Abstract
Purpose
Chimeric antigen receptor T-cell therapy (CART) prolongs survival for patients with relapsed/refractory B-cell non-Hodgkin’s lymphoma. The recently introduced International Metabolic Prognostic Index (IMPI) was shown to improve prognostication in the first-line treatment of large B-cell lymphoma. Here, we investigate the prognostic value of the IMPI for progression-free (PFS) and overall survival (OS) in the setting of CD19 CART.
Methods
Consecutively treated patients with baseline 18F-FDG PET/CT imaging and follow-up imaging at 30 days after CART were included. IMPI is composed of age, stage, and metabolic tumor volume (MTV) at baseline and was compared with the International Prognostic Index (IPI). Both indices were grouped into quartiles, as previously described for IPI. In addition, the continuous IMPI was subdivided into tertiaries for better separation of risk groups. Overall response rate (ORR), depth of response (DoR), and PFS were determined based on Lugano criteria. Proportional Cox regression analysis studied association of IMPI and IPI with PFS and OS.
Results
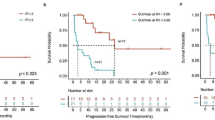
Thirty-nine patients were included. The IPI was 1 in 23%, 2 in 21%, 3 in 26%, 4 in 21%, and 5 in 10% of the patients. IMPIlow risk, IMPIintermediate risk, and IMPIhigh risk patients had 30-day ORR of 69%, 62%, and 62% and 30-day DoR of − 67%, − 66%, and − 54% with a PFS of 187 days, 97 days, and 87 days, respectively. ORR and DoR showed no correlation with lower IMPI (r = 0.065, p = 0.697). Dividing patients into three risk groups showed a significant trend for PFS stratification (p = 0.030), while IPI did not (p = 0.133). Neither IPI nor IMPI yielded a significant association with OS after CART (both p > 0.05).
Conclusion
In the context of CART, the IMPI yielded prognostic value regarding PFS estimation. In contrast with IMPI in the first-line DLBCL setting, we did not observe a significant association of IMPI at baseline with OS after CART.



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379:64–73. https://doi.org/10.1056/NEJMra1706169.
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jager U, Jaglowski S, Andreadis C, Westin JR, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. https://doi.org/10.1056/NEJMoa1804980.
Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak O, Brogdon JL, Pruteanu-Malinici I, Bhoj V, Landsburg D, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–54. https://doi.org/10.1056/NEJMoa1708566.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44. https://doi.org/10.1056/NEJMoa1707447.
Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, Timmerman JM, Holmes H, Jaglowski S, Flinn IW, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331–42. https://doi.org/10.1056/NEJMoa1914347.
Ghione P, Palomba ML, Patel AR, Bobillo S, Deighton K, Jacobson CA, Nahas M, Hatswell AJ, Jung AS, Kanters S, et al. Comparative effectiveness of ZUMA-5 (axi-cel) vs SCHOLAR-5 external control in relapsed/refractory follicular lymphoma. Blood. 2022;140:851–60. https://doi.org/10.1182/blood.2021014375.
International Non-Hodgkin’s Lymphoma Prognostic Factors P. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329:987–94. https://doi.org/10.1056/NEJM199309303291402.
Bethge WA, Martus P, Schmitt M, Holtick U, Subklewe M, von Tresckow B, Ayuk F, Wagner-Drouet EM, Wulf GG, Marks R, et al. GLA/DRST real-world outcome analysis of CAR-T cell therapies for large B-cell lymphoma in Germany. Blood. 2022. https://doi.org/10.1182/blood.2021015209.
Garcia-Recio M, Wudhikarn K, Pennisi M, Alonso-Trillo R, Flynn J, Shouval R, Afuye AO, Silverberg ML, Batlevi CW, Dahi P, et al. The International Prognostic Index is associated with outcomes in diffuse large B cell lymphoma after chimeric antigen receptor T cell therapy. Transplant Cell Ther. 2021;27:233–40. https://doi.org/10.1016/j.jtct.2020.10.022.
