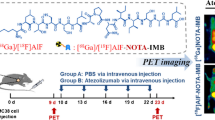
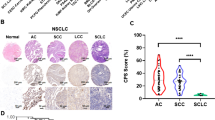
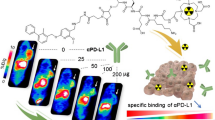
Abstract
Purpose
The aim of this study was to explore an effective 124I labeling strategy and improve the signal-to-noise ratio when evaluating the expression of PD-L1 using an 124I-iodinated durvalumab (durva) F(ab')2 fragment.
Methods
The prepared durva F(ab')2 fragments were incubated with N-succinimidyl-3-(4-hydroxyphenyl) propionate (SHPP); after purification, the HPP-durva F(ab')2 was iodinated using Iodo-Gen method. After the radiochemical purity, stability, and specific activities were determined, the binding affinities of probes prepared using different labeling strategies were compared in vitro. The clinical application value of [124I]I-HPP-durva-F(ab')2 was confirmed by PET imaging. To more objectively evaluate the in vivo distribution and clearance of tracers, the pharmacokinetics and biodistribution assays were also performed.
Results
After being modified with SHPP, the average conjugation number of SHPP per durva-F(ab')2 identified by LC–MS was about 8.92 ± 2.84. The prepared [124I]I-HPP-durva F(ab')2 was obtained with a satisfactory radiochemical purity of more than 98% and stability of more than 93% when incubated for 72 h. Compared with unmodified [124I]I-durva F(ab')2, the specific activity of [124I]I-HPP-durva-F(ab')2 was improved (52.91 ± 5.55 MBq/mg and 15.91 ± 0.74 MBq/mg), while the affinity did not significantly change. The biodistribution experiments and PET imaging showed that the prepared [124I]I-HPP-durva-F(ab')2 exhibited an accelerated clearance and improved tumor-to-background ratio compared with [124I]I-durva-F(ab')2. The specificity of [124I]I-HPP-durva-F(ab')2 to PD-L1 was well demonstrated both in vitro and in vivo.
Conclusions
A PD-L1 PET imaging probe [124I]I-HPP-durva F(ab')2 was successfully synthesized through the SHPP modification strategy. The prepared probe was able to accurately evaluate the PD-L1 expression level through high-contrast noninvasive imaging.








Similar content being viewed by others
Data Availability
The data used to support the findings of this study are available from the corresponding authors upon reasonable request.
References
Dermani FK, Samadi P, Rahmani G, Kohlan AK, Najafi R. PD-1/PD-L1 immune checkpoint: potential target for cancer therapy. J Cell Physiol. 2019;234:1313–25. https://doi.org/10.1002/jcp.27172.
Su D, Tsai HI, Xu Z, Yan F, Wu Y, Xiao Y, et al. Exosomal PD-L1 functions as an immunosuppressant to promote wound healing. J Extracell Vesicles. 2019;9:1709262. https://doi.org/10.1080/20013078.2019.1709262.
Wan W, Ao X, Chen Q, Yu Y, Ao L, Xing W, et al. METTL3/IGF2BP3 axis inhibits tumor immune surveillance by upregulating N(6)-methyladenosine modification of PD-L1 mRNA in breast cancer. Mol Cancer. 2022;21:60. https://doi.org/10.1186/s12943-021-01447-y.
Moik F, Chan WE, Wiedemann S, Hoeller C, Tuchmann F, Aretin MB, et al. Incidence, risk factors, and outcomes of venous and arterial thromboembolism in immune checkpoint inhibitor therapy. Blood. 2021;137:1669–78. https://doi.org/10.1182/blood.2020007878.
Carlisle JW, Steuer CE, Owonikoko TK, Saba NF. An update on the immune landscape in lung and head and neck cancers. CA Cancer J Clin. 2020;70:505–17. https://doi.org/10.3322/caac.21630.
Wen M, Cao Y, Wu B, Xiao T, Cao R, Wang Q, et al. PD-L1 degradation is regulated by electrostatic membrane association of its cytoplasmic domain. Nat Commun. 2021;12:5106. https://doi.org/10.1038/s41467-021-25416-7.
