Abstract
Introduction
Dopaminergic scintigraphic imaging is a cornerstone to support the diagnosis in dementia with Lewy bodies. To clarify the current state of knowledge on this imaging modality and its impact on clinical diagnosis, we performed an updated systematic review of the literature.
Methods
This systematic review was carried out according to PRISMA guidelines. A comprehensive computer literature search of PubMed/MEDLINE, EMBASE, and Cochrane Library databases for studies published through June 2022 was performed using the following search algorithm: (a) "Lewy body" [TI] OR "Lewy bodies" [TI] and (b) ("DaTscan" OR "ioflupane" OR "123ip" OR "123?ip" OR "123 ip" OR "123i-FP-CIT" OR "FPCIT" OR "FP-CIT" OR "beta?CIT" OR "beta CIT" OR "CIT?SPECT" OR "CIT SPECT" OR "Dat?scan*" OR "dat scan*" OR "dat?spect*" OR "SPECT"). Risk of bias and applicability concerns of the studies were evaluated using the QUADAS-2 tool.
Results
We performed a qualitative analysis of 59 studies. Of the 59 studies, 19 (32%) addressed the diagnostic performance of dopamine transporter imaging, 15 (25%) assessed the identification of dementia with Lewy bodies in the spectrum of Lewy body disease and 18 (31%) investigated the role of functional dopaminergic imaging in distinguishing dementia with Lewy bodies from other dementias. Dopamine transporter loss was correlated with clinical outcomes in 19 studies (32%) and with other functional imaging modalities in 15 studies (25%). Heterogeneous technical aspects were found among the studies through the use of various radioligands, the more prevalent being the [123I]N‑ω‑fluoropropyl‑2β‑carbomethoxy‑3β‑(4‑iodophenyl) nortropane (123I-FP-CIT) in 54 studies (91.5%). Image analysis used visual analysis (9 studies, 15%), semi-quantitative analysis (29 studies, 49%), or a combination of both (16 studies, 27%).
Conclusion
Our systematic review confirms the major role of dopaminergic scintigraphic imaging in the assessment of dementia with Lewy bodies. Early diagnosis could be facilitated by identifying the prodromes of dementia with Lewy bodies using dopaminergic scintigraphic imaging coupled with emphasis on clinical neuropsychiatric symptoms. Most published studies use a semi-quantitative analytical assessment of tracer uptake, while there are no studies using quantitative analytical methods to measure dopamine transporter loss. The superiority of a purely quantitative approach to assess dopaminergic transmission more accurately needs to be further clarified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dementia with Lewy bodies (DLB) is the second most frequent type of neurodegenerative dementia after Alzheimer’s disease (AD), comprising 15–25% of all dementias [1]. Neuropathological findings in patients with DLB show Lewy bodies and Lewy neurites that are positive for α-synuclein immunohistochemical staining, as well as neuronal degeneration in the neocortex, limbic system and brainstem [2]. Although a clear-cut distinction between the entities of the “Lewy body disease (LBD) spectrum” (dementia with Lewy bodies, idiopathic Parkinson’s disease (PD) and PD with dementia) is not always easy, diagnosis of DLB is made clinically through the identification of core clinical features. These include fluctuations of attention and cognitive impairment, visual hallucinations, rapid eye-movement (REM) sleep behavior disorder (RBD), and parkinsonism. Reduced dopamine transporter uptake in the basal ganglia shown by single-positron emission computed tomography (SPECT) is included as an indicative biomarker in the fourth and latest consensus on the diagnosis of Lewy body dementia [3]. Dopamine transporter (DAT) imaging is performed using specific radioligands. One such ligand is [123I]N‑ω‑fluoropropyl‑2β‑carbomethoxy‑3β‑(4‑iodophenyl) nortropane (123I-FP-CIT), a cocaine analogue that specifically binds to presynaptic DATs in the central nervous system, thus identifying the location and concentration of dopamine transporters in the synapses of dopamine-secreting neurons of the corpus striatum in the central nervous system. This allows imaging of the nigrostriatal pathway denervation that occurs in DLB. Other radiotracers can also be used [4]. Clinically, DLB can be difficult to differentiate from other forms of dementia. Furthermore, it is of paramount clinical importance to differentiate DLB from other etiologies as the subsequent clinical and therapeutical management of patients varies, especially to avoid any inappropriate use of neuroleptics in DLB patients [5]. The course of the disease is also different, as life expectancy is shorter in DLB. Literature covering these topics lack homogeneity.
A systematic review and a Bayesian latent class model (LCM) meta-analysis on the diagnostic accuracy of both DAT SPECT imaging as well as metaiodobenzylguanidine (MIBG) myocardial scintigraphy in DLB diagnosis has been published previously [6]. However, there were several limitations, as the literature was only reviewed up to the year 2018 and the number of analyzed studies was small (n = 27), out of which less than a third used the new criteria of DLB published in 2017 [3]. In the present systematic literature review, we present an updated analysis of dopaminergic transporter imaging in the diagnosis of DLB.
Methods
This study was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, which describe an evidence-based minimum set of items for reporting in systematic reviews and meta-analyses [7]. A predefined protocol was created by the authors (without registration).
Search strategy
Two authors (MJ and GKK) performed a comprehensive computer literature search of the PubMed/MEDLINE, EMBASE and Cochrane Library databases to identify relevant retrospective or prospective published studies on the diagnostic performance of functional dopaminergic scintigraphy in the diagnosis of Lewy body dementia. The search algorithm used was based on a combination of terms, as follows: (a) "Lewy body" [TI] OR "Lewy bodies" [TI] and (b) ("DaTscan" OR "ioflupane" OR "123ip" OR "123?ip" OR "123 ip" OR "123i-FP-CIT" OR "FPCIT" OR "FP-CIT" OR "beta?CIT" OR "beta CIT" OR "CIT?SPECT" OR "CIT SPECT" OR "Dat?scan*" OR "dat scan*" OR "dat?spect*" OR "SPECT"). The search was updated through June 2022. No language restriction was applied. To expand the search, references of the retrieved articles were also screened for additional studies.
Study selection
Studies or subsets of studies investigating the diagnostic performance of functional dopaminergic scintigraphy in the evaluation of patients with Dementia with Lewy bodies (DLB) were eligible for inclusion in the qualitative analysis (systematic review).
The exclusion criteria were as follows: (a) articles not within the field of interest of this review, such as those with outcomes unrelated to dopaminergic scintigraphic imaging for diagnosis of DLB (e.g., use of brain perfusion SPECT or PET or myocardial scintigraphy alone); (b) review articles, editorials or letters, comments, conference proceedings; (c) case reports or small case series (< 5 patients).
Two researchers (MJ and GKK) independently reviewed the titles and abstracts of the retrieved articles, applying the inclusion and exclusion criteria mentioned above. Articles were rejected if they were clearly ineligible. The same two researchers then independently reviewed the full-text versions of the remaining articles to determine their eligibility for inclusion. Disagreements were resolved in a consensus meeting.
Data extraction
Two researchers independently performed the data extraction. For each potentially eligible study, information was collected concerning basic study characteristics (authors, year of publication, country of origin, study design), patient characteristics (type and number of patients, mean age, sex ratio) and technical aspects (radiotracer used, hybrid imaging modality, mean injected activity, time interval between radiotracer injection and image acquisition, image analysis). Finally, information about the main outcome of this systematic review (diagnostic performance of dopaminergic scintigraphic imaging) was collected. Diagnostic performance was assessed according to clinical confirmation of DLB diagnosis as well as post-mortem neuropathological studies, the latter being rarely systematically documented. Differences between basic study characteristics, technical aspects and outcomes were reported and were analyzed.
