Abstract
Purpose
The emergence of chimeric antigen receptor (CAR) T-cell therapy fundamentally changed the management of individuals with relapsed and refractory large B-cell lymphoma (LBCL). However, real-world data have shown divergent outcomes for the approved products. The present study therefore set out to evaluate potential risk factors in a larger cohort.
Methods
Our analysis set included 88 patients, treated in four German university hospitals and one Italian center, who had undergone 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography (PET) before CAR T-cell therapy with tisagenlecleucel or axicabtagene ciloleucel. We first determined the predictive value of conventional risk factors, treatment lines, and response to bridging therapy for progression-free survival (PFS) through forward selection based on Cox regression. In a second step, the additive potential of two common PET parameters was assessed. Their optimal dichotomizing thresholds were calculated individually for each CAR T-cell product.
Results
Extra-nodal involvement emerged as the most relevant of the conventional tumor and patient characteristics. Moreover, we found that inclusion of metabolic tumor volume (MTV) further improves outcome prediction. The hazard ratio for a PFS event was 1.68 per unit increase of our proposed risk score (95% confidence interval [1.20, 2.35], P = 0.003), which comprised both extra-nodal disease and lymphoma burden. While the most suitable MTV cut-off among patients receiving tisagenlecleucel was 11 mL, a markedly higher threshold of 259 mL showed optimal predictive performance in those undergoing axicabtagene ciloleucel treatment.
Conclusion
Our analysis demonstrates that the presence of more than one extra-nodal lesion and higher MTV in LBCL are associated with inferior outcome after CAR T-cell treatment. Based on an assessment tool including these two factors, patients can be assigned to one of three risk groups. Importantly, as shown by our study, metabolic tumor burden might facilitate CAR T-cell product selection and reflect the individual need for bridging therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chimeric antigen receptor (CAR) T‐cell therapy provides a new option for patients with various lymphoma subtypes. Tisagenlecleucel and axicabtagene ciloleucel are anti-CD19 products that were approved in relapsed or refractory large B-cell lymphoma (LBCL) based on results of the JULIET and ZUMA-1 trials [1, 2]. While the design heterogeneity of these studies, mainly with respect to inclusion criteria and bridging therapy, precludes direct comparison, recently published real-world data show considerable progression-free survival (PFS) as well as overall survival (OS) differences between the two constructs [3, 4].
The importance of individualized treatment planning is already widely appreciated, since CAR T-cell therapy can cause clinically relevant side effects and requires complex patient management [5, 6]. However, a specific risk model on which to base treatment decisions has not yet been established. CAR T-cell product selection and indications for tumor debulking are contingent upon local standards of care and hence inconsistent. The available data suggest that, among other factors, lymphoma burden and response to bridging therapy may influence outcomes after CAR T-cell infusion [7, 8]. Moreover, intrinsic tumor factors and characteristics of the cells administered such as dose or kinetics have been discussed [9,10,11].
Positron emission tomography (PET) with 2-[18F]fluoro-2-deoxy-D-glucose ([18F]FDG) is a diagnostic modality routinely used for the staging of patients undergoing CAR T-cell therapy. Recently, retrospective studies including our own have demonstrated that quantitative image evaluation by parameters like metabolic tumor volume (MTV) may be helpful in pretreatment assessment [12,13,14,15]. We therefore initiated this multicenter analysis to find a risk assessment tool for the specific context of CAR T-cell therapy based on conventional disease characteristics, patient factors, and PET metrics, taking into account potential differences between products. Additionally, the role of lymphoma burden before treatment in the development of toxicities was examined.
Patients and methods
Data collection
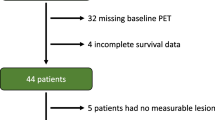
Our study cohort included patients with relapsed or refractory biopsy-proven LBCLs who had undergone CAR T-cell therapy through January 31, 2021, and met the following criteria:
-
(1)
PET examination performed within 30 days before infusion of tisagenlecleucel or axicabtagene ciloleucel
-
(2)
No systemic cytoreductive treatment after imaging besides a lymphodepleting regimen of fludarabine and cyclophosphamide
-
(3)
Absence of lesions only captured by another diagnostic modality or classified as non-measurable due to high physiologic [18F]FDG uptake in the surrounding tissue
After institutional ethics committee approval, the four participating German university hospitals and single Italian center identified 88 individuals who qualified for analysis. The study sites were asked to provide information about clinical stage, extra-nodal disease sites, patient age, Eastern Cooperative Oncology Group status, lactate dehydrogenase (LDH) levels, C-reactive protein values, therapy lines, and response status after bridging treatment. Product selection was based on the approved indications and local availability. All individuals or their representatives gave written informed consent for CAR T-cell therapy and the respective staging procedures. Forty-seven of the patients enrolled were also examined in a smaller analysis recently published elsewhere [14].