Neubert NJ, Schmittnaegel M, Bordry N, Nassiri S, Wald N, Martignier C, et al. T cell-induced CSF1 promotes melanoma resistance to PD1 blockade. Sci Transl Med. 2018;10. https://doi.org/10.1126/scitranslmed.aan3311.
Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM, Gnjatic S, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2021;18:345–62. https://doi.org/10.1038/s41571-021-00473-5.
Lu S, Stein JE, Rimm DL, Wang DW, Bell JM, Johnson DB, et al. Comparison of biomarker modalities for predicting response to PD-1/PD-L1 checkpoint blockade: a systematic review and meta-analysis. JAMA Oncol. 2019;5:1195–204. https://doi.org/10.1001/jamaoncol.2019.1549.
Niemeijer AN, Leung D, Huisman MC, Bahce I, Hoekstra OS, van Dongen G, et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat Commun. 2018;9:4664. https://doi.org/10.1038/s41467-018-07131-y.
Yan Y, Zheng L, Du Q, Cui X, Dong K, Guo Y, et al. Interferon regulatory factor 1 (IRF-1) downregulates checkpoint kinase 1 (CHK1) through miR-195 to upregulate apoptosis and PD-L1 expression in Hepatocellular carcinoma (HCC) cells. Br J Cancer. 2021;125:101–11. https://doi.org/10.1038/s41416-021-01337-6.
Zhang W, Liu Y, Yan Z, Yang H, Sun W, Yao Y, et al. IL-6 promotes PD-L1 expression in monocytes and macrophages by decreasing protein tyrosine phosphatase receptor type O expression in human hepatocellular carcinoma. J Immunother Cancer. 2020;8. https://doi.org/10.1136/jitc-2019-000285.
Siu LL, Even C, Mesia R, Remenar E, Daste A, Delord JP, et al. Safety and efficacy of durvalumab with or without tremelimumab in patients with PD-L1-low/negative recurrent or metastatic HNSCC: the phase 2 CONDOR randomized clinical trial. JAMA Oncol. 2019;5:195–203. https://doi.org/10.1001/jamaoncol.2018.4628.
Schofield DJ, Percival-Alwyn J, Rytelewski M, Hood J, Rothstein R, Wetzel L, et al. Activity of murine surrogate antibodies for durvalumab and tremelimumab lacking effector function and the ability to deplete regulatory T cells in mouse models of cancer. MAbs. 2021;13:1857100. https://doi.org/10.1080/19420862.2020.1857100.
Cheng Y, Shi D, Xu Z, Gao Z, Si Z, Zhao Y, et al. 124I-labeled monoclonal antibody and fragment for the noninvasive evaluation of tumor PD-L1 expression in vivo. Mol Pharm. 2022. https://doi.org/10.1021/acs.molpharmaceut.2c00084.
Tijink BM, Perk LR, Budde M, Stigter-van Walsum M, Visser GW, Kloet RW, et al. 124I–L19-SIP for immuno-PET imaging of tumour vasculature and guidance of 131I–L19-SIP radioimmunotherapy. Eur J Nucl Med Mol Imaging. 2009;36:1235–44. https://doi.org/10.1007/s00259-009-1096-y.
Shi D, Zhang Y, Xu Z, Si Z, Cheng Y, Cheng D, et al. Noninvasive evaluation of EGFR expression of digestive tumors using 99mTc-MAG3-Cet-F(ab’)2-based SPECT/CT imaging. Mol Imaging. 2022;2022:3748315. https://doi.org/10.1155/2022/3748315.
England CG, Ehlerding EB, Hernandez R, Rekoske BT, Graves SA, Sun H, et al. Preclinical pharmacokinetics and biodistribution studies of 89Zr-labeled pembrolizumab. J Nucl Med. 2017;58:162–8. https://doi.org/10.2967/jnumed.116.177857.
Dong W, Wu X, Ma S, Wang Y, Nalin AP, Zhu Z, et al. The mechanism of anti-PD-L1 antibody efficacy against PD-L1-negative tumors identifies NK cells expressing PD-L1 as a cytolytic effector. Cancer Discov. 2019;9:1422–37. https://doi.org/10.1158/2159-8290.CD-18-1259.
