Abstract
Purpose
The aim of the study was to evaluate extrastriatal dopaminergic and serotonergic pathways in patients with Parkinson’s disease (PD) and dementia with Lewy bodies (DLB) using 123I-FP-CIT SPECT imaging.
Methods
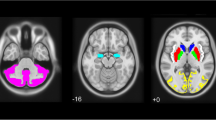
The study groups comprised 56 PD patients without dementia, 41 DLB patients and 54 controls. Each patient underwent a standardized neurological examination and 123I-FP-CIT SPECT. Binding in nigrostriatal and extrastriatal regions of interest was calculated in each patient from spatially normalized images. The occipital-adjusted specific to nondisplaceable binding ratio (SBR) in the different regions was compared among the PD patients, DLB patients and controls adjusting for the effects of age, sex, disease duration and serotonergic/dopaminergic treatment. Covariance analysis was used to determine the correlates of local and long-distance regions with extrastriatal 123I-FP-CIT deficits.
Results
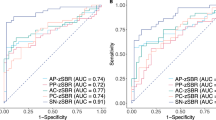
Both PD and DLB patients showed lower 123I-FP-CIT SPECT SBR in several regions beyond the nigrostriatal system, especially the insula, cingulate and thalamus. DLB patients showed significantly lower 123I-FP-CIT SBR in the thalamus than controls and PD patients. Thalamic and cingulate 123I-FP-CIT SBR deficits were correlated, respectively, with limbic serotonergic and widespread cortical monoaminergic projections only in DLB patients but exhibited only local correlations in PD patients and controls.
Conclusion
PD and DLB patients both showed insular dopamine deficits, whereas impairment of thalamic serotonergic pathways was specifically associated with DLB. Longitudinal studies are necessary to determine the clinical value of the assessment of extrastriatal 123I-FP-CIT SPECT.


Similar content being viewed by others
References
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30:1591–601.
McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor J-P, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies. Neurology. 2017;89(1):88–100. https://doi.org/10.1212/WNL.0000000000004058.
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. Abolishing the 1-year rule: how much evidence will be enough? Mov Disord. 2016;31(11):1623–7. https://doi.org/10.1002/mds.26796.
Maetzler W, Pilotto A, Apel A, Deuschle C, Kuebart G, Heinzel S, et al. In vivo markers of Parkinson’s disease and dementia with Lewy bodies: current value of the 5G4 α-synuclein antibody. Acta Neuropathol. 2014;128(6):893–5.
Halliday GM, Holton JL, Revesz T, Dickson DW. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol. 2011;122:187–204. https://doi.org/10.1007/s00401-011-0852-9.
Goedert M, Jakes R, Grazia M, Neurosciences C, Building CA. The synucleinopathies: twenty years on. J Parkinsons Dis. 2017;7(s1):S51–69. https://doi.org/10.3233/JPD-179005.
Roselli F, Pisciotta NM, Pennelli M, Aniello MS, Gigante A, De Caro MF, et al. Midbrain SERT in degenerative parkinsonisms: a 123I-FP-CIT SPECT study. Mov Disord. 2010;25:1853–9.
Koch W, Unterrainer M, Xiong G, Bartenstein P, Diemling M, Varrone A, et al. Extrastriatal binding of [123I]FP-CIT in the thalamus and pons: gender and age dependencies assessed in a European multicentre database of healthy controls. Eur J Nucl Med Mol Imaging. 2014;41(10):1938–46. https://doi.org/10.1007/s00259-014-2785-8.
Joling M, Vriend C, van der Zande JJ, Lemstra AW, van den Heuvel OA, Booij J, et al. Lower 123I-FP-CIT binding to the striatal dopamine transporter, but not to the extrastriatal serotonin transporter, in Parkinson’s disease compared with dementia with Lewy bodies. Neuroimage Clin. 2018;19:130–6. https://doi.org/10.1016/j.nicl.2018.04.009.
Walker Z, Costa DC, Walker RWH, Lee L, Livingston G, Jaros E, et al. Striatal dopamine transporter in dementia with Lewy bodies and Parkinson disease: a comparison. Neurology. 2004;62(9):1568–72.
Colloby SJ, O’Brien JT, Fenwick JD, Firbank MJ, Burn DJ, McKeith IG, et al. The application of statistical parametric mapping to 123I-FP-CIT SPECT in dementia with Lewy bodies, Alzheimer’s disease and Parkinson’s disease. Neuroimage. 2004;23:956–66.
McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology. 2005;65:1863–72. https://doi.org/10.1212/01.wnl.0000187889.17253.b1.
Pilotto A, Turrone R, Liepelt-Scarfone I, Bianchi M, Poli L, Borroni B, et al. Vascular risk factors and cognition in Parkinson’s disease. J Alzheimers Dis. 2016;51:563–70. https://doi.org/10.3233/JAD-150610.
Premi E, Pilotto A, Garibotto V, Bigni B, Turrone R, Alberici A, et al. Impulse control disorder in PD: a lateralized monoaminergic frontostriatal disconnection syndrome? Parkinsonism Relat Disord. 2016;30:62–6. https://doi.org/10.1016/j.parkreldis.2016.05.028.
Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22:1689–707. https://doi.org/10.1002/mds.21507.
Bhatia KP, Bain P, Bajaj N, Elble RJ, Hallett M, Louis ED, et al. Consensus statement on the classification of tremors. From the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord. 2018;33:75–87. https://doi.org/10.1002/mds.27121.
Premi E, Calhoun VD, Garibotto V, Turrone R, Alberici A, Cottini E, et al. Source-based morphometry multivariate approach to analyze [123I]FP-CIT SPECT imaging. Mol Imaging Biol. 2017;19(5):772–8.
Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–31.
Darcourt J, Booij J, Tatsch K, Varrone A, Vander Borght T, Kapucu ÖL, et al. EANM procedure guidelines for brain neurotransmission SPECT using 123I-labelled dopamine transporter ligands, version 2. Eur J Nucl Med Mol Imaging. 2010;37:443–50.
Calvini P, Rodriguez G, Inguglia F, Mignone A, Guerra UP, Nobili F. The basal ganglia matching tools package for striatal uptake semi-quantification: description and validation. Eur J Nucl Med Mol Imaging. 2007;34:1240–53.
Paternicó D, Premi E, Alberici A, Archetti S, Bonomi E, Gualeni V, et al. Dyslexia susceptibility genes influence brain atrophy in frontotemporal dementia. Neurol Genet. 2015;1:e24. https://doi.org/10.1212/NXG.0000000000000024.
Marquie M, Locascio JJ, Rentz DM, Becker JA, Hedden T, Johnson KA, et al. Striatal and extrastriatal dopamine transporter levels relate to cognition in Lewy body diseases: an 11C altropane positron emission tomography study. Alzheimers Res Ther. 2014;6:52. https://doi.org/10.1186/s13195-014-0052-7.
Politis M, Wu K, Loane C, Kiferle L, Molloy S, Brooks DJ, et al. Staging of serotonergic dysfunction in Parkinson’s disease: an in vivo 11C-DASB PET study. Neurobiol Dis. 2010;40:216–21. https://doi.org/10.1016/j.nbd.2010.05.028.
Lewis DA, Melchitzky DS, Sesack SR, Whitehead RE, Auh S, Sampson A. Dopamine transporter immunoreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. J Comp Neurol. 2001;136:119–36.
Papapetropoulos S, Mash DC. Insular pathology in Parkinson’s disease patients with orthostatic hypotension. Parkinsonism Relat Disord. 2007;13:308–11.
Christopher L, Koshimori Y, Lang AE, Criaud M, Strafella AP. Uncovering the role of the insula in non-motor symptoms of Parkinson’s disease. Brain. 2014;137:2143–54.
Fathy YY, Jonker AJ, Oudejans E, de Jong FJJ, van Dam AW, Rozemuller AJM, et al. Differential insular cortex subregional vulnerability to α-synuclein pathology in Parkinson’s disease and dementia with Lewy bodies. Neuropathol Appl Neurobiol. 2019;45:262–7. https://doi.org/10.1111/nan.12501.
Roquet D, Noblet V, Anthony P, Philippi N, Cretin B, Martin-Hunyadi C, et al. Insular atrophy at the prodromal stage of dementia with Lewy bodies: a VBM DARTEL study. Sci Rep. 2017;7:9437. https://doi.org/10.1038/s41598-017-08667-7.
