioma or predominantly low-grade astrocytoma/oligodendroglioma from dysembryoplastic neuroepithelial tumour (DNT) or FCD. Distinguishing between different pathologies has a significant impact on patient care as it may set the indication for surgery, as the latter are at risk for malignant progression, while the former usually have a benign course. Furthermore, postsurgical seizure outcome is dependent on complete excision of the lesion, and AA PET may determine the glioma extension that usually requires a more limited removal than FCD, where a wider cortical resection using invasive electroencephalography may be recommended. At the group level, DNT and FCD show lower AA uptake than astrocytoma, oligodendroglioma, and ganglioglioma [113, 176, 177], but the literature is not sufficiently large to consistently separate the two groups [178, 179]. Approximately 30% of low grade glioma are inactive using [18F]FET [180, 181], and it may be in the same order for [11C]MET and [18F]FDOPA [182, 183], while some DNT and FCD show up to moderately increased activity [113, 178, 179, 184, 185]. However, there is agreement that a marked increased AA activity uptake strongly supports the neoplastic nature of a lesion. The report of a particularly favourable diagnostic accuracy of [11C]MET (AUC: 0.95) may be caused by a disproportionately large fraction of glioma with an oligodendroglial component [178], which are generally more active than the astrocytoma [186]. Single case reports using [11C]MET PET show marked uptake in chronic progressive encephalitis such as Rasmussen syndrome that may be associated with inflammation or epileptogenic activity and similar marked uptake in meningio-angiomatosis (Sturge-Weber syndrome) [187, 188].
Serotonin receptor ligands
The 5-hydroxytryptamine receptor 1A (5-HT1A) receptor is a subtype of serotonin receptor located in presynaptic and postsynaptic regions. Several 5-HT1A receptor radioligands such as [18F]FCWAY100635, [11C]WAY100635, and [18F]MPPF have been developed over the years demonstrating a lower binding in the ipsilateral temporal lobe, which may help to identify the epileptogenic zone. Moreover, the overwhelming majority of studies also observed a reduced binding in the brainstem and limbic connections. The latter might explain the affective symptoms that can appear in patients with TLE. In contrast, an increased binding potential contralateral to the epileptogenic side in patients with TLE is described. This might be explained by differences in tracer affinity as [18F]MPPF is more sensitive to serotonin than [18F]FCWAY100635 and [11C]WAY100635 [189]. Additionally, ASM significantly influences the plasma free fraction of [18F]FCWAY100635 [190]. In summary, 5-HT1A receptor radioligand imaging is more of a scientific interest and has not yet been introduced into the clinical routine of epilepsy imaging.
Other PET tracers
Numerous other radiotracers for different targets have been also used in epilepsy imaging research, such as synaptic density, neuroinflammation, neurotransmitter systems including the opioid system, but are so far not used in clinical practice.
Synaptic density
Synaptic vesicle glycoprotein 2A (SV2A) is a marker of synaptic density. Moreover, it is the binding site for levetiracetam, an ASM [191]. Lower SV2A has been detected in epileptic lesions both in TLE with medial temporal lobe sclerosis (37% ± 19%) using [11C]UCB-J and in FCD type 2 (27% ± 10%) using [18F]SynVesT-1 [192, 193]. The decrease in Sv2A is correlated with a reduction in [18F]FDG uptake.
Neuroinflammation
It has been demonstrated that epilepsy can cause neuroinflammation but also the other way around. Several studies reported an increase in translocator protein (TSPO) signal in the epileptic lesions, as proxy for neuroinflammation. Similar to synaptic density, the increased TSPO signal is associated with a with a reduction in [18F]FDG uptake [194,195,196]. Additionally, TSPO increases in the contralateral side have been described, illustrating both focal and distributed neuroinflammation [196,197,198].
Neurotransmitter
Besides GABA and serotonin, which are described above, other neurotransmitter systems have also been investigated in epilepsy. Glutamate transport has been examined using a radioligand for NMDA receptors, [18F]GE-179, and a radioligand for metabotropic glutamate receptor type 5 (mGluR5), [11C]ABP688. In focal epilepsy, clusters of increased [18F]GE-179 binding were found. [199, 200] In addition, global [18F]GE-179 binding was higher, except in patients taking antidepressant drugs in whom [18F]GE-179 was decreased [199]. In focal cortical dysplasia lower [11C]ABP688 binding was found in the lesion [201]. Dopamine neurotransmission has been investigated using multiple targets such as [18F]FDOPA (analog of levodopa), [18F]fallypride (D2/D3 receptors), [11C]SCH23390 (D1 receptor), [11C]raclopride (D2 receptor), and [11C]PE2I (dopamine transporter) with generally reduced uptake [202,203,204,205,206,207,208]. Increased [18F]FDOPA uptake was described in TLE in the epileptogenic region and increased [11C]raclopride uptake in the striatum and thalamus in myoclonic epilepsies [209, 210]; subcortical decrease of [18F]FDOPA uptake was found in various types of drug resistant epilepsy, including TLE [202, 203]. Lastly, nicotinic neurotransmission using the α4β2-nicotinic acetylcholine receptor ligand [18F]F-A-85380 has been investigated in autosomal dominant nocturnal frontal lobe epilepsy and in idiopathic generalized epilepsy, in which regional binding increases were observed [199, 211].
Opioids
There are three main opioid receptor types, µ, κ, and δ [212]. Post-ictal increases in the nonselective (µ, κ, and δ) radioligand [11C]diprenorphine in TLE were observed in ipsilateral temporal region relative to the interictal state [213, 214]. Similarly, the δ antagonist [11C]methylnaltrindole ([11C]MeNTI) and the µ agonist [11C]carfentanil had higher binding in the epileptogenic region in TLE and the κ and µ antagonist [18F]cyclofoxy was higher in complex partial seizures in mesial temporal lobes, thalamus, basal ganglia, and frontal cortex. [215] In reading epilepsy [11C]diprenorphine binding was decreased in reading-associated cortical regions during reading-induced seizures [216].
Abbreviations
- [11C]AMT:
-
Alpha-[11C]methyl-L-tryptophan
- [11C]FMZ:
-
[11C]flumazenil
- [11C]MET:
-
L-([11C]methyl)methionine
- [18F]FDG:
-
[18F]fluorodeoxyglucose
- AA:
-
Amino acid
- ASM:
-
Anti-seizure medication
- EANM:
-
European Association of Nuclear Medicine
- ECD:
-
Ethyl cystine dimer
- EEG:
-
Electroencephalography
- ETLE:
-
Extra-temporal lobe epilepsy
- FCD:
-
Focal cortical dysplasia
- GABA:
-
Gamma-aminobutyric acid
- HMPAO:
-
Hexamethylpropyleneamine oxime
- MCD:
-
Malformation of cortical development
- MRI:
-
Magnetic resonance imaging
- NM:
-
Nuclear Medicine
- PET:
-
Positron emission tomography
- SISCOM:
-
Subtraction of ictal and inter-ictal SPECT co-registered to MRI
- SOZ:
-
Seizure onset zone
- SPECT:
-
Single-Photon Emission Computed Tomography
- TLE:
-
Temporal lobe epilepsy
References
World Health Organization. Epilepsy: a public health imperative [Internet]. Geneva: World Health Organization; 2019. Available from: https://apps.who.int/iris/handle/10665/325293.
Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–21.
Sultana B, Panzini M-A, Veilleux Carpentier A, Comtois J, Rioux B, Gore G, et al. Incidence and prevalence of drug-resistant epilepsy: a systematic review and meta-analysis. Neurology. 2021;96:805–17.