Quality assessment
The overall quality of the studies included in the systematic review was critically appraised based on the revised Quality Assessment of Diagnostic Accuracy Studies tool (QUADAS-2) [8]. This tool comprises four domains: patient selection, index test, reference standard, and flow and timing. Three independent reviewers (MJ, GKK, JP) assessed each domain in terms of risk of bias (i.e., selection bias, as well as biases concerning the index test, reference standard and timing of studies), and the first three domains were also assessed in terms of concerns regarding applicability [8] (Table 1 and Fig. 1).
Results
Literature search
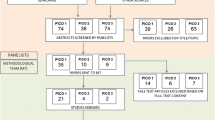
A comprehensive computer literature search of the PubMed/MEDLINE, EMBASE and Cochrane Library databases revealed 218 peer-reviewed articles. Upon review of titles and abstracts, 153 articles were excluded, as follows: 79 were not in the field of interest of this review, 44 were reviews, editorials or letters, and 30 were case reports or small case series (< 5 patients). 59 were selected and retrieved in full-text version [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61]. No additional studies were found by screening the references of these articles (Fig. 2).
Finally, 59 articles including data on the diagnostic performance of functional dopaminergic scintigraphic imaging in the diagnosis of dementia with Lewy bodies (DLB) dementia were eligible for the qualitative analysis (systematic review) [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61]. The characteristics of the studies included in the systematic review are summarized in Tables 2 and 3.
Qualitative analysis (systematic review)
Basic study and patient characteristics
Using the database search, 59 full-text articles reporting on the diagnostic performance of functional dopaminergic scintigraphic imaging in the diagnosis of Lewy body dementia were selected (Supplementary Table 1) [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68].
All 59 selected articles were published within the last 21 years (32 were published since 2017 with the new DLB criteria). Several countries from Europe, North America and Asia were represented. 46% (27/59) of the studies were retrospective and 42% (25/59) were prospective. (< 1% Case–control/cohort n = 4, cross-sectional n = 4). Most (81%) of the articles were single-center studies (48/59).
In 17 out of 59 studies, functional dopaminergic scintigraphic imaging was investigated as the single imaging modality in patients with DLB, whereas in the remaining studies, functional dopaminergic scintigraphic imaging was performed in addition to 18F-FDG PET (n = 6) [9,10,11,12,13,14], ß-Amyloid PET (n = 1) [15], metaiodobenzylguanidine myocardial scintigraphy (MIBG) (n = 9) [16,17,18,19,20,21,22,23,24], brain perfusion SPECT imaging with 99mtechnetium-exametazime (n = 1) [25], 99mtechnetium-ethyl cysteinate dimer (n = 1) [17], and N-isopropylp-[123I] iodoamphetamine (n = 1) [26], 44% (n = 26) of the studies included patients with LBD only, while a mixed patient population with different types of dementia were included in the rest of the studies, in particular Alzheimer’s disease (AD) (n = 33), frontotemporal dementia (FTD) (n = 8), corticobasal syndrome (CBS) (n = 4), multi-system atrophy (MSA) (n = 2), progressive supranuclear palsy (PSP) (n = 2), Creutzfeldt-Jakob disease (CJD) (n = 1), vascular dementia (VD) (n = 3), vascular parkinsonism (VP) (n = 2), and normal pressure hydrocephalus (NPH) (n = 1).
Only a few studies (n = 31) reported disease duration [9, 10, 12,13,14, 18, 21, 22, 25, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48].
The mean patient age was 74 years and ranged from 64 to 82 years. The mean percentage of male patients was approximately 60%.
The diagnostic performance of dopamine transporter imaging in the assessment of nigrostriatal function loss was investigated in 19 studies (32%) in patients with DLB [17, 19, 20, 22, 23, 27, 35,36,37, 45, 49,50,51,52,53,54,55,56,57], while the differentiation of DLB from other entities in the Lewy body disease spectrum (DLB, PD, PDD) was assessed in 15 studies (25%) [18, 24, 26, 27, 29, 30, 35, 37,38,39, 43, 47, 53, 58, 59]. The correlation of dopamine transporter imaging to clinical phenotypes, core symptoms and clinical scores in DLB was addressed in 19 studies (32%) [11, 14,15,16, 18, 32, 33, 35, 40, 41, 44, 48, 58, 60,61,62,63,64,65]. Other studies compared the outcome of functional dopaminergic scintigraphic imaging to perfusion SPECT (rCBF) or 18F-FDG PET/CT (n = 8) [9, 10, 12, 13, 17, 25, 26, 49] or MIBG myocardial scintigraphy (n = 7) [17, 20,21,22,23,24, 54]. A total of 31% (n = 18) of the studies investigated the role of functional dopaminergic scintigraphic imaging in differentiating DLB from other types of dementia [12, 13, 21,22,23, 31, 34, 35, 37, 42, 42, 43, 49, 54, 59, 62, 66, 67]. The comparison of striatal dopamine receptor binding with extrastriatal serotonin transporter binding was the subject of 4 studies only (7%) [29, 30, 38, 46].
Technical aspects
Heterogeneous technical aspects were found among the included studies (Table 2). The radiotracer used was [123I]N‑ω‑fluoropropyl‑2β‑carbomethoxy‑3β‑(4‑iodophenyl) nortropane (123I-FP-CIT) in 54 studies (91.5%) [all others], 2 beta-carboxymethoxy-3 beta-(4-iodophenyl)tropane (123I-ß-CIT) in 2 studies (3.4%) [12, 39], 123I-N-(3-iodoprop-2E-enyl)-2-b-carbomethoxy-3b-(4-methylphenyl) nortropane (123I-PE2I) in one study (1.7%) [48], and Technetium-99 m labeled tropane derivative (99mTc-TRODAT-1) in 2 studies (3.4%) [9, 60]. The hybrid imaging modality was SPECT/CT was used in 20 studies [9,10,11, 14, 15, 17, 19, 20, 22, 26, 29,30,31, 33, 35, 37, 52, 54, 58, 60], while SPECT alone was used in 29 studies [16, 18, 23,24,25, 34, 37,38,39,40,41,42,43,44,45, 47,48,49,50,51, 55, 56, 62,63,64,65,66, 68] and the combination of SPECT and MRI was used in 11 studies [13, 27,28,29,30, 32, 46, 53, 57, 60, 61]. The reported mean injected activity of radiolabelled 123I-FP-CIT ranged from 110 to 210 MBq (in absolute values). The time interval between radiotracer injection and image acquisition varied among studies, ranging from 2 to 6 h after injection. The duration of acquisition of images also varied among studies for FP-CIT, ranging from 24 to 60 min. Some studies (n = 27) did not report one or more of the aforementioned technical aspects.
Image analysis was performed using visual analysis in 9 studies (15%), semi-quantitative analysis 29 studies (49%) and a combination of semi-quantitative and visual analysis in 16 studies (27%) (Table 2). Briefly, semi-quantitative analysis is the quantification of specific binding ratios using the occipital lobe for intensity normalization from off-target binding of the radiotracer.
Main findings
DLB versus other dementia and LBD spectrum
The clinical differentiation of DLB from other forms of dementia like AD can be challenging, as clinical features may overlap and co-pathologies often occur [69, 70]. Multiple studies confirm that dopaminergic imaging can help distinguish DLB from AD [13, 21, 22, 35,36,37, 49, 59, 66].