PET scanning and quantitative image analysis
Imaging was performed at the academic medical centers that participated in our study, using Biograph mCT, Biograph mMR, Biograph Vision (Siemens Healthcare GmbH, Erlangen, Germany); Discovery MI (GE HealthCare, Milwaukee, WI); and Gemini TF (Koninklijke Philips N.V., Amsterdam, The Netherlands) PET systems. As part of clinical protocol, scans were acquired according to institutional standards.
MTV was calculated semi-automatically with a standardized uptake value (SUV) threshold of 4.0 in ACCURATE (PETRA consortium, Amsterdam, The Netherlands), LIFEx (Laboratory of Translational Imaging in Oncology, Orsay, France) [16], MIM Encore (MIM Software, Inc., Cleveland, OH), or syngo.via (Siemens Healthcare GmbH, Erlangen, Germany), depending on reader preferences. Recent studies indicate a satisfactory level of agreement between the software tools used [14, 17, 18]. The absolute cut-off chosen has been shown previously to achieve the highest delineation success rates in lymphoma patients [19]. Furthermore, we measured the maximum SUV (SUVmax) as an additional PET parameter.
Statistical evaluation and development of a predictive model
Main patient outcomes evaluated by our study were PFS, defined as time to imaging-detected progression, relapse, or any-cause death, and OS, which was calculated taking only deaths into account. We determined 1-year survival rates through Kaplan–Meier analysis and used Cox regression with likelihood ratio tests to assess differences between risk groups. Median follow-up time was calculated by the reverse Kaplan–Meier method. Moreover, hazard ratios of the identified risk factors and corresponding 95% Wald’s confidence limits were established.
Our model for prediction of PFS was developed by multivariable Cox regression based on forward selection, using the Akaike’s information criterion (AIC). All conventional disease parameters and basic patient characteristics collected were taken into account as factors potentially affecting outcome. In a second step, we assessed whether MTV and SUVmax could improve the generated model. The respective optimal threshold was determined individually for each CAR T-cell product based on AIC with the condition that at least 15% of patients should be allocated to a risk group. Factors which proved predictive were analyzed through univariable Cox regression and in the context of our final model. Additionally, our study evaluated whether grade of cytokine release (CRS) or immune effector cell-associated neurotoxicity syndrome (ICANS), assessed using the American Society for Blood and Marrow Transplantation consensus scoring system [20], correlates with metabolic tumor burden based on Pearson’s coefficients separately for both CAR T-cell constructs. An excessive response of immune cells following infusion primarily causes fever in CRS. It may also lead to hypotension, capillary leak, and end-organ dysfunction. CAR T-cell-related ICANS is a pathologic process involving the central nervous system, which can induce aphasia, impairment of consciousness or cognitive skills, motor weakness, seizures, and cerebral edema.
To examine potential differences in baseline characteristics between the two treatment subgroups receiving tisagenlecleucel and axicabtagene ciloleucel, we performed Mann–Whitney U as well as Fisher’s exact tests. All analyses were conducted with SAS software (SAS Institute, Inc., Cary, NC), treating the study sites as random effects in Cox models.
Results
Baseline characteristics of the study cohort
The median patient age was 59 years, with a male-to-female ratio of 1.67 (Table 1). A majority of them were treated for relapsed or refractory diffuse LBCL (n = 67, 76.1%), and elevated LDH was the most common established risk factor (n = 61, 69.3%). While 62 individuals underwent CAR T-cell therapy with tisagenlecleucel (70.5%), 26 received axicabtagene ciloleucel (29.5%). Though, generally, the baseline characteristics of our two treatment groups did not significantly differ, there were a few exceptions: first, a higher metabolic tumor burden (P = 0.024) but less frequent need for bridging therapy of patients who received axicabtagene ciloleucel (P < 0.001) and, second, differences in histologic subtype distribution (P = 0.023). A markedly lower proportion of individuals undergoing treatment with tisagenlecleucel had primary mediastinal B-cell lymphoma.