Neelapu SS, Dickinson M, Munoz J, Ulrickson ML, Thieblemont C, Oluwole OO, Herrera AF, Ujjani CS, Lin Y, Riedell PA, et al. Axicabtagene ciloleucel as first-line therapy in high-risk large B-cell lymphoma: the phase 2 ZUMA-12 trial. Nat Med. 2022. https://doi.org/10.1038/s41591-022-01731-4.
Cottereau AS, Versari A, Loft A, Casasnovas O, Bellei M, Ricci R, Bardet S, Castagnoli A, Brice P, Raemaekers J, et al. Prognostic value of baseline metabolic tumor volume in early-stage Hodgkin lymphoma in the standard arm of the H10 trial. Blood. 2018;131:1456–63. https://doi.org/10.1182/blood-2017-07-795476.
Meignan M, Cottereau AS, Versari A, Chartier L, Dupuis J, Boussetta S, Grassi I, Casasnovas RO, Haioun C, Tilly H, et al. Baseline metabolic tumor volume predicts outcome in high-tumor-burden follicular lymphoma: a pooled analysis of three multicenter studies. J Clin Oncol. 2016;34:3618–26. https://doi.org/10.1200/JCO.2016.66.9440.
Albano D, Bosio G, Bianchetti N, Pagani C, Re A, Tucci A, Giubbini R, Bertagna F. Prognostic role of baseline 18F-FDG PET/CT metabolic parameters in mantle cell lymphoma. Ann Nucl Med. 2019;33:449–58. https://doi.org/10.1007/s12149-019-01354-9.
Ceriani L, Gritti G, Cascione L, Pirosa MC, Polino A, Ruberto T, Stathis A, Bruno A, Moccia AA, Giovanella L, et al. SAKK38/07 study: integration of baseline metabolic heterogeneity and metabolic tumor volume in DLBCL prognostic model. Blood Adv. 2020;4:1082–92. https://doi.org/10.1182/bloodadvances.2019001201.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA, Alliance AL, Lymphoma G, Eastern Cooperative Oncology G, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68. https://doi.org/10.1200/JCO.2013.54.8800.
Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Mueller SP, Schwartz LH, Zucca E, Fisher RI, Trotman J, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32:3048–58. https://doi.org/10.1200/JCO.2013.53.5229.
Mikhaeel NG, Heymans MW, Eertink JJ, de Vet HCW, Boellaard R, Duhrsen U, Ceriani L, Schmitz C, Wiegers SE, Huttmann A, et al. Proposed new dynamic prognostic index for diffuse large B-cell lymphoma: International Metabolic Prognostic Index. J Clin Oncol. 2022. https://doi.org/10.1200/JCO.21.02063. (JCO2102063).
Vercellino L. High total metabolic tumor volume at baseline predicts survival independent of response to therapy. Blood. 2020;135(16):1396–405.
Nioche C, Orlhac F, Boughdad S, Reuze S, Goya-Outi J, Robert C, Pellot-Barakat C, Soussan M, Frouin F, Buvat I. LIFEx: a freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res. 2018;78:4786–9. https://doi.org/10.1158/0008-5472.CAN-18-0125.
Tutino F, Puccini G, Linguanti F, Puccini B, Rigacci L, Kovalchuk S, Sciagra R, Berti V. Baseline metabolic tumor volume calculation using different SUV thresholding methods in Hodgkin lymphoma patients: interobserver agreement and reproducibility across software platforms. Nucl Med Commun. 2021;42:284–91. https://doi.org/10.1097/MNM.0000000000001324.
Martin-Saladich Q, Reynes-Llompart G, Sabate-Llobera A, Palomar-Munoz A, Domingo-Domenech E, Cortes-Romera M. Comparison of different automatic methods for the delineation of the total metabolic tumor volume in I-II stage Hodgkin Lymphoma. Sci Rep. 2020;10:12590. https://doi.org/10.1038/s41598-020-69577-9.
Batlevi CL, Younes A. Surrogate end points in lymphoma. Ann Oncol. 2018;29:1622–3. https://doi.org/10.1093/annonc/mdy219.
Zhu J, Yang Y, Tao J, Wang SL, Chen B, Dai JR, Hu C, Qi SN, Li YX. Association of progression-free or event-free survival with overall survival in diffuse large B-cell lymphoma after immunochemotherapy: a systematic review. Leuk Off J Leuk Soc Am Leuk Res Fund UK. 2020;34:2576–91. https://doi.org/10.1038/s41375-020-0963-1.