DLB versus AD
Dopamine transport (DAT) imaging can help distinguish DLB from AD in vivo through the measure of specific binding ratios (SBRs) of the radioligand. The specific (i.e., bilateral caudate nuclei, putamen) to non-specific (i.e., occipital cortex) FP-CIT binding ratio in DLB patients is lower than in AD patients [49]. Uptake of FP-CIT in the putamen is significantly lower in patients with DLB compared to those with AD, and discordant cases (i.e., AD patients with very low putamen uptake) exist but often show mixed LB and AD pathologies in post-mortem neuropathological confirmation studies [13]. Compared to patients with AD, patients with DLB have reduced FP-CIT binding on all levels of the striatum, i.e., caudate nucleus, anterior and posterior putamen [36]. With regards to laterality, when analyzed using the mean right and left SBRs, FP-CIT uptake is markedly lower in patients with DLB as compared to patients with AD [21].
In addition to distinguishing DLB from AD, DAT SPECT imaging also allows the distinction of DLB from amnestic mild cognitive impairment, considered by some as a prodromal stage of AD with an accuracy of 88% [31]. Further, some authors provide evidence for distinguishing mild cognitive impairment associated DLB from that associated with AD [54, 62]
DLB versus FTD
Frontotemporal degeneration (FTD) can be difficult to distinguish from DLB by visual rating of FP-CIT alone [34]. However, semi-quantitative assessment of the putaminal binding and the binding ratio of FP-CIT, as well as the combination of these two parameters provides high accuracy to distinguish DLB from FTD (AUC 0.92, 0.91 and 0.97 respectively) [42]. Tiraboschi et al. recognize the possibility to rule out dementia subtypes like FTD and progressive supranuclear palsy (PSP) using DAT imaging as well as MIBG myocardial scintigraphy. They recognized in all such patients that striatal FP-CIT uptake was reduced, whereas uptake of 123I-MIBG was normal [23].
DLB versus PSP
In accordance with previous knowledge in the literature, PSP has a markedly decreased striatal DAT and a uniform involvement in the caudate and putamen [71, 72], but this is when comparing PSP to PD and MSA, not DLB, and is thus outside the scope of our systematic review.
DLB versus PD and PDD
There are considerable clinical and pathological similarities between dementia with Lewy bodies (DLB) and idiopathic Parkinson’s disease (PD). However, dopaminergic SPECT imaging may identify differences in patterns of dopaminergic deficit between each entity. For instance, Walker et al. showed that DLB patients have lower FP-CIT binding in the caudate nucleus than PD patients, and that PD patients have a greater asymmetry of uptake in the posterior putamen, confirming a selective pattern of dopaminergic degeneration in both entities (i.e., degeneration of ventrolateral nigral neurons in PD) [47].
Parkinson’s disease with dementia (PDD) shares very similar clinical and cognitive features with DLB. Colloby et al. performed serial FP-CIT SPECT studies, which found similar rates of dopaminergic loss in DLB, PD and PDD [27].
With regards to DLB versus PD, Ransmayr et al. found that DLB presented with more severe loss of dopaminergic transporter function than PD [39].
FP-CIT SPECT has a low specificity in differentiating PD and DLB from other degenerative parkinsonian syndromes, i.e., atypical parkinsonian syndromes like multisystem atrophy (MSA), corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP), as they all demonstrate striatal dopaminergic deficits [24]. In a prospective analysis, Nicastro et al. confirmed this as visual and semi-quantitative assessment of FP-CIT SPECT is normal in only a negligible proportion of patients with DLB and other degenerative parkinsonian syndromes [35].
Correlation of DAT imaging with clinical presentations & scores
Dopaminergic imaging and parkinsonism
The association between parkinsonian symptoms (i.e., extra-pyramidal motor symptoms like rigidity, brady-/akinesia) and FP-CIT uptake has been studied but results are controversial: some authors [48] found no significant difference between striatal dopamine transport availability and severity of motor parkinsonism measured by the Hoehn and Yahr score in DLB patients, whereas others like Siepel et al. did [41].
Chiu et al. demonstrated that a motor dysfunction questionnaire (MDQ) used to distinguish characteristic parkinsonian features of DLB patients positively correlates with the Unified Parkinson Disease Rating Scale motor scale (UPDRS-m) as well as with dopamine transporter imaging [60]. A composite scale of MDQ and visual rating of DaTscans is more accurate to distinguish DLB from AD or healthy controls than DaTscan or the MDQ questionnaire alone (see Table 3). The UPDRS-m also inversely correlates with FP-CIT uptake in the caudate and the putamen, and patients with even mild extra-pyramidal symptoms had similarly less abnormal FP-CIT uptake than those with severe parkinsonism [61]. Nicastro et al. showed that patients with DLB with parkinsonism features had more pronounced dysfunction of putaminal uptake versus a diffuse pattern and higher uptake values in patients with DLB and without parkinsonism features [35].
In summary, despite the initial ambivalence in the literature, these data show that dopaminergic scintigraphic imaging can correlate with the presence and severity of motor parkinsonism in DLB patients, even at early stages of symptoms, when used in combination of clinical scores and questionnaires. More importantly, parkinsonism in DLB can be highlighted through specific patterns of radiotracer uptake.
Prodromal DLB
Mild cognitive impairment (MCI) with one or more core features of DLB (fluctuations of attention and cognitive impairment, visual hallucinations, rapid eye-movement (REM) sleep behavior disorder (RBD), and parkinsonism) corresponds to the prodromal phase of DLB that may be present several years before a clinical diagnosis, referred as MCI with Lewy bodies (MCI-LB) [68]. A prospective longitudinal case study carried out over 2 to 5 years by Siepel et al. showed that visually assessed FP-CIT SPECT detects patients with DLB before they develop the complete clinical syndrome, and that the frequency and severity of parkinsonism and cognitive fluctuations increased during the follow-up period [65]. Other research criteria for prodromal DLB include psychiatric-onset DLB and delirium-onset DLB, but these entities have not been extensively studied with regards to dopaminergic imaging and are not included in the scope of this review [73].
Neuropsychiatric symptoms (cognition, awareness and hallucinations)
Neuropsychiatric presentation of patients is a key feature in dementia with Lewy bodies. Visual hallucinations are the psychiatric symptoms that are included among the core clinical features of DLB, but other neuropsychiatric features are now considered as supportive features, such as non-visual hallucinations such as presence hallucinations, delusions, depression, and anxiety [3]. Donaghy et al. compared prodromal DLB and AD patients and showed that MCI-LB patients were four times more likely than MCI-AD patients to present two or more of the five supportive neuropsychiatric symptoms. [62]. However, studies that show a link between dopaminergic imaging and neuropsychiatric symptoms are scarce [74]. Nonetheless, evidence shows that the onset of symptoms in DLB patients who show dopaminergic dysfunction through positive FP-CIT SPECT occurs more often with psychiatric symptoms than cognitive impairment. Furthermore, it is generally accepted that the neuropsychological profile of DLB patients will show impacted attentional, executive and visuospatial deficits with relatively preserved episodic memory, unlike AD [64]. When episodic memory is affected in DLB patients, it suggests the presence of a concomitant AD [74]. Although less prevalent in patients with DLB than AD, Iizuka et al. showed that the awareness of memory-deficits in DLB patients, measured by the discrepancy between subjective and objective memory scores, is more impaired than in patients with normal cognition. Interestingly, the awareness index does not correlate with striatal DAT density, but does with hypometabolism of cortical midline structures (i.e., bilateral occipital and parietal association cortices, bilateral temporal cortex, precuneus, and posterior cingulate cortex) shown by 18F-FDG-PET [11].