Optimal PET parameter thresholds and risk categorization
The most suitable MTV and SUVmax cut-offs to predict PFS were 11 mL and 9.7, respectively, for patients treated with tisagenlecleucel. In contrast, markedly higher thresholds of 259 mL and 15.1 provided optimal predictive power among those receiving axicabtagene ciloleucel.
Extra-nodal disease emerged as the most relevant of the conventional tumor and patient characteristics (Fig. 1A, B) with a hazard ratio (HR) of 1.83 (95% confidence interval (CI) [1.10, 3.04], P = 0.021). No other factors considered for the first risk modeling step were of any added value in our study cohort. However, with a univariable HR of 2.04 (95% CI [1.16, 3.58], P = 0.014), metabolic tumor burden was identified as a PET parameter that can further improve PFS prediction and formed the developed assessment tool’s second category (Fig. 1C, D). Accordingly, patients were assigned to one of three risk groups based on a numerical scale ranging between 0 and 2.
In our study, which had a 17-month median follow-up, individuals with no more than one extra-nodal lesion plus smaller MTV achieved 1-year PFS and OS probabilities of 49.0% (95% CI [28.4, 84.5]) and 85.7% (95% CI [69.2, 100]), respectively. The HR for a PFS event per unit increase of our sum score comprising these two risk factors was 1.68 (95% CI [1.20, 2.35]; P = 0.003; Fig. 2). Accordingly, 1-year PFS and OS rates in patients who had extra-nodal disease as well as a higher metabolic lymphoma burden were significantly lower at 15.8% (95% CI [7.6, 32.9]) and 48.2% (95% CI [34.3, 67.7]), respectively.
Correlation between tumor burden and grades of toxicity
We observed a weak correlation of metabolically active tumor volume with CRS (r = 0.359; P = 0.004) and ICANS grade (r = 0.298; P = 0.019) for individuals receiving tisagenlecleucel. No relevant associations of MTV to CRS (r = 0.242; P = 0.235) or ICANS severity (r = 0.059; P = 0.776) after CAR T-cell treatment were found in axicabtagene ciloleucel-treated patients. The lymphoma burden distribution of individuals with negligible or moderate versus more severe toxicity is illustrated by Fig. 3.
Box plots comparing the distribution of MTV in patients with negligible to moderate versus more severe CRS (A, C) and ICANS (B, D) by CAR T-cell product. CAR, chimeric antigen receptor; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; MTV, metabolic tumor volume
Discussion
We here present a product-specific risk assessment tool for CAR T-cell therapy in LBCL that includes extra-nodal disease reflecting lesion dissemination and pretreatment MTV as a measure of tumor load. The PET parameter cut-offs were chosen taking into account potential differences between tisagenlecleucel and axicabtagene ciloleucel. Our two-factor score accurately identified patients at risk of shorter PFS and OS after CAR T-cell treatment. In those with more than one extra-nodal lesion and elevated MTV, the rate of progression or death was three times higher than for individuals without.
Extra-nodal spread was the most relevant of conventional patient characteristics and basic disease parameters, a finding consistent to previous studies [13, 14]. Shorter survival in these individuals could be explained by the observed hindrance of T-cell migration into non-lymphatic tissues [21]. Hence, optimization of the approved products and tailored bridging strategies have the potential to improve therapy outcomes.
Interestingly, extra-nodal disease and MTV compared favorably with several established risk factors. This may be explained by the circumstance that Ann Arbor stage and LDH are only surrogates of tumor burden. Using large clinical trial data sets, Mikhaeel et al. developed a risk score based on metabolic tumor burden, patient age, and clinical stage, which predicted the treatment response of diffuse LBCL patients undergoing first-line therapy more accurately than the International Prognostic Index [18, 22]. In the setting of CAR T-cell treatment, that novel risk assessment tool was found to be predictive for PFS but not OS [23].