Picot M-C, Baldy-Moulinier M, Daurs J-P, Dujols P, Crespel A. The prevalence of epilepsy and pharmacoresistant epilepsy in adults: a population-based study in a Western European country. Epilepsia. 2008;49:1230–8.
Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–9.
Semah F, Picot M-C, Adam C, Broglin D, Arzimanoglou A, Bazin B, et al. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology. 1998;51:1256–62.
Rosati A, De Masi S, Guerrini R. Antiepileptic drug treatment in children with epilepsy. CNS Drugs. 2015;29:847–63.
Witt J-A, Elger CE, Helmstaedter C. Adverse cognitive effects of antiepileptic pharmacotherapy: each additional drug matters. Eur Neuropsychopharmacol. 2015;25:1954–9.
de Tisi J, Bell GS, Peacock JL, McEvoy AW, Harkness WF, Sander JW, et al. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet. 2011;378:1388–95.
Dwivedi R, Ramanujam B, Chandra PS, Sapra S, Gulati S, Kalaivani M, et al. Surgery for drug-resistant epilepsy in children. N Engl J Med. 2017;377:1639–47.
Lamberink HJ, Otte WM, Blümcke I, Braun KPJ, Aichholzer M, Amorim I, et al. Seizure outcome and use of antiepileptic drugs after epilepsy surgery according to histopathological diagnosis: a retrospective multicentre cohort study. Lancet Neurol. 2020;19:748–57.
Picot M-C, Jaussent A, Neveu D, Kahane P, Crespel A, Gelisse P, et al. Cost-effectiveness analysis of epilepsy surgery in a controlled cohort of adult patients with intractable partial epilepsy: a 5-year follow-up study. Epilepsia. 2016;57:1669–79.
Jayakar P, Gaillard WD, Tripathi M, Libenson MH, Mathern GW, Cross JH, et al. Diagnostic test utilization in evaluation for resective epilepsy surgery in children. Epilepsia. 2014;55:507–18.
Zanotti-Fregonara P, Laforest R, Wallis JW. Fetal radiation dose from 18 F-FDG in pregnant patients imaged with PET, PET/CT, and PET/MR. J Nucl Med. 2015;56:1218–22.
Zanotti-Fregonara P. Radiation absorbed dose to the embryo and fetus from radiopharmaceuticals. Semin Nucl Med. 2022;52:140–8.
Mattsson S, Johansson L, Leide Svegborn S, Liniecki J, Noßke D, Riklund KÅ, et al. ICRP publication 128: radiation dose to patients from radiopharmaceuticals: a compendium of current information related to frequently used substances. Ann ICRP. 2015;44:7–321.
Marshall DSC, Newberry NR, Ryan PJ. Measurement of the secretion of technetium-99m hexamethylpropylene amine oxime into breast milk. Eur J Nucl Med. 1996;23:1634–5.
Hicks RJ, Binns D, Stabin MG. Pattern of uptake and excretion of (18)F-FDG in the lactating breast. J Nucl Med. 2001;42:1238–42.
Jahreis I, Bascuñana P, Ross TL, Bankstahl JP, Bankstahl M. Choice of anesthesia and data analysis method strongly increases sensitivity of 18F-FDG PET imaging during experimental epileptogenesis. Bauckneht M, editor. PLoS ONE. 2021;16:e0260482.
Guedj E, Varrone A, Boellaard R, Albert NL, Barthel H, van Berckel B, et al. EANM procedure guidelines for brain PET imaging using [18F]FDG, version 3. Eur J Nucl Med Mol Imaging. 2022;49:632–51.
de Laat NN, Tolboom N, Leijten FSS. Optimal timing of interictal FDG-PET for epilepsy surgery: a systematic review on time since last seizure. Epilepsia Open. 2022;7:512–7.
Leonard JP, Nowotnik DP, Neirinckx RD. Technetium-99m-d, 1-HM-PAO: a new radiopharmaceutical for imaging regional brain perfusion using SPECT–a comparison with iodine-123 HIPDM. J Nucl Med. 1986;27:1819–23.
Tousseyn S, Krishnan B, Wang ZI, Wongwiangjunt S, Nayak CS, Mosher JC, et al. Connectivity in ictal single photon emission computed tomography perfusion: a cortico-cortical evoked potential study. Brain. 2017;140:1872–84.
Devous MD, Thisted RA, Morgan GF, Leroy RF, Rowe CC. SPECT brain imaging in epilepsy: a meta-analysis. J Nucl Med. 1998;39:285–93.
Newton MR, Berkovic SF, Austin MC, Rowe CC, McKay WJ, Bladin PF. SPECT in the localisation of extratemporal and temporal seizure foci. J Neurol Neurosurg Psychiatry. 1995;59:26–30.
Krsek P, Kudr M, Jahodova A, Komarek V, Maton B, Malone S, et al. Localizing value of ictal SPECT is comparable to MRI and EEG in children with focal cortical dysplasia. Epilepsia. 2013;54:351–8.
Kim DW, Lee SK, Moon H-J, Jung K-Y, Chu K, Chung C-K. Surgical treatment of nonlesional neocortical epilepsy: long-term longitudinal study. JAMA Neurol. 2017;74:324–31.
Van Paesschen W, Dupont P, Van Driel G, Van Billoen H, Maes A. SPECT perfusion changes during complex partial seizures in patients with hippocampal sclerosis. Brain. 2003;126:1103–11.
O’Brien TJ, So EL, Mullan BP, Hauser MF, Brinkmann BH, Bohnen NI, et al. Subtraction ictal SPECT co-registered to MRI improves clinical usefulness of SPECT in localizing the surgical seizure focus. Neurology. 1998;50:445–54.
O’Brien TJ, So EL, Mullan BP, Cascino GD, Hauser MF, Brinkmann BH, et al. Subtraction peri-ictal SPECT is predictive of extratemporal epilepsy surgery outcome. Neurology. 2000;55:1668–77.
O’Brien TJ, So EL, Cascino GD, Hauser MF, Marsh WR, Meyer FB, et al. Subtraction SPECT coregistered to MRI in focal malformations of cortical development: localization of the epileptogenic zone in epilepsy surgery candidates. Epilepsia. 2004;45:367–76.
Kaiboriboon K, Lowe VJ, Chantarujikapong SI, Hogan RE. The usefulness of subtraction ictal SPECT coregistered to MRI in single- and dual-headed SPECT cameras in partial epilepsy. Epilepsia. 2002;43:408–14.
Véra P, Kaminska A, Cieuta C, Hollo A, Stiévenart JL, Gardin I, et al. Use of subtraction ictal SPECT co-registered to MRI for optimizing the localization of seizure foci in children. J Nucl Med. 1999;40:786–92.
Bouilleret V, Valenti MP, Hirsch E, Semah F, Namer IJ. Correlation between PET and SISCOM in temporal lobe epilepsy. J Nucl Med. 2002;43:991–8.
Sierra-Marcos A, Maestro I, Falcón C, Donaire A, Setoain J, Aparicio J, et al. Ictal EEG-fMRI in localization of epileptogenic area in patients with refractory neocortical focal epilepsy. Epilepsia. 2013;54:1688–98.
Desai A, Bekelis K, Thadani VM, Roberts DW, Jobst BC, Duhaime A-C, et al. Interictal PET and ictal subtraction SPECT: sensitivity in the detection of seizure foci in patients with medically intractable epilepsy. Epilepsia. 2013;54:341–50.
Schneider F, Irene Wang Z, Alexopoulos AV, Almubarak S, Kakisaka Y, Jin K, et al. Magnetic source imaging and ictal SPECT in MRI-negative neocortical epilepsies: additional value and comparison with intracranial EEG: MSI/SPECT in Nonlesional Neocortical Epilepsy. Epilepsia. 2013;54:359–69.