Liepelt-Scarfone I, Pilotto A, Müller K, Bormann C, Gauss K, Wurster I, et al. Autonomic dysfunction in subjects at high risk for Parkinson’s disease. J Neurol. 2015;262(12):2643–52.
Coon EA, Cutsforth-Gregory JK, Benarroch EE. Neuropathology of autonomic dysfunction in synucleinopathies. Mov Disord. 2018;33(3):349–58. https://doi.org/10.1002/mds.27186.
Shennhav A, Botvinick M, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79:217–40.
Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32.
Haber SN, Calzavara R. The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res Bull. 2009;78(2-3):69–74.
Mitchell AS, Sherman SM, Sommer MA, Mair RG, Vertes RP, Chudasama Y. Advances in understanding mechanisms of thalamic relays in cognition and behavior. J Neurosci. 2014;34:15340–6. https://doi.org/10.1523/JNEUROSCI.3289-14.2014.
Delli Pizzi S, Franciotti R, Taylor JP, Thomas A, Tartaro A, Onofrj M, et al. Thalamic involvement in fluctuating cognition in dementia with Lewy bodies: magnetic resonance evidences. Cereb Cortex. 2015;25:3682–9.
Watson R, Colloby SJ, Blamire AM, Wesnes KA, Wood J, O’Brien JT. Does attentional dysfunction and thalamic atrophy predict decline in dementia with Lewy bodies? Parkinsonism Relat Disord. 2017;45:69–74. https://doi.org/10.1016/j.parkreldis.2017.10.006.
Gazzina S, Premi E, Turrone R, Acosta-Cabronero J, Rizzetti MC, Cotelli MS, et al. Subcortical matter in the α-synucleinopathies spectrum: an MRI pilot study. J Neurol. 2016;263:1575–82. https://doi.org/10.1007/s00415-016-8173-5.
Jellinger KA, Attems J. Does striatal pathology distinguish Parkinson disease with dementia and dementia with Lewy bodies? Acta Neuropathol. 2006;112:253–60.
Erskine D, Ding J, Thomas AJ, Kaganovich A, Khundakar AA, Hanson PS, et al. Molecular changes in the absence of severe pathology in the pulvinar in dementia with Lewy bodies. Mov Disord. 2018;33:982–91.
Stokholm MG, Iranzo A, Østergaard K, Serradell M, Otto M, Bacher Svendsen K, et al. Extrastriatal monoaminergic dysfunction and enhanced microglial activation in idiopathic rapid eye movement sleep behaviour disorder. Neurobiol Dis. 2018;115:9–16. https://doi.org/10.1016/j.nbd.2018.02.017.
Pilotto A, Heinzel S, Suenkel U, Lerche S, Brockmann K, Roeben B, et al. Application of the Movement Disorder Society prodromal Parkinson’s disease research criteria in 2 independent prospective cohorts. Mov Disord. 2017;2:1025–34. https://doi.org/10.1002/mds.27035.
Moore RY, Whone AL, Brooks DJ. Extrastriatal monoamine neuron function in Parkinson’s disease: an 18F-dopa PET study. Neurobiol Dis. 2008;29:381–90.
Pavese N, Rivero-Bosch M, Lewis SJ, Whone AL, Brooks DJ. Progression of monoaminergic dysfunction in Parkinson’s disease: a longitudinal 18F-dopa PET study. Neuroimage. 2011;56:1463–8.
Albin RL, Koeppe RA, Bohnen NI, Wernette K, Kilbourn MA, Frey KA. Spared caudal brainstem SERT binding in early Parkinson’s disease. J Cereb Blood Flow Metab. 2008;28:441–4.
Pilotto A, Premi E, Paola Caminiti S, Presotto L, Turrone R, Alberici A, et al. Single-subject SPM FDG-PET patterns predict risk of dementia progression in Parkinson disease. Neurology. 2018;90(12):e1029–37. https://doi.org/10.1212/WNL.0000000000005161.
Booij J, de Jong J, de Bruin K, Knol R, de Win MM, van Eck-Smit BL. Quantification of striatal dopamine transporters with 123I-FP-CIT SPECT is influenced by the selective serotonin reuptake inhibitor paroxetine: a double-blind, placebo-controlled, crossover study in healthy control subjects. J Nucl Med. 2007;48:359–66.