To date, there is no randomized study comparing the efficacy of tisagenlecleucel and axicabtagene ciloleucel. However, real-world data have recently shown significant differences regarding survival rates [3, 4]. Our analysis indicates that axicabtagene ciloleucel may be particularly beneficial in patients with high lymphoma load, as the optimal MTV threshold of tisagenlecleucel was markedly lower. Metabolic tumor burden would thus seem useful for the selection of both CAR T-cell product and bridging therapy. Of note, unlike others [12, 24], we did not use the MTV median as a threshold to identify individuals at risk, but determined specific cut-offs based on model selection methods, providing increased accuracy. Several studies indicate that effective debulking is most important in high-tumor volume patients, while those who have low MTV should be protected from chemo- or radiotherapy-associated toxicity. The decrease of metabolic tumor burden between leukapheresis and CAR T-cell infusion significantly correlated with PFS in a study by Sesques et al. [24]. Surprisingly, we did not find any predictive value of response status after bridging treatment. This might be explained by the fact that disease stratification based on Lugano criteria [25] is less precise than the exact measurement of volume changes.
While other authors have reported an association between MTV and CRS development [26, 27], the present study showed only weak correlations of lymphoma load with CAR T-cell-specific adverse events. However, it is important to note that the number of higher-grade toxicities was limited in our cohort. Furthermore, management of these potentially life-threatening side effects has significantly improved over time, presumably through the use of tocilizumab and corticosteroids at an increasingly early stage [28, 29].
Implementation of standardized measuring methods and automated workflows will be essential if MTV or other PET-derived biomarkers are to become available in routine clinical practice. Radiomics may also play a relevant role within the management of patients undergoing CAR T-cell therapy [30]. Recent developments suggest that more convenient tools such as plug-ins for commercial imaging software can be expected soon. The deep-learning algorithm presented by Jemaa et al. is just one of several with sufficient accuracy in lymphoma [31]. We ourselves used the absolute SUV threshold of 4.0 for semi-automatic tumor burden calculation. This cut-off is currently the most promising candidate, since it was found to be the least influenced by image reconstruction and choice of segmentation tool [17, 32].
One potential weakness of our study lies in the retrospective data collection. Nevertheless, the findings appear generalizable to a broad spectrum of patients, as individuals from five university centers and two European countries were enrolled. Another strength was the inclusion of two different CAR T-cell products. However, it should be noted that the treatment groups were not matched for all baseline characteristics. Although cautious interpretation is needed due to the limited number of patients who received either tisagenlecleucel or axicabtagene ciloleucel, respectively, we believe that both risk factors identified will become increasingly important (Supplementary Fig. 1). Thus, there remains a particular need for reliable data on the efficacy of different bridging strategies in cases with higher tumor load. Moreover, prospective studies should be carried out to confirm the prognostic value of extra-nodal disease as well as MTV and refine treatment recommendations not only for tisagenlecleucel or axicabtagene ciloleucel but also other CAR T-cell products like lisocabtagene maraleucel [33].
Conclusions
Our study demonstrates that extra-nodal disease and higher MTV identified by [18F]FDG PET are associated with a significantly worse outcome following CAR T-cell treatment in LBCL. Using an assessment tool, which includes these two factors, patients can be assigned to one of three risk groups. Moreover, as shown by the present analysis, metabolic tumor burden might be a valuable parameter for selection of the optimal CAR T-cell product and bridging therapy.
Data availability
All data generated and analyzed during our study are available from the corresponding author on reasonable request.
References
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. https://doi.org/10.1056/NEJMoa1804980.
Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640–54. https://doi.org/10.1056/NEJMoa2116133.
Bethge WA, Martus P, Schmitt M, Holtick U, Subklewe M, von Tresckow B, et al. GLA/DRST real-world outcome analysis of CAR T-cell therapies for large B-cell lymphoma in Germany. Blood. 2022;140(4):349–58. https://doi.org/10.1182/blood.2021015209.
Bachy E, Le Gouill S, Di Blasi R, Sesques P, Manson G, Cartron G, et al. A real-world comparison of tisagenlecleucel and axicabtagene ciloleucel CAR T cells in relapsed or refractory diffuse large B cell lymphoma. Nat Med. 2022;28(10):2145–54. https://doi.org/10.1038/s41591-022-01969-y.
Gödel P, Shimabukuro-Vornhagen A, von Bergwelt-Baildon M. Understanding cytokine release syndrome. Intensive Care Med. 2018;44(3):371–3. https://doi.org/10.1007/s00134-017-4943-5.
Perica K, Curran KJ, Brentjens RJ, Giralt SA. Building a CAR garage: preparing for the delivery of commercial CAR T cell products at Memorial Sloan Kettering Cancer Center. Biol Blood Marrow Transplant. 2018;24(6):1135–41. https://doi.org/10.1016/j.bbmt.2018.02.018.