Bell ML, Rao S, So EL, Trenerry M, Kazemi N, Matt Stead S, et al. Epilepsy surgery outcomes in temporal lobe epilepsy with a normal MRI. Epilepsia. 2009;50:2053–60.
Ahnlide J-A, Rosén I, Lindén-Mickelsson Tech P, Källén K. Does SISCOM contribute to favorable seizure outcome after epilepsy surgery? Epilepsia. 2007;48:579–88.
Kudr M, Krsek P, Marusic P, Tomasek M, Trnka J, Michalova K, et al. SISCOM and FDG-PET in patients with non-lesional extratemporal epilepsy: correlation with intracranial EEG, histology, and seizure outcome. Epileptic Disord. 2013;15:3–13.
Alim-Marvasti A, Vakharia VN, Duncan JS. Multimodal prognostic features of seizure freedom in epilepsy surgery. J Neurol Neurosurg Psychiatry. 2022;93:499–508.
von Oertzen TJ, Mormann F, Urbach H, Reichmann K, Koenig R, Clusmann H, et al. Prospective use of subtraction ictal SPECT coregistered to MRI (SISCOM) in presurgical evaluation of epilepsy: SISCOM in Epilepsy Surgery. Epilepsia. 2011;52:2239–48.
Chen T, Guo L. The role of SISCOM in preoperative evaluation for patients with epilepsy surgery: a meta-analysis. Seizure. 2016;41:43–50.
Spencer SS. The relative contributions of MRI, SPECT, and PET imaging in epilepsy. Epilepsia. 1994;35:S72-89.
Bordonne M, Chawki MB, Marie P-Y, Zaragori T, Roch V, Grignon R, et al. High-quality brain perfusion SPECT images may be achieved with a high-speed recording using 360° CZT camera. EJNMMI Phys. 2020;7:65.
Kapucu ÖL, Nobili F, Varrone A, Booij J, Vander Borght T, Någren K, et al. EANM procedure guideline for brain perfusion SPECT using 99mTc-labelled radiopharmaceuticals, version 2. Eur J Nucl Med Mol Imaging. 2009;36:2093–102.
Kim S, Holder DL, Laymon CM, Tudorascu DL, Deeb EL, Panigrahy A, et al. Clinical value of the first dedicated, commercially available automatic injector for ictal brain SPECT in presurgical evaluation of pediatric epilepsy: comparison with manual injection. J Nucl Med. 2013;54:732–8.
Setoain X, Campos F, Donaire A, Mayoral M, Perissinotti A, Niñerola-Baizan A, et al. How to inject ictal SPECT? From manual to automated injection. Epilepsy Res. 2021;175:106691.
O’brien TJ, O’connor MK, Mullan BP, Brinkmann BH, Hanson D, Jack CR, et al. Subtraction ictal SPET co-registered to MRI in partial epilepsy: description and technical validation of the method with phantom and patient studies. Nucl Med Commun. 1998;19:31–46.
Van Paesschen W. Ictal SPECT. Epilepsia. 2004;45:35–40.
De Coster L, Van Laere K, Cleeren E, Baete K, Dupont P, Van Paesschen W, et al. On the optimal z-score threshold for SISCOM analysis to localize the ictal onset zone. EJNMMI Res. 2018;8:34.
Rosenow F. Presurgical evaluation of epilepsy. Brain. 2001;124:1683–700.
Nelissen N, Van Paesschen W, Baete K, Van Laere K, Palmini A, Van Billoen H, et al. Correlations of interictal FDG-PET metabolism and ictal SPECT perfusion changes in human temporal lobe epilepsy with hippocampal sclerosis. NeuroImage. 2006;32:684–95.
Henry TR, Babb TL, Engel J, Mazziotta JC, Phelps ME, Crandall PH. Hippocampal neuronal loss and regional hypometabolism in temporal lobe epilepsy. Ann Neurol. 1994;36:925–7.
Vielhaber S, Von Oertzen JH, Kudin AF, Schoenfeld A, Menzel C, Biersack H, et al. Correlation of hippocampal glucose oxidation capacity and interictal FDG-PET in temporal lobe epilepsy. Epilepsia. 2003;44:193–9.
Theodore WH. Antiepileptic drugs and cerebral glucose metabolism. Epilepsia. 1988;29:S48-55.
Gaillard WD, Zeffiro T, Fazilat S, DeCarli C, Theodore WH. Effect of valproate on cerebral metabolism and blood flow: an 18F-2-deoxyglusose and 15O water positron emission tomography study. Epilepsia. 1996;37:515–21.
Savic I, Altshuler L, Baxter L, Engel J. Pattern of interictal hypometabolism in PET scans with fludeoxyglucose F 18 reflects prior seizure types in patients with mesial temporal lobe seizures. Arch Neurol. 1997;54:129–36.
Gaillard WD, Weinstein S, Conry J, Pearl PL, Fazilat S, Fazilat S, et al. Prognosis of children with partial epilepsy: MRI and serial 18FDG-PET. Neurology. 2007;68:655–9.
Chassoux F, Artiges E, Semah F, Desarnaud S, Laurent A, Landre E, et al. Determinants of brain metabolism changes in mesial temporal lobe epilepsy. Epilepsia. 2016;57:907–19.
Chassoux F. Metabolic changes and electro-clinical patterns in mesio-temporal lobe epilepsy: a correlative study. Brain. 2004;127:164–74.
Lagarde S, Boucekine M, McGonigal A, Carron R, Scavarda D, Trebuchon A, et al. Relationship between PET metabolism and SEEG epileptogenicity in focal lesional epilepsy. Eur J Nucl Med Mol Imaging. 2020;47:3130–42.
Tomás J, Pittau F, Hammers A, Bouvard S, Picard F, Vargas MI, et al. The predictive value of hypometabolism in focal epilepsy: a prospective study in surgical candidates. Eur J Nucl Med Mol Imaging. 2019;46:1806–16.
Salvadori J, Imbert L, Perrin M, Karcher G, Lamiral Z, Marie P-Y, et al. Head-to-head comparison of image quality between brain 18F-FDG images recorded with a fully digital versus a last-generation analog PET camera. EJNMMI Res. 2019;9:61.
Boscolo Galazzo I, Mattoli MV, Pizzini FB, De Vita E, Barnes A, Duncan JS, et al. Cerebral metabolism and perfusion in MR-negative individuals with refractory focal epilepsy assessed by simultaneous acquisition of 18 F-FDG PET and arterial spin labeling. NeuroImage: Clin. 2016;11:648–57.
Guo K, Cui B, Shang K, Hou Y, Fan X, Yang H, et al. Assessment of localization accuracy and postsurgical prediction of simultaneous 18F-FDG PET/MRI in refractory epilepsy patients. Eur Radiol. 2021;31:6974–82.
Guo K, Wang J, Cui B, Wang Y, Hou Y, Zhao G, et al. [18F]FDG PET/MRI and magnetoencephalography may improve presurgical localization of temporal lobe epilepsy. Eur Radiol. 2022;32:3024–34.
Mendes Coelho VC, Morita ME, Amorim BJ, Ramos CD, Yasuda CL, Tedeschi H, et al. Automated online quantification method for 18F-FDG positron emission tomography/CT improves detection of the epileptogenic zone in patients with pharmacoresistant epilepsy. Front Neurol. 2017;8:453.
Menon RN, Radhakrishnan A, Parameswaran R, Thomas B, Kesavadas C, Abraham M, et al. Does F-18 FDG-PET substantially alter the surgical decision-making in drug-resistant partial epilepsy? Epilepsy Behav. 2015;51:133–9.