Koopman KE, la Fleur SE, Fliers E, Serlie MJ, Booij J. Assessing the optimal time point for the measurement of extrastriatal serotonin transporter binding with 123I-FP-CIT SPECT in healthy, male subjects. J Nucl Med. 2012;53:1087–90. https://doi.org/10.2967/jnumed.111.102277.
Matsuoka K, Yasuno F, Shinkai T, Miyasaka T, Takahashi M, Kiuchi K, et al. Test-retest reproducibility of extrastriatal binding with 7#I-FP-CIT SPECT in healthy male subjects. Psychiatry Res Neuroimaging. 2016;258:10–5. https://doi.org/10.1016/j.pscychresns.2016.10.007.
Walker Z, Moreno E, Thomas A, Inglis F, Tabet N, Stevens T, et al. Evolution of clinical features in possible DLB depending on FP-CIT SPECT result. Neurology. 2016;87:1045–51.
Iranzo A, Valldeoriola F, Lomeña F, Molinuevo JL, Serradell M, Salamero M, et al. Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol. 2011;10:797–805. https://doi.org/10.1016/S1474-4422(11)70152-1.
Author information
Authors and Affiliations
Contributions
Andrea Pilotto: study concept and design, acquisition of data, statistical analysis design and execution, interpretation of data, drafting/revising the manuscript for content.
Francesca Schiano di Cola: acquisition of data, statistical analysis execution, interpretation of data, drafting/revising the manuscript for content.
Enrico Premi: statistical analysis design and interpretation of data, revising the manuscript for content.
Roberto Grasso: acquisition of data, revising the manuscript for content.
Rosanna Turrone: acquisition of data, revising the manuscript for content.
Stefano Gipponi: acquisition of data, revising the manuscript for content.
Andrea Scalvini acquisition of data, revising the manuscript for content.
Elisabetta Cottini: acquisition of data, revising the manuscript for content.
Barbara Paghera: acquisition of data, revising the manuscript for content.
Valentina Garibotto: revising the manuscript for content.
Maria Cristina Rizzetti: revising the manuscript for content.
Laura Bonanni: revising the manuscript for content.
Barbara Borroni: revising the manuscript for content.
Silvia Morbelli: revising the manuscript for content.
Flavio Nobili: revising the manuscript for content.
Ugo Paolo Guerra: revising the manuscript for content.
Daniela Perani: revising the manuscript for content.
Alessandro Padovani: study concept and design, acquisition of data, analysis and interpretation of data, drafting/revising the manuscript for content.
Corresponding author
Ethics declarations
Conflicts of interest
Andrea Pilotto received speaker honoraria from BioMarin Pharmaceutical, Chiesi Pharmaceuticals, Nutricia Pharmaceuticals, UCB Pharma and Zambon Pharmaceuticals. He received travel grants from AbbVie Pharmaceuticals, BioMarin Pharmaceutical, Nutricia Pharmaceuticals, Zambon Pharmaceuticals and the Italian Movement Disorder Society.
Valentina Garibotto is funded by a grant from the Swiss National Science Foundation (SNF 320030_169876) and from the Velux Foundation (project 1123).
Silvia Morbelli acted as consultant for Eli Lilly in 2014 and for Avid Radiopharmaceuticals in 2016. She received speaker honoraria from General Electric Healthcare in 2017.
Daniela Perani is funded by a grant from Fondazione Cariplo, Bando Ricerca 2014 Malattie Invecchiamento, project title “Evaluation of autonomic, genetic, imaging and biochemical markers for Parkinson-related dementia,” 2015–2017, and the EU FP7 INMIND project (FP7-HEALTH-2013, grant agreement 278,850).
Alessandro Padovani is a consultant for and served on the scientific advisory board of GE Healthcare, Eli Lilly, and Actelion Ltd. Pharmaceuticals, received speaker honoraria from Nutricia, PIAM, Langstone Technology, GE Healthcare, Lilly, UCB Pharma, Zambon and Chiesi Pharmaceuticals. He is funded by a grant from the Ministry of University and Research (MURST).
All other authors declare no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Pilotto, A., Schiano di Cola, F., Premi, E. et al. Extrastriatal dopaminergic and serotonergic pathways in Parkinson’s disease and in dementia with Lewy bodies: a 123I-FP-CIT SPECT study. Eur J Nucl Med Mol Imaging 46, 1642–1651 (2019). https://doi.org/10.1007/s00259-019-04324-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04324-5