Locke FL, Rossi JM, Neelapu SS, Jacobson CA, Miklos DB, Ghobadi A, et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4(19):4898–911. https://doi.org/10.1182/bloodadvances.2020002394.
Bethge WA, Martus P, Schmitt M, Holtick U, Borchmann P, Subklewe M, et al. Standard of care CAR-T cell therapy for large B-cell lymphoma (LBCL): does bridging efficacy matter? A German GLA/DRST real world analysis. Blood. 2021;138(Suppl 1):3822. https://doi.org/10.1182/blood-2021-146120.
Cherng HJ, Sun R, Sugg B, Irwin R, Yang H, Le CC, et al. Risk assessment with low-pass whole-genome sequencing of cell-free DNA before CD19 CAR T-cell therapy for large B-cell lymphoma. Blood. 2022;140(5):504–15. https://doi.org/10.1182/blood.2022015601.
Bishop MR, Dickinson M, Purtill D, Barba P, Santoro A, Hamad N, et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med. 2022;386(7):629–39. https://doi.org/10.1056/NEJMoa2116596.
Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20(1):31–42. https://doi.org/10.1016/S1470-2045(18)30864-7.
Dean EA, Mhaskar RS, Lu H, Mousa MS, Krivenko GS, Lazaryan A, et al. High metabolic tumor volume is associated with decreased efficacy of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4(14):3268–76. https://doi.org/10.1182/bloodadvances.2020001900.
Galtier J, Vercellino L, Chartier L, Olivier P, Tabouret-Viaud C, Mesguich C, et al. Positron emission tomography-imaging assessment for guiding strategy in patients with relapsed/refractory large B-cell lymphoma receiving CAR T cells. Haematologica. 2023;108(1):171–80. https://doi.org/10.3324/haematol.2021.280550.
Voltin CA, Gödel P, Beckmann L, Heger JM, Kobe C, Kutsch N, et al. Outcome prediction in patients with large B-cell lymphoma undergoing chimeric antigen receptor T-cell therapy. Hemasphere. 2023;7(1):e817. https://doi.org/10.1097/HS9.0000000000000817.
Ababneh H, Frigault M, Ng AK, Patel CG. 18FDG PET/CT parameters for the prediction of CAR T-cell therapy response among patients with large B-cell lymphoma. Int J Radiat Oncol Biol Phys. 2022;114(3 Suppl):251. https://doi.org/10.1016/j.ijrobp.2022.07.489.
Nioche C, Orlhac F, Boughdad S, Reuzé S, Goya-Outi J, Robert C, et al. LIFEx: a freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res. 2018;78(16):4786–9. https://doi.org/10.1158/0008-5472.CAN-18-0125.
Tutino F, Puccini G, Linguanti F, Puccini B, Rigacci L, Kovalchuk S, et al. Baseline metabolic tumor volume calculation using different SUV thresholding methods in Hodgkin lymphoma patients: interobserver agreement and reproducibility across software platforms. Nucl Med Commun. 2021;42(3):284–91. https://doi.org/10.1097/MNM.0000000000001324.
Mikhaeel NG, Heymans MW, Eertink JJ, de Vet HCW, Boellaard R, Dührsen U, et al. Proposed new dynamic prognostic index for diffuse large B-cell lymphoma: international metabolic prognostic index. J Clin Oncol. 2022;40(21):2352–60. https://doi.org/10.1200/JCO.21.02063.
Barrington SF, Zwezerijnen BGJC, de Vet HCW, Heymans MW, Mikhaeel NG, Burggraaff CN, et al. Automated segmentation of baseline metabolic total tumor burden in diffuse large B-cell lymphoma: which method is most successful? A study on behalf of the PETRA consortium. J Nucl Med. 2021;62(3):332–7. https://doi.org/10.2967/jnumed.119.238923.
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–38. https://doi.org/10.1016/j.bbmt.2018.12.758.
Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A, et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest. 2012;122(3):899–910. https://doi.org/10.1172/JCI45817.
International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329(14):987–94. https://doi.org/10.1056/NEJM199309303291402.
Winkelmann M, Blumenberg V, Rejeski K, Bücklein VL, Ruzicka M, Unterrainer M, et al. Prognostic value of the international metabolic prognostic index for lymphoma patients receiving chimeric antigen receptor T-cell therapy. Eur J Nucl Med Mol Imaging. 2023;50(5):1406–13. https://doi.org/10.1007/s00259-022-06075-2.