Reduced FP-CIT SPECT binding is useful in predicting the development of LBD within five years in patients presenting with isolated or idiopathic RBD (iRBD), as shown by Kaplan–Meier survival analysis by Miyamoto and colleagues [58]. Important non-visual hallucinations that DLB patients frequently present are presence hallucinations (PH), corresponding to a vivid sensation of somebody nearby in the absence of any physical person [14]. PH occurs frequently in PD, especially at early stages [75,76,77]. Nicastro et al. showed that DLB patients with PH have widespread frontoparietal 18F-FDG hypometabolism, and that 18F-FDG uptake in the ventral premotor cortex (vPMC) is negatively correlated with FP-CIT uptake in the caudate nucleus. As for visual hallucinations (VH), Roselli et al. have reported that FP-CIT uptake is inversely associated with their severity and frequency [40]. Among other non-motor symptoms associated with DLB, duration of olfactory dysfunction negatively correlates with striatal specific binding ratios of FP-CIT SPECT [32]. Furthermore, clinical scores that test olfactory decline and susceptibility to visual hallucinations, the odor stick identification and pareidolia tests respectively, can aid in differentiating DLB from AD, albeit less sensitive and specific than FP-CIT uptake [16] (see Table 3). In a more recent study, Nakahara et al. showed that olfactory dysfunction correlates with lower FP-CIT binding independently of cerebral blood flow in the frontal lobe (assessed through perfection SPECT), unlike clinically assessed frontal lobe dysfunction which only showed a negative correlation in patients with frontal lobe hypoperfusion [18].
In DLB patients, a higher level of education is associated with better scores in neuropsychological tests that assess visuoconstructive functions and retrieval strategies, and correlates with higher dopamine transporter binding in the striatum, caudate nucleus and putamen bilaterally [33].
Diagnostic performance of DAT imaging and other modalities
A retrospective analysis assessing the impact of dopamine transporter imaging on patients with suspected DLB during their diagnostic workup showed significant impact on diagnosis and subsequent management, as 90% of patient with an abnormal DaTscan had a postscan clinical diagnosis of DLB, and 95% of patients with normal imaging had an alternative clinical diagnosis [50]. Similarly, a randomized multi-center trial by Walker et al. showed that DAT imaging significantly helps clinicians change their diagnosis from possible DLB to probable DLB [57].
Patients who meet clinical criteria for DLB but have a normal DaTscan remain a challenge. In this context, a retrospective study from the Amsterdam Dementia Cohort [45] found that in almost all DLB patients with negative DaTscans, a follow-up 123I-FP-CIT SPECT (average 1.5 years after first DaTscan) was abnormal emphasizing the importance of repeating DaTscans if the clinical diagnosis is difficult.
There seems to be a benefit in combining visual and semi-quantitative assessments to discriminate between DLB and AD patients, with a combined sensitivity of 100% [35]. Oliveira et al. computed the bihemispheric caudate binding potentials (CBP), putamen binding potentials (PBP) and putamen-to-caudate ratios (PCR) (derived from the ratio of mean counts across voxels of the regions of interest over the mean counts across voxels of the background reference region), finding that DLB patients had lower CBP and PBPs than AD patients and higher PCR than PD patients, providing an accuracy of 94% in classifying DLB versus AD and DLB versus PD [59].
The use of other technical methods to measure FP-CIT binding, such as through the use of software packages for brain imaging analyses (e.g., Statistical Parametrical Mapping), has been shown to have comparable discriminatory power as visual rating [28].
Multiple studies compared the diagnostic accuracy of dopaminergic imaging using FP-CIT against perfusion SPECT/PET modalities, or their combined use. In comparison to dopaminergic transporter imaging, 18F-FDG PET imaging was less accurate and had a lower effect size, but regional hypometabolism in the lateral occipital cortex can be used to exclude the diagnosis of DLB, and the so-called “cingulate island sign” (relative preservation of the mid or posterior cingulate gyrus) is very specific to DLB [12]. Huber et al. showed an inverse relationship between FP-CIT uptake and glucose metabolism in the basal ganglia and limbic regions, referred to relative glucose hypermetabolism [10]. Other tracers such as 99mTc-exametazime also has lower accuracy than FP-CIT in distinguishing between AD and DLB (AUC of 0.64 and 0.83) [25]. However, this radiotracer can identify selective occipital hypoperfusion on LBD, as compared to decreased temporo-parietal blood flow of AD [49]. Other studies confirm these regional differences in hypometabolism, i.e., reduced 18F-FDG PET uptake in the visual cortex in DLB patients, and specific decreased blood flow in parieto-temporo-occipital association cortices in a form of AD [9].
Patients in the LBD spectrum have regional reduction in striatal FP-CIT uptake and changes in brain perfusion, as measured by 123I-IMP SPECT, such that decreased putamen-to-caudate ration correlates with hypoperfusion in the brainstem whereas decreased caudate-to-putamen ratio correlates with right temporal cortex hypoperfusion [26].
Cardiac 123I-metaiodobenzylguanidine sympathetic innervation imaging (MIBG) is included in the diagnostic criteria in the most recent consensus criteria for DLB [3] as an indicative biomarker. Overall, FP-CIT SPECT and MIBG myocardial scintigraphy have similar diagnostic accuracies when distinguishing DLB from other dementias, although FP-CIT SPECT has the highest sensitivity [24]. Other studies show that MIBG scintigraphy is more specific for excluding non-DLB dementias and is particularly useful when the only core feature exhibited by the patient is parkinsonism [23]. Concerning the prodromal stage of DLB, MIBG myocardial scintigraphy has a specificity, sensitivity and accuracy of 59%, 88%, and 75%, respectively, to distinguish patients with MCI-LB from those with MCI-AD [54].
Multimodal imaging shows high accuracy in diagnosing DLB. Miyagawa et al. demonstrated almost perfect areas under the curve (AUC), ranging from 0.987 to 0.996, in differentiating DLB from AD when using FP-SPECT combined with 18F-FDG PET and 11C-Pittsburgh compound B (PiB)-PET [13]. It also seems useful to combine dopamine transporter imaging, myocardial scintigraphy and brain-perfusion SPECT for the diagnosis of DLB, which yields a sensitivity of 100% [17]. Sakamoto et al. show that MIBG myocardial scintigraphy alone is superior (sensitivity, specificity and accuracy of 85, 91%, and 89%) to a combined index of FP-CIT SPECT and MIBG SPECT (76.6%, 74.3%, and 75.2%) [20]. Another study by Shimizu et al. compared the diagnostic performance of FP-CIT SPECT, MIBG, perfusion SPECT, and MRI (for quantification of atrophy), and found that FP-CIT SPECT is the most accurate modality overall (sensitivity and specificity of 93.8% and 93.8%, respectively) to differentiate between DLB and AD, and MIBG myocardial scintigraphy has low sensitivity but high specificity (62.5% and 100%, respectively) [22]. Combining DAT SPECT and MIBG myocardial scintigraphy surpass the accuracy of either modalities alone according to some authors [21].