Steinbrenner M, Duncan JS, Dickson J, Rathore C, Wächter B, Aygun N, et al. Utility of 18F-fluorodeoxyglucose positron emission tomography in presurgical evaluation of patients with epilepsy: a multicenter study. Epilepsia. 2022;63:1238–52.
Chassoux F, Landré E, Mellerio C, Turak B, Mann MW, Daumas-Duport C, et al. Type II focal cortical dysplasia: electroclinical phenotype and surgical outcome related to imaging: phenotype and imaging in TTFCD. Epilepsia. 2012;53:349–58.
Chandra PS, Salamon N, Huang J, Wu JY, Koh S, Vinters HV, et al. FDG-PET/MRI coregistration and diffusion-tensor imaging distinguish epileptogenic tubers and cortex in patients with tuberous sclerosis complex: a preliminary report. Epilepsia. 2006;47:1543–9.
Kumar A, Chugani HT. The role of radionuclide imaging in epilepsy, part 1: sporadic temporal and extratemporal lobe epilepsy. J Nucl Med Technol. 2017;45:14–21.
Chassoux F, Rodrigo S, Semah F, Beuvon F, Landre E, Devaux B, et al. FDG-PET improves surgical outcome in negative MRI Taylor-type focal cortical dysplasias. Neurology. 2010;75:2168–75.
Theodore WH, Sato S, Kufta CV, Gaillard WD, Kelley K. FDG-positron emission tomography and invasive EEG: seizure focus detection and surgical outcome. Epilepsia. 1997;38:81–6.
Kamm J, Boles Ponto LL, Manzel K, Gaasedelen OJ, Nagahama Y, Abel T, et al. Temporal lobe asymmetry in FDG-PET uptake predicts neuropsychological and seizure outcomes after temporal lobectomy. Epilepsy Behav. 2018;78:62–7.
Niu N, Xing H, Wu M, Ma Y, Liu Y, Ba J, et al. Performance of PET imaging for the localization of epileptogenic zone in patients with epilepsy: a meta-analysis. Eur Radiol. 2021;31:6353–66.
Guedj E, Bonini F, Gavaret M, Trébuchon A, Aubert S, Boucekine M, et al. 18 FDG-PET in different subtypes of temporal lobe epilepsy: SEEG validation and predictive value. Epilepsia. 2015;56:414–21.
Bartolomei F, Lagarde S, Wendling F, McGonigal A, Jirsa V, Guye M, et al. Defining epileptogenic networks: contribution of SEEG and signal analysis. Epilepsia. 2017;58:1131–47.
Doyen M, Chawki MB, Heyer S, Guedj E, Roch V, Marie P-Y, et al. Metabolic connectivity is associated with seizure outcome in surgically treated temporal lobe epilepsies: a 18F-FDG PET seed correlation analysis. NeuroImage: Clin. 2022;36:103210.
Chassoux F, Artiges E, Semah F, Laurent A, Landré E, Turak B, et al. 18 F-FDG-PET patterns of surgical success and failure in mesial temporal lobe epilepsy. Neurology. 2017;88:1045–53.
Cahill V, Sinclair B, Malpas CB, McIntosh AM, Chen Z, Vivash LE, et al. Metabolic patterns and seizure outcomes following anterior temporal lobectomy: Cahill et al: Metabolic Patterns. Ann Neurol. 2019;85:241–50.
Choi JY, Kim SJ, Hong SB, Seo DW, Hong SC, Kim B-T, et al. Extratemporal hypometabolism on FDG PET in temporal lobe epilepsy as a predictor of seizure outcome after temporal lobectomy. Eur J Nucl Med Mol Imaging. 2003;30:581–7.
Jokeit H. Prefrontal asymmetric interictal glucose hypometabolism and cognitive impairment in patients with temporal lobe epilepsy. Brain. 1997;120:2283–94.
Koutroumanidis M, Hennessy MJ, Seed PT, Elwes RDC, Jarosz J, Morris RG, et al. Significance of interictal bilateral temporal hypometabolism in temporal lobe epilepsy. Neurology. 2000;54:1811–21.
Griffith HR, Richardson E, Pyzalski RW, Bell B, Dow C, Hermann BP, et al. Memory for famous faces and the temporal pole: functional imaging findings in temporal lobe epilepsy. Epilepsy Behav. 2006;9:173–80.
Fonseca AT-D, Guedj E, Alario F-X, Laguitton V, Mundler O, Chauvel P, et al. Brain regions underlying word finding difficulties in temporal lobe epilepsy. Brain. 2009;32:2772–84.
Bouilleret V, Dupont S, Spelle L, Baulac M, Samson Y, Semah F. Insular cortex involvement in mesiotemporal lobe epilepsy: a positron emission tomography study. Ann Neurol. 2002;51:202–8.
Newberg AB, Alavi A, Berlin J, Mozley PD, O’Connor M, Sperling M. Ipsilateral and contralateral thalamic hypometabolism as a predictor of outcome after temporal lobectomy for seizures. J Nucl Med. 2000;41:1964–8.
Laurent A, Artiges E, Mellerio C, Boutin-Watine M, Landré E, Semah F, et al. Metabolic correlates of cognitive impairment in mesial temporal lobe epilepsy. Epilepsy Behav. 2020;105:106948.
Rathore C, Dickson JC, Teotónio R, Ell P, Duncan JS. The utility of 18F-fluorodeoxyglucose PET (FDG PET) in epilepsy surgery. Epilepsy Res. 2014;108:1306–14.
Lee SK, Lee SY, Kim K-K, Hong K-S, Lee D-S, Chung C-K. Surgical outcome and prognostic factors of cryptogenic neocortical epilepsy. Ann Neurol. 2005;58:525–32.
Wong CH, Bleasel A, Wen L, Eberl S, Byth K, Fulham M, et al. Relationship between preoperative hypometabolism and surgical outcome in neocortical epilepsy surgery: Preoperative Hypometabolism and Outcome. Epilepsia. 2012;53:1333–40.
Kim YK, Lee DS, Lee SK, Chung CK, Chung J-K, Lee MC. (18)F-FDG PET in localization of frontal lobe epilepsy: comparison of visual and SPM analysis. J Nucl Med. 2002;43:1167–74.
Kim DW, Lee SK, Yun C-H, Kim K-K, Lee DS, Chung C-K, et al. Parietal lobe epilepsy: the semiology, yield of diagnostic workup, and surgical outcome. Epilepsia. 2004;45:641–9.
Kun Lee S, Young Lee S, Kim D-W, Soo Lee D, Chung C-K. Occipital lobe epilepsy: clinical characteristics, surgical outcome, and role of diagnostic modalities. Epilepsia. 2005;46:688–95.
Kim S, Lee DS, Lee SK, Kim YK, Kang KW, Chung CK, et al. Diagnostic performance of [18 F]FDG-PET and Ictal [99m Tc]-HMPAO SPECT in occipital lobe epilepsy. Epilepsia. 2001;42:1531–40.
Fernández S, Donaire A, Serès E, Setoain X, Bargalló N, Falcón C, et al. PET/MRI and PET/MRI/SISCOM coregistration in the presurgical evaluation of refractory focal epilepsy. Epilepsy Res. 2015;111:1–9.
Desarnaud S, Mellerio C, Semah F, Laurent A, Landre E, Devaux B, et al. 18F-FDG PET in drug-resistant epilepsy due to focal cortical dysplasia type 2: additional value of electroclinical data and coregistration with MRI. Eur J Nucl Med Mol Imaging. 2018;45:1449–60.
Ding Y, Zhu Y, Jiang B, Zhou Y, Jin B, Hou H, et al. 18F-FDG PET and high-resolution MRI co-registration for pre-surgical evaluation of patients with conventional MRI-negative refractory extra-temporal lobe epilepsy. Eur J Nucl Med Mol Imaging. 2018;45:1567–72.