Cyriac G, Gandhi L. Emerging biomarkers for immune checkpoint inhibition in lung cancer. Semin Cancer Biol. 2018;52:269–77. https://doi.org/10.1016/j.semcancer.2018.05.006.
Sha D, Jin Z, Budczies J, Kluck K, Stenzinger A, Sinicrope FA. Tumor mutational burden as a predictive biomarker in solid tumors. Cancer Discov. 2020;10:1808–25. https://doi.org/10.1158/2159-8290.CD-20-0522.
Zettlitz KA, Tavare R, Knowles SM, Steward KK, Timmerman JM, Wu AM. ImmunoPET of malignant and normal B cells with 89Zr- and 124I-labeled obinutuzumab antibody fragments reveals differential CD20 internalization in vivo. Clin Cancer Res. 2017;23:7242–52. https://doi.org/10.1158/1078-0432.CCR-17-0855.
Oliveira MC, Correia JDG. Biomedical applications of radioiodinated peptides. Eur J Med Chem. 2019;179:56–77. https://doi.org/10.1016/j.ejmech.2019.06.014.
Sugiura G, Kuhn H, Sauter M, Haberkorn U, Mier W. Radiolabeling strategies for tumor-targeting proteinaceous drugs. Molecules. 2014;19:2135–65. https://doi.org/10.3390/molecules19022135.
Vorobyeva A, Schulga A, Rinne SS, Gunther T, Orlova A, Deyev S, et al. Indirect radioiodination of DARPin G3 using N-succinimidyl-para-iodobenzoate improves the contrast of HER2 molecular imaging. Int J Mol Sci. 2019;20. https://doi.org/10.3390/ijms20123047.
Tolmachev V, Mume E, Sjoberg S, Frejd FY, Orlova A. Influence of valency and labelling chemistry on in vivo targeting using radioiodinated HER2-binding affibody molecules. Eur J Nucl Med Mol Imaging. 2009;36:692–701. https://doi.org/10.1007/s00259-008-1003-y.
Li H, Frankenfield AM, Houston R, Sekine S, Hao L. Thiol-cleavable biotin for chemical and enzymatic biotinylation and its application to mitochondrial TurboID proteomics. J Am Soc Mass Spectrom. 2021;32:2358–65. https://doi.org/10.1021/jasms.1c00079.
Wang Y, Liu X, Hnatowich DJ. An improved synthesis of NHS-MAG3 for conjugation and radiolabeling of biomolecules with (99m)Tc at room temperature. Nat Protoc. 2007;2:972–8. https://doi.org/10.1038/nprot.2007.144.
Vorobyeva A, Ssmall es CA, Konovalova E, Guler R, Mitran B, Garousi J, et al. Comparison of tumortargeting properties of directly and indirectly radioiodinated designed ankyrin repeat protein (DARPin) G3 variants for molecular imaging of HER2. Int J Oncol 2019;54:1209–20. https://doi.org/10.3892/ijo.2019.4712.
Mitran B, Guler R, Roche FP, Lindstrom E, Selvaraju RK, Fleetwood F, et al. Radionuclide imaging of VEGFR2 in glioma vasculature using biparatopic affibody conjugate: proof-of-principle in a murine model. Theranostics. 2018;8:4462–76. https://doi.org/10.7150/thno.24395.
Funding
This study was supported by the General Programs of the National Nature Science Foundation of China (Nos. 11875114, 82172002, and 82272058) and the Shanghai Municipal Science and Technology Committee of the Shanghai Outstanding Young Academic Leaders Plan (No. 21XD1423500). Clinical Research Project of Zhongshan Hospital, Fudan University (No. 2020ZSLC20).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Animal care and experimental procedures were conducted in compliance with the Helsinki Declaration and approved by the Ethical Committee of Zhongshan Hospital, Fudan University. The use of NSCLC patients tissue microarray was approved by the ethics committee of Outdo Biotech.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Preclinical Imaging.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cheng, Y., Shi, D., Ye, R. et al. Noninvasive evaluation of PD-L1 expression in non-small cell lung cancer by immunoPET imaging using an acylating agent–modified antibody fragment. Eur J Nucl Med Mol Imaging 50, 1585–1596 (2023). https://doi.org/10.1007/s00259-023-06130-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06130-6