Finally, amyloid PET imaging, using the 11C-Pittsburgh compound B (PiB) radiotracer, has been compared to standard FP-CIT imaging and do not have a higher diagnostic accuracy (measured by AUC) to distinguish DLB from AD [13]. In MCI-LB, it is possible to study the co-existence of β-amyloid pathology through Amyloid PET, but the phenotype of both β-amyloid positive and FP-CIT positive is rare, as the majority of the studied MCI-LB patients have decreased dopaminergic activity and low β-amyloid deposition [15]. Comparative performance between more recent AmyPET radiotracers such as flutemetamol and florbetapir and FP-CIT imaging has not been studied in the literature.
DAT versus SERT
FP-CIT has affinity to both dopamine (DAT) and serotonin (SERT) transporters, therefore it is possible to image both the integrity of dopaminergic striatal and serotoninergic extrastriatal systems simultaneously [30]. However, extrastriatal serotonin transporter (SERT) is seldom studied along with striatal dopaminergic transporter (DAT) binding using FP-CIT SPECT imaging. Some authors found no difference in extrastriatal SERT binding between DLB and PD patients using FP-CIT [29], but others showed that only DLB patients had impairments in serotoninergic pathways of the thalamus [38]. Further, Joling et al. showed that DLB patients have lower hypothalamic SERT availability as compared to standard reference [30]. Finally, Van der Zande et al. studied DLB patients with concomitant AD pathology (defined with cerebrospinal fluid tau/aβ-42 ratio) and found that these patients had lower extrastriatal FP-CIT SERT binding in limbic brain regions (i.e., left amygdala) [46].
Medication such as chronic cholinesterase inhibitors (ChEi) do not influence the radioligand’s binding to striatal DATs therefore do not influence the diagnostic performance of 123I-FP-CIT imaging [43]. The authors took into account the effect of selective serotonin reuptake inhibitors (SSRIs) like citalopram and paroxetine on striatal FP-CIT binding, increasing its availability: they had similar proportions of subjects taking antidepressants in those taking ChEi and those without ChEi. The interaction between serotonin and dopamine systems in the striatum is of interest since depression is one of the prodromal symptoms of DLB and AD, and the use of SSRIs is thus frequent in these patients.
Discussion
Summary of evidence
The present study is an updated systematic literature review involving 59 primary studies, constituting the largest collection of studies relating to the diagnosis of dementia with Lewy bodies using scintigraphic dopaminergic imaging to this day. Based on the body of evidence that was hereby studied, the use of dopamine transporter imaging provides support in the diagnosis of DLB from other forms of dementia, and within the larger spectrum of Lewy body diseases. Dopaminergic scintigraphic imaging enables accurate discrimination between DLB and AD. As for other forms of neurodegenerative parkinsonian syndromes such as FTD, PSP, and CBD, semi-quantitative measures of DAT uptake cannot clearly differentiate them from DLB. Within the spectrum of Lewy body disease, some patterns of FP-CIT uptake (i.e., lower FP-CIT binding in the caudate nucleus in DLB than PD patients and greater asymmetry of uptake in the posterior putamen with degeneration of ventrolateral nigral in PD patients) have been proposed to specifically identify PD from DLB and PDD, whereas discriminating between the latter two is more challenging. Whether this is due to the different modalities of pharmacological treatments and the patients’ clinical response remains unclear and requires further investigation. Similarly, we could ask ourselves what effects cognitive fluctuations have on FP-CIT binding. Clear patterns of radioligand uptake can be identified using semi-quantitative and/or simple visual rating, and this can be done in prodromal stages of dementia. There is solid evidence to consider motor symptoms and parkinsonism, measured by the validated clinical scores, as adjunct factors to FP-CIT SPECT imaging. The same goes for non-motor symptoms, especially behavioral symptoms. These clinical variables greatly aid the diagnostic accuracy of functional imaging, even at the prodromal stage of DLB. Specifically for the neuropsychiatric symptoms that DLB initially present, such as visual and non-visual hallucinations, relevance of FP-CIT SPECT imaging in the early stages of the disease exists and has been shown in only a few studies, and further investigations are required. For instance, it is unclear whether patterns of ligand uptake can be differentially identified for patients presenting major or minor hallucinations, as the methods of classifying and reporting of these symptoms is not standardized and has been insufficiently studied with regards to dopaminergic scintigraphic imaging.
DAT imaging can be complemented by other imaging modalities, namely by myocardial MIBG scintigraphy, brain-perfusion SPECT and 18F-FDG-PET. Essentially, MIBG myocardial scintigraphy is more specific than DAT SPECT imaging, whereas the latter is more sensitive in detecting DLB. FDG-PET can be used to highlight certain signs that are highly specific to DLB, such as the relative preservation of the posterior cingulate (cingulate island sign) and occipital hypometabolism. Combinations of striatal scintigraphy, as well as brain-perfusion SPECT and FDG-PET can identify regional correlations of hypoperfusion and striatal DAT availability and ascertain the diagnosis of DLB with greater sensitivity and specificity.
The current review updates the meta-analysis performed by Nihashi et al. in 2018, which itself was an update of their 2015 meta-analysis [6, 78]. Since we did not perform a meta-analysis, we did not compare specificities and sensitivities with these previous studies. Admittedly, we considered there to be too much heterogeneity in the studied populations and subsequent imperfection in comparing reference results. However, our review adds 23 new studies, all but two (n = 21) including the use of semi-quantitative assessment. In the study by Nihashi and colleagues, semi-quantitative image analysis was still relatively new, thus limiting the number of analyzed articles. In our review, we propose groups of study findings that are pooled according to their main outcomes (see Fig. 3). This allows identification of clinically relevant contexts (i.e., facing pathologies in the LBD spectrum, other forms of dementia, or having specific clinical scores) in which dopaminergic scintigraphic imaging is efficient.
According to the 2017 DLB consensus criteria, decreased uptake on SPECT is an indicative biomarker that supports the diagnosis of DLB, in addition to the four core clinical features [3]. In our systematic review, we noted that these criteria were respected, and the use of indicative biomarkers for DLB is clearly supported by direct biological biomarkers. There has been no updated consensus criterion since 2017. We identified studies where prodromal DLB could be identified and form a clinical entity, as some studies have shown that screening using SPECT imaging is possible in healthy or paucisymptomatic patients, even years before the diagnosis of DLB [62, 65]. Future research perspectives and biomarker-based research could be anchored towards potential treatment trials in the identified prodromal DLB patients and pave the way for early intervention in pre-dementia syndromes.
Limitations
We identified several limitations in the various studies we analyzed, such as the heterogeneity of radiotracers that were sometimes used. Furthermore, study designs and outcome measures varied considerably between studies. Extraction of accurate data on true negatives/positives and false negatives/positives was not systematically possible, and a pooled analysis of the studies would most probably entail a large heterogeneity, which is why we decided not to pursue a meta-analysis.
Regarding the technical aspects, image acquisition was usually precisely reported, with details on the injected doses of radiotracers, time-intervals between injection and imaging, types of reconstructions, and algorithms used.
In all the reviewed studies, image analysis was performed either by visual rating alone, semi-quantitative measures using specific binding ratios of the radiotracer in the striatum, or a combination of both methods. However, these methods have the limitation of being user-dependent and lack anatomical standardization. In fact, a few studies in the literature address this issue and point to a promising role of quantitative assessment of DAT loss in the striatum using computer tomography (CT) data acquired on hybrid SPECT/CT equipment [79]. Using CT in order to apply anatomical standardization to dopaminergic scintigraphic imaging, authors like Yokoyama et al. proposed a method that avoids deformation errors due to DaTscan-specific templates lacking structural information [79]. Further research using quantitative assessment is thus required in order to more accurately discriminate Dementia with Lewy bodies and better understand the physiopathology of its distinct clinical features.