Traub-Weidinger T, Muzik O, Sundar LKS, Aull-Watschinger S, Beyer T, Hacker M, et al. Utility of absolute quantification in non-lesional extratemporal lobe epilepsy using FDG PET/MR imaging. Front Neurol. 2020;11:54.
Lee N, Radtke RA, Gray L, Burger PC, Montine TJ, DeLong GR, et al. Neuronal migration disorders: positron emission tomography correlations. Ann Neurol. 1994;35:290–7.
Poduri A, Golja A, Takeoka M, Bourgeois BFD, Connolly L, Riviello JJ. Focal cortical malformations can show asymmetrically higher uptake on interictal fluorine-18 fluorodeoxyglucose positron emission tomography (PET). J Child Neurol. 2007;22:232–7.
Franceschi M, Lucignani G, Del Sole A, Grana C, Bressi S, Minicucci F, et al. Increased interictal cerebral glucose metabolism in a cortical-subcortical network in drug naive patients with cryptogenic temporal lobe epilepsy. J Neurol Neurosurg Psychiatr. 1995;59:427–31.
Juhász C, Chugani DC, Muzik O, Watson C, Shah J, Shah A, et al. Is epileptogenic cortex truly hypometabolic on interictal positron emission tomography? Ann Neurol. 2000;48:88–96.
Alkonyi B, Juhász C, Muzik O, Asano E, Saporta A, Shah A, et al. Quantitative brain surface mapping of an electrophysiologic/metabolic mismatch in human neocortical epilepsy. Epilepsy Res. 2009;87:77–87.
Blumcke I, Spreafico R, Haaker G, Coras R, Kobow K, Bien CG, et al. Histopathological findings in brain tissue obtained during epilepsy surgery. N Engl J Med. 2017;377:1648–56.
Guerrini R, Dobyns WB. Malformations of cortical development: clinical features and genetic causes. Lancet Neurol. 2014;13:710–26.
Oegema R, Barakat TS, Wilke M, Stouffs K, Amrom D, Aronica E, et al. International consensus recommendations on the diagnostic work-up for malformations of cortical development. Nat Rev Neurol. 2020;16:618–35.
Krsek P, Maton B, Jayakar P, Dean P, Korman B, Rey G, et al. Incomplete resection of focal cortical dysplasia is the main predictor of poor postsurgical outcome. Neurology. 2009;72:217–23.
Kim SK, Na DG, Byun HS, Kim SE, Suh YL, Choi JY, et al. Focal cortical dysplasia: comparison of MRI and FDG-PET. J Comput Assist Tomogr. 2000;24:296–302.
Halac G, Delil S, Zafer D, Isler C, Uzan M, Comunoglu N, et al. Compatibility of MRI and FDG-PET findings with histopathological results in patients with focal cortical dysplasia. Seizure. 2017;45:80–6.
Phi JH, Paeng JC, Lee HS, Wang K-C, Cho B-K, Lee J-Y, et al. Evaluation of focal cortical dysplasia and mixed neuronal and glial tumors in pediatric epilepsy patients using 18 F-FDG and 11 C-methionine PET. J Nucl Med. 2010;51:728–34.
Bulteau C, Otsuki T, Delalande O. Epilepsy surgery for hemispheric syndromes in infants: hemimegalencepahly and hemispheric cortical dysplasia. Brain Dev. 2013;35:742–7.
Raghavendra S, Nooraine J, Shivakumar R, Iyer R, Rao R. Posterior quadrant disconnection for refractory epilepsy: a case series. Ann Indian Acad Neurol. 2014;17:392.
Kalbhenn T, Cloppenborg T, Wörmann FG, Blümcke I, Coras R, May TW, et al. Operative posterior disconnection in epilepsy surgery: experience with 29 patients. Epilepsia. 2019;60:1973–83.
Moosa ANV, Jehi L, Marashly A, Cosmo G, Lachhwani D, Wyllie E, et al. Long-term functional outcomes and their predictors after hemispherectomy in 115 children. Epilepsia. 2013;54:1771–9.
Traub-Weidinger T, Weidinger P, Gröppel G, Karanikas G, Wadsak W, Kasprian G, et al. Presurgical evaluation of pediatric epilepsy patients prior to hemispherotomy: the prognostic value of 18F-FDG PET. PED. 2016;18:683–8.
Weil AG, Lewis EC, Ibrahim GM, Kola O, Tseng C-H, Zhou X, et al. Hemispherectomy Outcome Prediction Scale: development and validation of a seizure freedom prediction tool. Epilepsia. 2021;62:1064–73.
Wirrell EC, Nabbout R, Scheffer IE, Alsaadi T, Bogacz A, French JA, et al. Methodology for classification and definition of epilepsy syndromes with list of syndromes: report of the ILAE Task Force on Nosology and Definitions. Epilepsia. 2022;63:1333–48.
Chu-Shore CJ, Major P, Camposano S, Muzykewicz D, Thiele EA. The natural history of epilepsy in tuberous sclerosis complex: epilepsy in TSC. Epilepsia. 2009;51:1236–41.
Jansen FE, Van Huffelen AC, Algra A, Van Nieuwenhuizen O. Epilepsy Surgery in tuberous sclerosis: a systematic review. Epilepsia. 2007;48:1477–84.
Koh S, Jayakar P, Resnick T, Alvarez L, Liit RE, Duchowny M. The localizing value of ictal SPECT in children with tuberous sclerosis complex and refractory partial epilepsy. Epileptic Disord. 1999;1:41–6.
Aboian MS, Wong-Kisiel LC, Rank M, Wetjen NM, Wirrell EC, Witte RJ. SISCOM in children with tuberous sclerosis complex-related epilepsy. Pediatr Neurol. 2011;45:83–8.
Rintahaka PJ, Chugani HT. Clinical role of positron emission tomography in children with tuberous sclerosis complex. J Child Neurol. 1997;12:42–52.
Szelies B, Herholz K, Heiss WD, Rackl A, Pawlik G, Wagner R, et al. Hypometabolic cortical lesions in tuberous sclerosis with epilepsy: demonstration by positron emission tomography. J Comput Assist Tomogr. 1983;7:946–53.
Asano E, Chugani DC, Muzik O, Shen C, Juhász C, Janisse J, et al. Multimodality imaging for improved detection of epileptogenic foci in tuberous sclerosis complex. Neurology. 2000;54:1976–84.
Chugani HT, Mazziotta JC, Phelps ME. Sturge-Weber syndrome: a study of cerebral glucose utilization with positron emission tomography. J Pediatr. 1989;114:244–53.
Juhasz C, Batista CEA, Chugani DC, Muzik O, Chugani HT. Evolution of cortical metabolic abnormalities and their clinical correlates in Sturge-Weber syndrome. Eur J Paediatr Neurol. 2007;11:277–84.
Alkonyi B, Chugani HT, Juhász C. Transient focal cortical increase of interictal glucose metabolism in Sturge-Weber syndrome: implications for epileptogenesis: hypermetabolism in Sturge-Weber Syndrome. Epilepsia. 2011;52:1265–72.
Chugani HT, Mazziotta JC, Engel J, Phelps ME. The lennox-gastaut syndrome: metabolic subtypes determined by 2-deoxy-2 [18F]fluoro-d-glucose positron emission tomography. Ann Neurol. 1987;21:4–13.
You SJ, Lee J-K, Ko T-S. Epilepsy surgery in a patient with Lennox-Gastaut syndrome and cortical dysplasia. Brain Dev. 2007;29:167–70.