Sesques P, Tordo J, Ferrant E, Safar V, Wallet F, Dhomps A, et al. Prognostic impact of 18F-FDG PET/CT in patients with aggressive B-cell lymphoma treated with anti-CD19 chimeric antigen receptor T cells. Clin Nucl Med. 2021;46(8):627–34. https://doi.org/10.1097/RLU.0000000000003756.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–68. https://doi.org/10.1200/JCO.2013.54.8800.
Wang J, Hu Y, Yang S, Wei G, Zhao X, Wu W, et al. Role of fluorodeoxyglucose positron emission tomography/computed tomography in predicting the adverse effects of chimerics antigen receptor T cell therapy in patients with non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2019;25(6):1092–8. https://doi.org/10.1016/j.bbmt.2019.02.008.
Hong R, Tan Su Yin E, Wang L, Zhao X, Zhou L, Wang G, et al. Tumor burden measured by 18F-FDG PET/CT in predicting efficacy and adverse effects of chimeric antigen receptor T-cell therapy in non-Hodgkin lymphoma. Front Oncol. 2021;11:713577. https://doi.org/10.3389/fonc.2021.713577.
Oluwole OO, Bouabdallah K, Muñoz J, De Guibert S, Vose JM, Bartlett NL, et al. Prophylactic corticosteroid use in patients receiving axicabtagene ciloleucel for large B-cell lymphoma. Br J Haematol. 2021;194(4):690–700. https://doi.org/10.1111/bjh.17527.
Caimi PF, Pacheco Sanchez G, Sharma A, Otegbeye F, Ahmed N, Rojas P, et al. Prophylactic tocilizumab prior to anti-CD19 CAR-T cell therapy for non-Hodgkin lymphoma. Front Immunol. 2021;12:745320. https://doi.org/10.3389/fimmu.2021.745320.
Zhou Y, Zhang B, Han J, Dai N, Jia T, Huang H, et al. Development of a radiomic-clinical nomogram for prediction of survival in patients with diffuse large B-cell lymphoma treated with chimeric antigen receptor T cells. J Cancer Res Clin Oncol. 2023;149(13):11549–60. https://doi.org/10.1007/s00432-023-05038-w.
Jemaa S, Fredrickson J, Carano RAD, Nielsen T, de Crespigny A, Bengtsson T. Tumor segmentation and feature extraction from whole-body FDG-PET/CT using cascaded 2D and 3D convolutional neural networks. J Digit Imaging. 2020;33(4):888–94. https://doi.org/10.1007/s10278-020-00341-1.
Ferrández MC, Eertink JJ, Golla SSV, Wiegers SE, Zwezerijnen GJC, Pieplenbosch S, et al. Combatting the effect of image reconstruction settings on lymphoma [18F]FDG PET metabolic tumor volume assessment using various segmentation methods. EJNMMI Res. 2022;12(1):44. https://doi.org/10.1186/s13550-022-00916-9.
Kamdar M, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399(10343):2294–308. https://doi.org/10.1016/S0140-6736(22)00662-6.
Acknowledgements
We dedicate our work to Michel Meignan, in memory of his exceptional contributions for the establishment of PET in lymphoma.
Funding
Open Access funding enabled and organized by Projekt DEAL. Our work was supported by German Research Foundation grant no. 451580403 (MS), the Bavarian Elite Graduate Training Network (MS), Wilhelm Sander Foundation (project no. 2018.087.1, MS), Else Kröner-Fresenius Foundation (VB), and German Cancer Consortium (MS and VB), as well as Bavarian Center for Cancer Research and conducted partly in the Collaborative Research Center SFB-TRR 388/1 202 1–452881907.
Author information
Authors and Affiliations
Contributions
CAV, PG, and CH designed the present study; SFl led the statistical analysis; CAV, SFl, PG, and CH wrote the first article draft; all authors contributed to data acquisition as well as interpretation, reviewed the manuscript, and approved its final version.
Corresponding author
Ethics declarations
Ethics approval
This retrospective study was conducted after obtaining institutional ethics committee approvals.
Consent to participate
Written informed consent was obtained from all patients prior to enrollment in this study.