Conclusions
Dopaminergic scintigraphic imaging is an efficient method to diagnose dementia with Lewy bodies and distinguish it from other forms of dementia. This is done through semi-quantitative and visual methods, and very little work has been done including the use of absolute tracer uptake quantification or the CT-guided anatomically standardized methods to accurately measure dopamine transporter decrease in the striatum. Therefore, further research is needed in order to assess dopaminergic degeneration more accurately and to possibly predict the degree of severity and progression of dementia with Lewy bodies.
References
Geser F, Wenning GK, Poewe W, McKeith I. How to diagnose dementia with Lewy bodies: State of the art. Mov Disord. 2005;20:S11–20.
Walker Z, Possin KL, Boeve BF, Aarsland D. Lewy body dementias. Lancet. 2015;386:1683–97.
McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor J-P, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89:88–100.
Morbelli S, Esposito G, Arbizu J, Barthel H, Boellaard R, Bohnen NI, et al. EANM practice guideline/SNMMI procedure standard for dopaminergic imaging in Parkinsonian syndromes 1.0. Eur J Nucl Med Mol Imaging. 2020;47:1885–912.
Taylor J-P, McKeith IG, Burn DJ, Boeve BF, Weintraub D, Bamford C, et al. New evidence on the management of Lewy body dementia. Lancet Neurol. 2020;19:157–69.
Nihashi T, Ito K, Terasawa T. Diagnostic accuracy of DAT-SPECT and MIBG scintigraphy for dementia with Lewy bodies: an updated systematic review and Bayesian latent class model meta-analysis. Eur J Nucl Med Mol Imaging. 2020;47:1984–97.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021:n71.
Whiting PF. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529.
Gupta V, Verma R, Ranjan R, Belho ES, Seniaray N, Dinand V, et al. Metabolic imaging patterns in posterior cortical atrophy and Lewy body dementia. Nucl Med Commun. 2019;40:1275–82.
Huber M, Beyer L, Prix C, Schönecker S, Palleis C, Rauchmann B, et al. Metabolic correlates of dopaminergic loss in dementia with Lewy bodies. Mov Disord. 2020;35:595–605.
Iizuka T, Kameyama M. Metabolic correlate of memory-deficit awareness in dementia with Lewy bodies: implication in cortical midline structure. Psychiatry Res Neuroimaging. 2017;269:43–7.
Lim SM, Katsifis A, Villemagne VL, Best R, Jones G, Saling M, et al. The 18 F-FDG PET cingulate island sign and comparison to 123 I-β-CIT SPECT for diagnosis of dementia with Lewy bodies. J Nucl Med. 2009;50:1638–45.
Miyagawa T, Przybelski SA, Maltais D, Min H-K, Jordan L, Lesnick TG, et al. The value of multimodal imaging with 123I-FP-CIT SPECT in differential diagnosis of dementia with Lewy bodies and Alzheimer’s disease dementia. Neurobiol Aging. 2021;99:11–8.
Nicastro N, Stripeikyte G, Assal F, Garibotto V, Blanke O. Premotor and fronto-striatal mechanisms associated with presence hallucinations in dementia with Lewy bodies. NeuroImage Clin. 2021;32:102791.
Chen Q, Lowe VJ, Boeve BF, Przybelski SA, Miyagawa T, Senjem ML, et al. β-Amyloid PET and 123 I-FP-CIT SPECT in mild cognitive impairment at risk for Lewy body dementia. Neurology. 2021;96:e1180–9.
Inagawa Y, Kanetaka H, Tsugawa A, Sakurai S, Serisawa S, Shimizu S, et al. Efficacy of olfactory and pareidolia tests compared with that of indicative biomarkers in diagnosis of dementia with Lewy bodies. Front Neurol. 2020;11:540291.
Kobayashi S, Makino K, Hatakeyama S, Ishii T, Tateno M, Iwamoto T, et al. The usefulness of combined brain perfusion single-photon emission computed tomography, dopamine-transporter single-photon emission computed tomography, and 123 I-metaiodobenzylguanidine myocardial scintigraphy for the diagnosis of dementia with Lewy bodies: The usefulness of neuroimaging for DLB. Psychogeriatrics. 2017;17:247–55.
Nakahara A, Sengoku R, Umehara T, Matsuno H, Yamazaki M, Oka H. Frontal lobe dysfunction is associated with reduced DAT-SPECT accumulation in Lewy body disease. J Neurol Sci. 2021;430:119998.
Roberts G, Durcan R, Donaghy PC, Lawley S, Ciafone J, Hamilton CA, et al. Accuracy of cardiac innervation scintigraphy for mild cognitive impairment with Lewy bodies. Neurology. 2021;96:e2801–11.
Sakamoto F, Shiraishi S, Ogasawara K, Tsuda N, Nakagawa M, Tomiguchi S, et al. A diagnostic strategy for Lewy body disease using DAT-SPECT, MIBG and Combined index. Ann Nucl Med. 2020;34:415–23.
Shimizu S, Hirao K, Kanetaka H, Namioka N, Hatanaka H, Hirose D, et al. Utility of the combination of DAT SPECT and MIBG myocardial scintigraphy in differentiating dementia with Lewy bodies from Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2016;43:184–92.
Shimizu S, Kanetaka H, Hirao K, Fukasawa R, Namioka N, Hatanaka H, et al. Neuroimaging for diagnosing dementia with Lewy bodies: what is the best neuroimaging technique in discriminating dementia with Lewy bodies from Alzheimer’s disease?: Neuroimaging for diagnosing DLB. Geriatr Gerontol Int. 2017;17:819–24.
Tiraboschi P, Corso A, Guerra UP, Nobili F, Piccardo A, Calcagni ML, et al. 123 I-2β-carbomethoxy-3β-(4-iodophenyl)- N -(3-fluoropropyl) nortropane single photon emission computed tomography and 123 I-metaiodobenzylguanidine myocardial scintigraphy in differentiating dementia with lewy bodies from other dementias: A comparative study. Ann Neurol. 2016;80:368–78.
Treglia G, Cason E, Cortelli P, Gabellini A, Liguori R, Bagnato A, et al. Iodine-123 metaiodobenzylguanidine scintigraphy and iodine-123 Ioflupane single photon emission computed tomography in Lewy Body diseases: complementary or alternative techniques?: MIBG and FP-CIT Scintigraphy in Lewy Body Diseases. J Neuroimaging. 2014;24:149–54.
Colloby SJ, Firbank MJ, Pakrasi S, Lloyd JJ, Driver I, McKeith IG, et al. A comparison of 99mTc-exametazime and 123I-FP-CIT SPECT imaging in the differential diagnosis of Alzheimer’s disease and dementia with Lewy bodies. Int Psychogeriatr. 2008;20:1124.
Iwabuchi Y, Shiga T, Kameyama M, Miyazawa R, Seki M, Ito D, et al. Striatal dopaminergic depletion pattern reflects pathological brain perfusion changes in lewy body diseases. Mol Imaging Biol [Internet]. 2022 [cited 2022 Jul 4]; Available from: https://link.springer.com/10.1007/s11307-022-01745-x
Colloby SJ, Williams ED, Burn DJ, Lloyd JJ, McKeith IG, O’Brien JT. Progression of dopaminergic degeneration in dementia with Lewy bodies and Parkinson’s disease with and without dementia assessed using 123I-FP-CIT SPECT. Eur J Nucl Med Mol Imaging. 2005;32:1176–85.