Rintahaka PJ, Chugani HT, Messa C, Phelps ME. Hemimegalencephaly: evaluation with positron emission tomography. Pediatr Neurol. 1993;9:21–8.
Burke GJ, Fifer SA, Yoder J. Early detection of Rasmussen´s syndrome by brain SPECT imaging. Clin Nucl Med. 1992;17:730–1.
Lee JS, Juhász C, Kaddurah AK, Chugani HT. Patterns of cerebral glucose metabolism in early and late stages of Rasmussen’s syndrome. J Child Neurol. 2001;16:798–805.
Burneo JG, Hamilton M, Vezina W, Parrent A. Utility of Ictal SPECT in the presurgical evaluation of Rasmussen’s encephalitis. Can J Neurol Sci. 2006;33:107–10.
Fiorella DJ, Provenzale JM, Coleman RE, Crain BJ, Al-Sugair AA. (18)F-fluorodeoxyglucose positron emission tomography and MR imaging findings in Rasmussen encephalitis. AJNR Am J Neuroradiol. 2001;22:1291–9.
Tian M, Watanabe Y, Kang KW, Murakami K, Chiti A, Carrio I, et al. International consensus on the use of [18F]-FDG PET/CT in pediatric patients affected by epilepsy. Eur J Nucl Med Mol Imaging. 2021;48:3827–34.
Lemmens C, Montandon M-L, Nuyts J, Ratib O, Dupont P, Zaidi H. Impact of metal artefacts due to EEG electrodes in brain PET/CT imaging. Phys Med Biol. 2008;53:4417–29.
Zhou L, Dupont P, Baete K, Vanpaesschen W, Vanlaere K, Nuyts J. Detection of inter-hemispheric metabolic asymmetries in FDG-PET images using prior anatomical information. NeuroImage. 2009;44:35–42.
Rubí S, Setoain X, Donaire A, Bargalló N, Sanmartí F, Carreño M, et al. Validation of FDG-PET/MRI coregistration in nonlesional refractory childhood epilepsy. Epilepsia. 2011;52:2216–24.
Paldino MJ, Yang E, Jones JY, Mahmood N, Sher A, Zhang W, et al. Comparison of the diagnostic accuracy of PET/MRI to PET/CT-acquired FDG brain exams for seizure focus detection: a prospective study. Pediatr Radiol. 2017;47:1500–7.
Zhang M, Liu W, Huang P, Lin X, Huang X, Meng H, et al. Utility of hybrid PET/MRI multiparametric imaging in navigating SEEG placement in refractory epilepsy. Seizure. 2020;81:295–303.
Tóth M, Barsi P, Tóth Z, Borbély K, Lückl J, Emri M, et al. The role of hybrid FDG-PET/MRI on decision-making in presurgical evaluation of drug-resistant epilepsy. BMC Neurol. 2021;21:363.
Grouiller F, Delattre BMA, Pittau F, Heinzer S, Lazeyras F, Spinelli L, et al. All-in-one interictal presurgical imaging in patients with epilepsy: single-session EEG/PET/(f)MRI. Eur J Nucl Med Mol Imaging. 2015;42:1133–43.
Flaus A, Mellerio C, Rodrigo S, Brulon V, Lebon V, Chassoux F. 18F-FDG PET/MR in focal epilepsy: a new step for improving the detection of epileptogenic lesions. Epilepsy Res. 2021;178:106819.
Kim MA, Heo K, Choo MK, Cho JH, Park SC, Lee JD, et al. Relationship between bilateral temporal hypometabolism and EEG findings for mesial temporal lobe epilepsy: analysis of 18F-FDG PET using SPM. Seizure. 2006;15:56–63.
Wang K, Liu T, Zhao X, Xia X, Zhang K, Qiao H, et al. Comparative study of voxel-based epileptic foci localization accuracy between statistical parametric mapping and three-dimensional stereotactic surface projection. Front Neurol [Internet]. 2016 [cited 2022 Nov 27];7. Available from: http://journal.frontiersin.org/Article/https://doi.org/10.3389/fneur.2016.00164/abstract.
Archambaud F, Bouilleret V, Hertz-Pannier L, Chaumet-Riffaud P, Rodrigo S, Dulac O, et al. Optimizing statistical parametric mapping analysis of 18F-FDG PET in children. EJNMMI Res. 2013;3:2.
De Blasi B, Barnes A, Galazzo IB, Hua C, Shulkin B, Koepp M, et al. Age-specific 18 F-FDG image processing pipelines and analysis are essential for individual mapping of seizure foci in pediatric patients with intractable epilepsy. J Nucl Med. 2018;59:1590–6.
Mérida I, Jung J, Bouvard S, Le Bars D, Lancelot S, Lavenne F, et al. CERMEP-IDB-MRXFDG: a database of 37 normal adult human brain [18F]FDG PET, T1 and FLAIR MRI, and CT images available for research. EJNMMI Res. 2021;11:91.
Goffin K, Van Paesschen W, Dupont P, Baete K, Palmini A, Nuyts J, et al. Anatomy-based reconstruction of FDG-PET images with implicit partial volume correction improves detection of hypometabolic regions in patients with epilepsy due to focal cortical dysplasia diagnosed on MRI. Eur J Nucl Med Mol Imaging. 2010;37:1148–55.
Flaus A, Deddah T, Reilhac A, Leiris ND, Janier M, Merida I, et al. PET image enhancement using artificial intelligence for better characterization of epilepsy lesions. Front Med. 2022;9:1042706.
Tan Y-L, Kim H, Lee S, Tihan T, Ver Hoef L, Mueller SG, et al. Quantitative surface analysis of combined MRI and PET enhances detection of focal cortical dysplasias. Neuroimage. 2018;166:10–8.
Beheshti I, Sone D, Maikusa N, Kimura Y, Shigemoto Y, Sato N, et al. Pattern analysis of glucose metabolic brain data for lateralization of MRI-negative temporal lobe epilepsy. Epilepsy Res. 2020;167:106474.
Zhang Q, Liao Y, Wang X, Zhang T, Feng J, Deng J, et al. A deep learning framework for 18F-FDG PET imaging diagnosis in pediatric patients with temporal lobe epilepsy. Eur J Nucl Med Mol Imaging. 2021;48:2476–85.
For the EANM/SNMMI Paediatric Dosage Harmonization Working Group, Lassmann M, Treves ST. Paediatric radiopharmaceutical administration: harmonization of the 2007 EANM paediatric dosage card (version 1.5.2008) and the 2010 North American consensus guidelines. Eur J Nucl Med Mol Imaging. 2014;41:1036–41.
Sieghart W. Pharmacology of benzodiazepine receptors: an update. J Psychiatry Neurosci. 1994;19:24–9.
Ryvlin P. Clinical utility of flumazenil-PET versus [18F]fluorodeoxyglucose-PET and MRI in refractory partial epilepsy. A prospective study in 100 patients. Brain. 1998;121:2067–81.
Maziere M, Hantraye P, Prenant C, Sastre J, Comar D. Synthesis of ethyl 8-fluoro-5,6-dihydro5- [11C]methyl-6-oxo-4H-imidazo[1,5-a] [1,4]benzodiazepine-3-carboxylate (RO 15.1788–11C): a specific radioligand for the in vivo study of central benzodiazepine receptors by positron emission tomography. Int J Appl Radiat Isot. 1984;35:973–6.
Koepp MJ, Hammers A, Labbe C, Woermann FG, Brooks DJ, Duncan JS. 11C-flumazenil PET in patients with refractory temporal lobe epilepsy and normal MRI. Neurology. 2000;54:332–332.