Competing interests
JMH: Miltenyi Biotec—advisory role; Incyte, Novartis—research funding; Gilead Pharmaceuticals, Novartis—travel support. NK: AstraZeneca, Roche—paid honoraria; AstraZeneca, Gilead Sciences, Inc.—research funding; AbbVie, AstraZeneca, Gilead Sciences, Inc., Janssen, Celgene—travel support. PB: Bristol Myers Squibb, Celgene, Gilead Sciences, Janssen, Miltenyi Biotec, Novartis—paid honoraria. MS: AvenCell, CDR-Life, Ichnos Sciences, Incyte Biosciences, Janssen, Miltenyi Biotec, Molecular Partners, Novartis, Pfizer, Takeda—consultant role; Amgen, AstraZeneca, Celgene, Gilead Sciences, GlaxoSmithKline, Janssen, Novartis, Pfizer, Roche, Takeda—lectureship honoraria; Amgen, Celgene, Gilead Sciences, Janssen, Miltenyi Biotec, Novartis, Roche, Seattle Genetics, Takeda—research funding. PLZ: ADC Therapeutics, AstraZeneca, BeiGene, Bristol Myers Squibb, Celltrion Healthcare, EUSA Pharma, Gilead Sciences, Incyte, Janssen-Cilag, Kyowa Kirin, Merck Sharp & Dohme, Novartis, Roche, Sandoz, Secura Bio, Servier, Takeda—advisory role; EUSA Pharma, Merck Sharp & Dohme, Novartis—consultant role; AstraZeneca, BeiGene, Bristol Myers Squibb, Celltrion Healthcare, EUSA Pharma, Gilead Sciences, Incyte, Janssen-Cilag, Kyowa Kirin, Merck Sharp & Dohme, Novartis, Roche, Servier, Takeda—lectureship honoraria. AD: Bayer Vital GmbH, Biogen, GE Healthcare, Invicro, Novartis, Novo Nordisk, Sanofi, Siemens Healthineers—lectureship honoraria or advisory role; 18F-JK–prostate-specific membrane antigen–7, 2-alkoxy-6-18F-fluoronicotinoyl-substituted Lys-C(O)-Glu derivatives—patent holder; Avid Radiopharmaceuticals, Inc., Eisai, GE Healthcare, Life Molecular Imaging, Novartis, Siemens Healthineers, SOFIE—research funding; Lantheus Holdings, Inc., Siemens Healthineers AG—stockholder. KR: Advanced Accelerator Applications, Amgen, AstraZeneca, Bayer, Janssen-Cilag, Sirtex Medical—paid honoraria; ABX advanced biochemical compounds GmbH, ABX-CRO, Advanced Accelerator Applications, Bayer—consultant or advisory role. HCR: CDL Therapeutics GmbH—co-founder; AbbVie, AstraZeneca, Bristol Myers Squibb, Merck, Noscendo GmbH, Novartis, Roche, Vertex Pharmaceuticals—consultant role and lectureship honoraria; AstraZeneca, Gilead Pharmaceuticals—research funding. BvT: Allogene Therapeutics, Amgen, Bristol Myers Squibb, Cerus Corporation, Gilead Sciences, Incyte, IQVIA, Merck Sharp & Dohme, Miltenyi Biotec, Noscendo GmbH, Novartis, PentixaPharm GmbH, Roche, Pfizer, Takeda—consultant or advisory role; AstraZeneca, Bristol Myers Squibb, Incyte, Merck Sharp & Dohme, Novartis, Roche Pharma AG, Takeda—paid honoraria; Merck Sharp & Dohme, Novartis, Takeda—research funding; AbbVie, AstraZeneca, Gilead Sciences, Merck Sharp & Dohme, Novartis, Roche, Takeda—travel support. RS: Boehringer Ingelheim Fonds, Else Kröner-Fresenius Foundation—research funding. VB: Gilead Sciences, Roche—consultant role; Gilead Sciences, Janssen, Novartis—paid honoraria; Bristol Myers Squibb, Gilead Sciences, Janssen, Novartis, Roche, Takeda—research funding. PG: Gilead Sciences, Novartis—travel support. CH: AstraZeneca, Bristol Myers Squibb, Janssen, Takeda—paid honoraria; AbbVie, Janssen—travel support.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Voltin, CA., Paccagnella, A., Winkelmann, M. et al. Multicenter development of a PET-based risk assessment tool for product-specific outcome prediction in large B-cell lymphoma patients undergoing CAR T-cell therapy. Eur J Nucl Med Mol Imaging 51, 1361–1370 (2024). https://doi.org/10.1007/s00259-023-06554-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06554-0