Colloby SJ, O’Brien JT, Fenwick JD, Firbank MJ, Burn DJ, McKeith IG, et al. The application of statistical parametric mapping to 123I-FP-CIT SPECT in dementia with Lewy bodies, Alzheimer’s disease and Parkinson’s disease. NeuroImage. 2004;23:956–66.
Joling M, Vriend C, van der Zande JJ, Lemstra AW, van den Heuvel OA, Booij J, et al. Lower 123I-FP-CIT binding to the striatal dopamine transporter, but not to the extrastriatal serotonin transporter, in Parkinson’s disease compared with dementia with Lewy bodies. NeuroImage Clin. 2018;19:130–6.
Joling M, Vriend C, Raijmakers PGHM, van der Zande JJ, Lemstra AW, Berendse HW, et al. Striatal DAT and extrastriatal SERT binding in early-stage Parkinson’s disease and dementia with Lewy bodies, compared with healthy controls: An 123I-FP-CIT SPECT study. NeuroImage Clin. 2019;22:101755.
Kamagata K, Nakatsuka T, Sakakibara R, Tsuyusaki Y, Takamura T, Sato K, et al. Diagnostic imaging of dementia with Lewy bodies by susceptibility-weighted imaging of nigrosomes versus striatal dopamine transporter single-photon emission computed tomography: a retrospective observational study. Neuroradiology. 2017;59:89–98.
Kasanuki K, Iseki E, Ota K, Kondo D, Ichimiya Y, Sato K, et al. 123I-FP-CIT SPECT findings and its clinical relevance in prodromal dementia with Lewy bodies. Eur J Nucl Med Mol Imaging. 2017;44:358–65.
Lamotte G, Morello R, Lebasnier A, Agostini D, Bouvard G, De La Sayette V, et al. Influence of education on cognitive performance and dopamine transporter binding in dementia with Lewy bodies. Clin Neurol Neurosurg. 2016;146:138–43.
Morgan S, Kemp P, Booij J, Costa DC, Padayachee S, Lee L, et al. Differentiation of frontotemporal dementia from dementia with Lewy bodies using FP-CIT SPECT. J Neurol Neurosurg Psychiatry. 2012;83:1063–70.
Nicastro N, Garibotto V, Allali G, Assal F, Burkhard PR. Added value of combined semi-quantitative and visual [123I]FP-CIT SPECT analyses for the diagnosis of dementia with Lewy bodies. Clin Nucl Med. 2017;42:e96–102.
O’Brien JT, Colloby S, Fenwick J, Williams ED, Firbank M, Burn D, et al. Dopamine transporter loss visualized with FP-CIT SPECT in the differential diagnosis of dementia with Lewy bodies. Arch Neurol. 2004;61:919.
O’Brien JT, McKeith IG, Walker Z, Tatsch K, Booij J, Darcourt J, et al. Diagnostic accuracy of 123 I-FP-CIT SPECT in possible dementia with Lewy bodies. Br J Psychiatry. 2009;194:34–9.
Pilotto A, Schiano di Cola F, Premi E, Grasso R, Turrone R, Gipponi S, et al. Extrastriatal dopaminergic and serotonergic pathways in Parkinson’s disease and in dementia with Lewy bodies: a 123I-FP-CIT SPECT study. Eur J Nucl Med Mol Imaging. 2019;46:1642–51.
Ransmayr G, Seppi K, Donnemiller E, Luginger E, Marksteiner J, Riccabona G, et al. Striatal dopamine transporter function in dementia with Lewy bodies and Parkinson’s disease. Eur J Nucl Med Mol Imaging. 2001;28:1523–8.
Roselli F, Pisciotta NM, Perneczky R, Pennelli M, Aniello MS, De Caro MF, et al. Severity of neuropsychiatric symptoms and dopamine transporter levels in dementia with Lewy bodies: A 123 I-FP-CIT SPECT study: DAT Levels and Neuropsychiatric Symptoms in DLB. Mov Disord. 2009;24:2097–103.
Siepel FJ, Dalen I, Grüner R, Booij J, Brønnick KS, Buter TC, et al. Loss of Dopamine Transporter Binding and Clinical Symptoms in Dementia With Lewy Bodies: DAT scan in Dementia with Lewy Bodies. Mov Disord. 2016;31:118–25.
Spehl TS, Frings L, Hellwig S, Weiller C, Hüll M, Meyer PT, et al. Role of semiquantitative assessment of regional binding potential in 123I-FP-CIT SPECT for the differentiation of frontotemporal dementia, dementia with Lewy bodies, and Alzheimer’s dementia. Clin Nucl Med. 2015;40:e27–33.
Taylor J-P, Colloby SJ, McKeith IG, Burn DJ, Williams D, Patterson J, et al. Cholinesterase inhibitor use does not significantly influence the ability of 123I-FP-CIT imaging to distinguish Alzheimer’s disease from dementia with Lewy bodies. J Neurol Neurosurg Amp Psychiatry. 2007;78:1069–71.
van de Beek M, van Steenoven I, van der Zande JJ, Porcelijn I, Barkhof F, Stam CJ, et al. Characterization of symptoms and determinants of disease burden in dementia with Lewy bodies: DEvELOP design and baseline results. Alzheimers Res Ther. 2021;13:53.
van der Zande JJ, Booij J, Scheltens P, Raijmakers PGHM, Lemstra AW. [123]FP-CIT SPECT scans initially rated as normal became abnormal over time in patients with probable dementia with Lewy bodies. Eur J Nucl Med Mol Imaging. 2016;43:1060–6.
van der Zande JJ, Joling M, Happach IG, Vriend C, Scheltens Ph, Booij J, et al. Serotonergic deficits in dementia with Lewy bodies with concomitant Alzheimer’s disease pathology: An 123I-FP-CIT SPECT study. NeuroImage Clin. 2020;25:102062.
Walker Z, Costa DC, Walker RWH, Lee L, Livingston G, Jaros E, et al. Striatal dopamine transporter in dementia with Lewy bodies and Parkinson disease: a comparison. Neurology. 2004;62:1568–72.
Ziebell M, Andersen BB, Pinborg LH, Knudsen GM, Stokholm J, Thomsen G, et al. Striatal dopamine transporter binding does not correlate with clinical severity in dementia with Lewy bodies. J Nucl Med. 2013;54:1072–6.
Ceravolo R, Volterrani D, Gambaccini G, Rossi C, Logi C, Manca G, et al. Dopaminergic degeneration and perfusional impairment in Lewy body dementia and Alzheimer?s disease. Neurol Sci. 2003;24:162–3.
Kemp PM, Clyde K, Holmes C. Impact of 123I-FP-CIT (DaTSCAN) SPECT on the diagnosis and management of patients with dementia with Lewy bodies: a retrospective study. Nucl Med Commun. 2011;32:298–302.
Lloyd JJ, Petrides G, Donaghy PC, Colloby SJ, Attems J, O’Brien JT, et al. A new visual rating scale for Ioflupane imaging in Lewy body disease. NeuroImage Clin. 2018;20:823–9.