Hammers A, Koepp MJ, Richardson MP, Hurlemann R, Brooks DJ, Duncan JS. Grey and white matter flumazenil binding in neocortical epilepsy with normal MRI. A PET study of 44 patients. Brain. 2003;126:1300–18.
Hammers A, Panagoda P, Heckemann RA, Kelsch W, Turkheimer FE, Brooks DJ, et al. [11 C]Flumazenil PET in temporal lobe epilepsy: do we need an arterial input function or kinetic modeling? J Cereb Blood Flow Metab. 2008;28:207–16.
Bouvard S, Costes N, Bonnefoi F, Lavenne F, Mauguière F, Delforge J, et al. Seizure-related short-term plasticity of benzodiazepine receptors in partial epilepsy: a [11C]flumazenil-PET study. Brain. 2005;128:1330–43.
Laufs H, Richardson MP, Salek-Haddadi A, Vollmar C, Duncan JS, Gale K, et al. Converging PET and fMRI evidence for a common area involved in human focal epilepsies. Neurology. 2011;77:904–10.
Ryvlin P, Bouvard S, Le Bars D, Mauguiere F. Transient and falsely lateralizing flumazenil-PET asymmetries in temporal lobe epilepsy. Neurology. 1999;53:1882–1882.
Vivash L, Gregoire M-C, Lau EW, Ware RE, Binns D, Roselt P, et al. 18F-flumazenil: a γ-aminobutyric acid A-specific PET radiotracer for the localization of drug-resistant temporal lobe epilepsy. J Nucl Med. 2013;54:1270–7.
Maeda J, Suhara T, Kawabe K, Okauchi T, Obayashi S, Hojo J, et al. Visualization of ?5 subunit of GABAA/benzodiazepine receptor by [11C]Ro15-4513 using positron emission tomography. Synapse. 2003;47:200–8.
McGinnity CJ, Riaño Barros DA, Hinz R, Myers JF, Yaakub SN, Thyssen C, et al. Αlpha 5 subunit-containing GABAA receptors in temporal lobe epilepsy with normal MRI. Brain Commun. 2021;3:fcaa190.
Fedi M, Reutens DC, Andermann F, Okazawa H, Boling W, White C, et al. α-[11C]-Methyl-l-tryptophan PET identifies the epileptogenic tuber and correlates with interictal spike frequency. Epilepsy Res. 2003;52:203–13.
Kagawa K, Chugani DC, Asano E, Juhász C, Muzik O, Shah A, et al. Epilepsy surgery outcome in children with tuberous sclerosis complex evaluated with α-[11C]methyl-L-tryptophan positron emission tomography (PET). J Child Neurol. 2005;20:429–38.
Chugani HT, Luat AF, Kumar A, Govindan R, Pawlik K, Asano E. [11C]-Methyl-L-tryptophan-PET in 191 patients with tuberous sclerosis complex. Neurology. 2013;81:674–80.
Chugani DC, Muzik O, Chakraborty P, Mangner T, Chugani HT. Human brain serotonin synthesis capacity measured in vivo with alpha-[C-11]methyl-L-tryptophan. Synapse. 1998;28:33–43.
Law I, Albert NL, Arbizu J, Boellaard R, Drzezga A, Galldiks N, et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 2019;46:540–57.
Piccardo A, Albert NL, Borgwardt L, Fahey FH, Hargrave D, Galldiks N, et al. Joint EANM/SIOPE/RAPNO practice guidelines/SNMMI procedure standards for imaging of paediatric gliomas using PET with radiolabelled amino acids and [18F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 2022;49:3852–69.
Maehara T, Nariai T, Arai N, Kawai K, Shimizu H, Ishii K, et al. Usefulness of [11C]methionine PET in the diagnosis of dysembryoplastic neuroepithelial tumor with temporal lobe epilepsy. Epilepsia. 2004;45:41–5.
Bund C, Heimburger C, Imperiale A, Lhermitte B, Chenard M-P, Lefebvre F, et al. FDOPA PET-CT of nonenhancing brain tumors. Clin Nucl Med. 2017;42:250–7.
Rheims S, Rubi S, Bouvard S, Bernard E, Streichenberger N, Guenot M, et al. Accuracy of distinguishing between dysembryoplastic neuroepithelial tumors and other epileptogenic brain neoplasms with [11C]methionine PET. Neuro-Oncology. 2014;16:1417–26.
Kasper BS, Struffert T, Kasper EM, Fritscher T, Pauli E, Weigel D, et al. 18Fluoroethyl-l-tyrosine-PET in long-term epilepsy associated glioneuronal tumors: FET in tumor epilepsy. Epilepsia. 2011;52:35–44.
Floeth FW, Pauleit D, Sabel M, Stoffels G, Reifenberger G, Riemenschneider MJ, et al. Prognostic value of O-(2–18F-fluoroethyl)-L-tyrosine PET and MRI in low-grade glioma. J Nucl Med. 2007;48:519–27.
Albert NL, Winkelmann I, Suchorska B, Wenter V, Schmid-Tannwald C, Mille E, et al. Early static 18F-FET-PET scans have a higher accuracy for glioma grading than the standard 20–40 min scans. Eur J Nucl Med Mol Imaging. 2016;43:1105–14.
Glaudemans AWJM, Enting RH, Heesters MAAM, Dierckx RAJO, van Rheenen RWJ, Walenkamp AME, et al. Value of 11C-methionine PET in imaging brain tumours and metastases. Eur J Nucl Med Mol Imaging. 2013;40:615–35.
Zaragori T, Castello A, Guedj E, Girard A, Galldiks N, Albert NL, et al. Photopenic defects in gliomas with amino-acid PET and relative prognostic value: a multicentric 11C-methionine and 18F-FDOPA PET experience. Clin Nucl Med. 2021;46:e36-7.
Rosenberg DS, Demarquay G, Jouvet A, Le Bars D, Streichenberger N, Sindou M, et al. [11C]-Methionine PET: dysembryoplastic neuroepithelial tumours compared with other epileptogenic brain neoplasms. J Neurol Neurosurg Psychiatry. 2005;76:1686–92.
Sasaki M, Kuwabara Y, Yoshida T, Fukumura T, Morioka T, Nishio S, et al. Carbon-11-methionine PET in focal cortical dysplasia: a comparison with fluorine-18-FDG PET and technetium-99m-ECD SPECT. J Nucl Med. 1998;39:974–7.
Kim D, Chun J-H, Kim SH, Moon JH, Kang S-G, Chang JH, et al. Re-evaluation of the diagnostic performance of 11C-methionine PET/CT according to the 2016 WHO classification of cerebral gliomas. Eur J Nucl Med Mol Imaging. 2019;46:1678–84.
Maeda Y, Oguni H, Saitou Y, Mutoh A, Imai K, Osawa M, et al. Rasmussen syndrome: multifocal spread of inflammation suggested from MRI and PET findings: MRI and PET in Rasmussen syndrome. Epilepsia. 2003;44:1118–21.
Ohta Y, Nariai T, Ishii K, Ishiwata K, Senda M, Okeda R, et al. Meningio-angiomatosis in a patient with focal epilepsy: value of PET in diagnoses and preoperative planning of surgery. Acta Neurochir (Wien). 2003;145:587–90 (discussion 590-591).
Martinez A, Finegersh A, Cannon DM, Dustin I, Nugent A, Herscovitch P, et al. The 5-HT1A receptor and 5-HT transporter in temporal lobe epilepsy. Neurology. 2013;80:1465–71.
Theodore WH, Giovacchini G, Bonwetsch R, Bagic A, Reeves-Tyer P, Herscovitch P, et al. The effect of antiepileptic drugs on 5-HT-receptor binding measured by positron emission tomography. Epilepsia. 2006;47:499–503.
Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, et al. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci U S A. 2004;101:9861–6.
Finnema SJ, Toyonaga T, Detyniecki K, Chen M-K, Dias M, Wang Q, et al. Reduced synaptic vesicle protein 2A binding in temporal lobe epilepsy: a [11 C]UCB-J positron emission tomography study. Epilepsia. 2020;61:2183–93.
Tang Y, Yu J, Zhou M, Li J, Long T, Li Y, et al. Cortical abnormalities of synaptic vesicle protein 2A in focal cortical dysplasia type II identified in vivo with 18F-SynVesT-1 positron emission tomography imaging. Eur J Nucl Med Mol Imaging. 2022;49:3482–91.
Kagitani-Shimono K, Kato H, Kuwayama R, Tominaga K, Nabatame S, Kishima H, et al. Clinical evaluation of neuroinflammation in child-onset focal epilepsy: a translocator protein PET study. J Neuroinflammation. 2021;18:8.
Butler T, Ichise M, Teich AF, Gerard E, Osborne J, French J, et al. Imaging inflammation in a patient with epilepsy due to focal cortical dysplasia. J Neuroimaging. 2013;23:129–31.
Dickstein LP, Liow J-S, Austermuehle A, Zoghbi S, Inati SK, Zaghloul K, et al. Neuroinflammation in neocortical epilepsy measured by PET imaging of translocator protein. Epilepsia. 2019;60:1248–54.
Hirvonen J, Kreisl WC, Fujita M, Dustin I, Khan O, Appel S, et al. Increased in vivo expression of an inflammatory marker in temporal lobe epilepsy. J Nucl Med. 2012;53:234–40.
Butler T, Li Y, Tsui W, Friedman D, Maoz A, Wang X, et al. Transient and chronic seizure-induced inflammation in human focal epilepsy. Epilepsia. 2016;57:e191-194.
McGinnity CJ, Koepp MJ, Hammers A, Riaño Barros DA, Pressler RM, Luthra S, et al. NMDA receptor binding in focal epilepsies. J Neurol Neurosurg Psychiatry. 2015;86:1150–7.
Vibholm AK, Dietz MJ, Beniczky S, Christensen J, Højlund A, Jacobsen J, et al. Activated N-methyl-D-aspartate receptor ion channels detected in focal epilepsy with [18 F]GE-179 positron emission tomography. Epilepsia. 2021;62:2899–908.
DuBois JM, Rousset OG, Guiot M-C, Hall JA, Reader AJ, Soucy J-P, et al. Metabotropic glutamate receptor type 5 (mGluR5) cortical abnormalities in focal cortical dysplasia identified in vivo with [11C]ABP688 positron-emission tomography (PET) imaging. Cereb Cortex. 2016;26:4170–9.
Bouilleret V, Semah F, Biraben A, Taussig D, Chassoux F, Syrota A, et al. Involvement of the basal ganglia in refractory epilepsy: an 18F-fluoro-L-DOPA PET study using 2 methods of analysis. J Nucl Med. 2005;46:540–7.
Bouilleret V, Semah F, Chassoux F, Mantzaridez M, Biraben A, Trebossen R, et al. Basal ganglia involvement in temporal lobe epilepsy: a functional and morphologic study. Neurology. 2008;70:177–84.
Fedi M, Berkovic SF, Scheffer IE, O’Keefe G, Marini C, Mulligan R, et al. Reduced striatal D1 receptor binding in autosomal dominant nocturnal frontal lobe epilepsy. Neurology. 2008;71:795–8.
Werhahn KJ, Landvogt C, Klimpe S, Buchholz H-G, Yakushev I, Siessmeier T, et al. Decreased dopamine D2/D3-receptor binding in temporal lobe epilepsy: an [18F]fallypride PET study. Epilepsia. 2006;47:1392–6.
Bernedo Paredes VE, Buchholz H-G, Gartenschläger M, Breimhorst M, Schreckenberger M, Werhahn KJ. Reduced D2/D3 receptor binding of extrastriatal and striatal regions in temporal lobe epilepsy. PLoS One. 2015;10:e0141098.
Ciumas C, Wahlin T-BR, Espino C, Savic I. The dopamine system in idiopathic generalized epilepsies: identification of syndrome-related changes. Neuroimage. 2010;51:606–15.
Odano I, Varrone A, Savic I, Ciumas C, Karlsson P, Jucaite A, et al. Quantitative PET analyses of regional [11C]PE2I binding to the dopamine transporter—application to juvenile myoclonic epilepsy. Neuroimage. 2012;59:3582–93.
Feraco P, Donner D, Picori L, Rozzanigo U. Unusual diagnostic findings in temporal lobe epilepsy: a combined MRI and 18F-dopa case study. Eur J Radiol Open. 2020;7:100241.
Korja M, Kaasinen V, Lamusuo S, Parkkola R, Någren K, Marttila RJ. Substantial thalamostriatal dopaminergic defect in Unverricht-Lundborg disease. Epilepsia. 2007;48:1768–73.
Picard F, Bruel D, Servent D, Saba W, Fruchart-Gaillard C, Schöllhorn-Peyronneau M-A, et al. Alteration of the in vivo nicotinic receptor density in ADNFLE patients: a PET study. Brain. 2006;129:2047–60.
Hammers A, Lingford-Hughes A. Opioid imaging. PET Clin. 2007;2:67–89.
McGinnity CJ, Shidahara M, Feldmann M, Keihaninejad S, Riaño Barros DA, Gousias IS, et al. Quantification of opioid receptor availability following spontaneous epileptic seizures: correction of [11C]diprenorphine PET data for the partial-volume effect. Neuroimage. 2013;79:72–80.
Hammers A, Asselin M-C, Hinz R, Kitchen I, Brooks DJ, Duncan JS, et al. Upregulation of opioid receptor binding following spontaneous epileptic seizures. Brain. 2007;130:1009–16.
Theodore WH, Carson RE, Andreasen P, Zametkin A, Blasberg R, Leiderman DB, et al. PET imaging of opiate receptor binding in human epilepsy using [18F]cyclofoxy. Epilepsy Res. 1992;13:129–39.
Koepp MJ, Richardson MP, Brooks DJ, Duncan JS. Focal cortical release of endogenous opioids during reading-induced seizures. Lancet. 1998;352:952–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Consent to participate
Not applicable.
Conflict of interest
JA has received research support from Life Molecular Imaging and Siemens Healthineers, and speaker honoraria from Roche, GE Healthcare, Novartis/Advanced Accelerator Application-, Life Molecular Imaging and Biogen. AV has received speaker honoraria from Curium, GE Healthcare, and Philips. HB has received speaker honoraria from AAA/Novartis. DC has received a research grant from Life Molecular Imaging and participated in a research grant offered by Ge Healthcare concerning Amyloid PET imaging. MB has received speaker honoraria from GE healthcare, Roche, and LMI and is an advisor of LMI. VG has received research/teaching funding through her institution from Siemens Healthineers, GE Healthcare and Novo Nordisk. AH has received research funding through his institution from Siemens Healthineers, speaker honoraria from Siemens Healthineers, consultant honoraria from GE, and royalties to institutions and himself for commercial use of Hammers Atlas databases (academic use is free). IL has received speaker honoraria from Siemens Healthcare. SM received speaker honoraria from GE Healthcare, life molecular imaging and Eli-Lilly. NT has received research support from ZONMw. The other authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Traub-Weidinger, T., Arbizu, J., Barthel, H. et al. EANM practice guidelines for an appropriate use of PET and SPECT for patients with epilepsy. Eur J Nucl Med Mol Imaging 51, 1891–1908 (2024). https://doi.org/10.1007/s00259-024-06656-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-024-06656-3