Adams HJ, de Klerk JM, Fijnheer R, Heggelman BG, Dubois SV, Nievelstein RA, Kwee TC. Where does diffuse large B-cell lymphoma relapse? J Comput Assist Tomogr. 2016;40:531–6. https://doi.org/10.1097/RCT.0000000000000395.
Figura NB, Robinson TJ, Sim AJ, Wang X, Cao B, Chavez JC, Shah BD, Khimani F, Lazaryan A, Davila M, et al. Patterns and predictors of failure in recurrent or refractory large B-cell lymphomas after chimeric antigen receptor T-cell therapy. Int J Radiat Oncol Biol Phys. 2021. https://doi.org/10.1016/j.ijrobp.2021.06.038.
Rejeski K, Perez A, Iacoboni G, Penack O, Bucklein V, Jentzsch L, Mougiakakos D, Johnson G, Arciola B, Carpio C, et al. The CAR-HEMATOTOX risk-stratifies patients for severe infections and disease progression after CD19 CAR-T in R/R LBCL. J Immunother Cancer. 2022. https://doi.org/10.1136/jitc-2021-004475.
Rejeski K, Perez A, Sesques P, Hoster E, Berger C, Jentzsch L, Mougiakakos D, Frolich L, Ackermann J, Bucklein V, et al. CAR-HEMATOTOX: a model for CAR T-cell-related hematologic toxicity in relapsed/refractory large B-cell lymphoma. Blood. 2021;138:2499–513. https://doi.org/10.1182/blood.2020010543.
Jain MD, Zhao H, Wang X, Atkins R, Menges M, Reid K, Spitler K, Faramand R, Bachmeier C, Dean EA, et al. Tumor interferon signaling and suppressive myeloid cells are associated with CAR T-cell failure in large B-cell lymphoma. Blood. 2021;137:2621–33. https://doi.org/10.1182/blood.2020007445.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Funding
The work was supported by funding from the research program “Förderung für Forschung und Lehre (FöFoLe) project number 1147” of the Medical Faculty of Ludwig Maximilian University (LMU) Munich and the Bavarian Cancer Research Center (BZKF) to M.W. The work was further supported by the Else-Kröner-Fresenius Stiftung (to V.B.) and the German Cancer Consortium DKTK (to V.B.).
Author information
Authors and Affiliations
Contributions
M.W. and W.G.K. conceived and design the study; V.B., K.R., V.L.B., M.R., M.U., and C.S. collected the data; M.W., V.B., K.R., V.L.B., and W.G.K. analyzed and interpreted the data; and M.W. and W.G.K. drafted the manuscript; and V.B., K.R., F.J.D., P.B., J.R., M.v.B.-B., and M.S. revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All medical records and imaging studies were reviewed with the approval of the LMU Munich Institutional Review Board (LMU Ethics Committee, project number 19–817).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Competing interests
V.B. has received industry research support from Gilead, Novartis, Celgene, and Roche. K.R. declares having received research funding and travel support from Kite/Gilead and honoraria from Novartis. C.S. received travel support from Kite/Gilead. M.v.B.-B. received research funding and honoraria from Novartis, Kite Pharma, Miltenyi Biotec, Mologen, MSD, Astellas, and Roche. M.S. received industry research support from Amgen, Gilead, Miltenyi Biotec, MorphoSys, Roche, and Seattle Genetics; served as a consultant or advisor to Amgen, Bristol Myers Squibb, Celgene, Gilead, Pfizer, Novartis, and Roche; is on the advisory boards of Amgen, Celgene, Gilead, Janssen, Novartis, Pfizer, and Seattle Genetics; and serves on the speaker’s bureau at Amgen, Celgene, Gilead, Janssen, and Pfizer. The remaining authors declare no competing financial interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hematology.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Winkelmann, M., Blumenberg, V., Rejeski, K. et al. Prognostic value of the International Metabolic Prognostic Index for lymphoma patients receiving chimeric antigen receptor T-cell therapy. Eur J Nucl Med Mol Imaging 50, 1406–1413 (2023). https://doi.org/10.1007/s00259-022-06075-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-06075-2