Maltais DD, Jordan LG, Min H-K, Miyagawa T, Przybelski SA, Lesnick TG, et al. Confirmation of 123 I-FP-CIT SPECT Quantification methods in dementia with Lewy bodies and other neurodegenerative disorders. J Nucl Med. 2020;61:1628–35.
Nicastro N, Burkhard PR, Garibotto V. Scan without evidence of dopaminergic deficit (SWEDD) in degenerative parkinsonism and dementia with Lewy bodies: a prospective study. J Neurol Sci. 2018;385:17–21.
Roberts G, Donaghy PC, Lloyd J, Durcan R, Petrides G, Colloby SJ, et al. Accuracy of dopaminergic imaging as a biomarker for mild cognitive impairment with Lewy bodies. Br J Psychiatry. 2021;218:276–82.
Thomas AJ, Donaghy P, Roberts G, Colloby SJ, Barnett NA, Petrides G, et al. Diagnostic accuracy of dopaminergic imaging in prodromal dementia with Lewy bodies. Psychol Med. 2019;49:396–402.
Walker Z, Jaros E, Walker RWH, Lee L, Costa DC, Livingston G, et al. Dementia with Lewy bodies: a comparison of clinical diagnosis, FP-CIT single photon emission computed tomography imaging and autopsy. J Neurol Neurosurg Amp Psychiatry. 2007;78:1176–81.
Walker Z, Moreno E, Thomas A, Inglis F, Tabet N, Rainer M, et al. Clinical usefulness of dopamine transporter SPECT imaging with 123 I-FP-CIT in patients with possible dementia with Lewy bodies: Randomised study. Br J Psychiatry. 2015;206:145–52.
Miyamoto T, Miyamoto M, Numahata K, Onoue H, Akaiwa Y, Sairenchi T. Reduced dopamine transporter binding predicts early transition to Lewy body disease in Japanese patients with idiopathic rapid eye movement sleep behavior disorder. J Neurol Sci. 2020;414:116821.
Oliveira FPM, Walker Z, Walker RWH, Attems J, Castanheira JC, Silva Â, et al. 123 I-FP-CIT SPECT in dementia with Lewy bodies, Parkinson’s disease and Alzheimer’s disease: a new quantitative analysis of autopsy confirmed cases. J Neurol Neurosurg Psychiatry. 2021;92:662–7.
Chiu P-Y, Wei C-Y, Hung G-U, Wu S-L. Motor dysfunction questionnaire and dopamine transporter imaging composite scale improve differentiating dementia with Lewy bodies from Alzheimer’s disease with motor dysfunction. Front Aging Neurosci. 2021;13:709215.
Del Sole A, Perini G, Lecchi M, Mariani C, Lucignani G, Clerici F. correlation Between 123I-FP-CIT brain SPECT and Parkinsonism in dementia with Lewy bodies: caveat for clinical use. Clin Nucl Med. 2015;40:32–5.
Donaghy PC, Taylor J-P, O’Brien JT, Barnett N, Olsen K, Colloby SJ, et al. Neuropsychiatric symptoms and cognitive profile in mild cognitive impairment with Lewy bodies. Psychol Med. 2018;48:2384–90.
Durcan R, Donaghy PC, Barnett NA, Olsen K, Yarnall AJ, Taylor J-P, et al. Prevalence and severity of symptoms suggestive of gastroparesis in prodromal dementia with Lewy bodies. Int J Geriatr Psychiatry. 2019;34:990–8.
Hansen N, Lange C, Timäus C, Wiltfang J, Bouter C. Assessing nigrostriatal dopaminergic pathways via 123I-FP-CIT SPECT in dementia with Lewy bodies in a psychiatric patient cohort. Front Aging Neurosci. 2021;13:672956.
Siepel FJ, Rongve A, Buter TC, Beyer MK, Ballard CG, Booij J, et al. (123 I)FP-CIT SPECT in suspected dementia with Lewy bodies: a longitudinal case study. BMJ Open. 2013;3:e002642.
Walker Z. Differentiation of dementia with Lewy bodies from Alzheimer’s disease using a dopaminergic presynaptic ligand. J Neurol Neurosurg Psychiatry. 2002;73:134–40.
Tiraboschi P, Salmon DP, Hansen LA, Hofstetter RC, Thal LJ, Corey-Bloom J. What best differentiates Lewy body from Alzheimer’s disease in early-stage dementia? Brain. 2006;129:729–35.
McKeith I, O’Brien J, Walker Z, Tatsch K, Booij J, Darcourt J, et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol. 2007;6:305–13.
Howard E, Irwin DJ, Rascovsky K, Nevler N, Shellikeri S, Tropea TF, et al. Cognitive profile and markers of Alzheimer disease–type pathology in patients with Lewy body dementias. Neurology. 2021;96:e1855–64.
Schumacher J, Gunter JL, Przybelski SA, Jones DT, Graff-Radford J, Savica R, et al. Dementia with Lewy bodies: association of Alzheimer pathology with functional connectivity networks. Brain. 2021;144:3212–25.
Treglia G, Cason E, Gabellini A, Giordano A, Fagioli G. Recent developments in innervation imaging using iodine-123-metaiodobenzylguanidine scintigraphy in Lewy body diseases. Neurol Sci. 2010;31:417–22.
Antonini A, Benti R, De Notaris R, Tesei S, Zecchinelli A, Sacilotto G, et al. 123I-Ioflupane/SPECT binding to striatal dopamine transporter (DAT) uptake in patients with Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Neurol Sci. 2003;24:149–50.
McKeith IG, Ferman TJ, Thomas AJ, Blanc F, Boeve BF, Fujishiro H, et al. Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology. 2020;94:743–55.
Coughlin DG, Ittyerah R, Peterson C, Phillips JS, Miller S, Rascovsky K, et al. Hippocampal subfield pathologic burden in Lewy body diseases vs. Alzheimer’s disease Neuropathol Appl Neurobiol. 2020;46:707–21.
Bernasconi F, Blondiaux E, Potheegadoo J, Stripeikyte G, Pagonabarraga J, Bejr-Kasem H, et al. Robot-induced hallucinations in Parkinson’s disease depend on altered sensorimotor processing in fronto-temporal network. Sci Transl Med. 2021;13:eabc8362.
Fenelon G, Soulas T, de Langavant LC, Trinkler I, Bachoud-Levi A-C. Feeling of presence in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2011;82:1219–24.
Llorca PM, Pereira B, Jardri R, Chereau-Boudet I, Brousse G, Misdrahi D, et al. Hallucinations in schizophrenia and Parkinson’s disease: an analysis of sensory modalities involved and the repercussion on patients. Sci Rep. 2016;6:38152.
Mishima A, Nihashi T, Ando Y, Kawai H, Kato T, Ito K, et al. Biomarkers Differentiating dementia with lewy bodies from other dementias: a meta-analysis. J Alzheimers Dis. 2015;50:161–74.
Yokoyama K, Imabayashi E, Sumida K, Sone D, Kimura Y, Sato N, et al. Computed-tomography-guided anatomic standardization for quantitative assessment of dopamine transporter SPECT. Eur J Nucl Med Mol Imaging. 2017;44:366–72.
Funding
Open access funding provided by University of Lausanne
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jreige, M., Kurian, G.K., Perriraz, J. et al. The diagnostic performance of functional dopaminergic scintigraphic imaging in the diagnosis of dementia with Lewy bodies: an updated systematic review. Eur J Nucl Med Mol Imaging 50, 1988–2035 (2023). https://doi.org/10.1007/s00259-023-06154-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06154-y