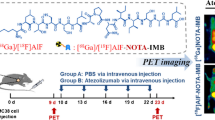
Abstract
Purpose
Programmed cell death protein ligand 1 (PD-L1) is a crucial biomarker for immunotherapy. However, nearly 70% of patients do not respond to PD-L1 immune checkpoint therapy. Accurate monitoring of PD-L1 expression and quantification of target binding during treatment are essential. In this study, a series of small-molecule radiotracers were developed to assess PD-L1 expression and direct immunotherapy.
Methods
Radiotracers of [68Ga]Ga-D-PMED, [68Ga]Ga-D-PEG-PMED, and [68Ga]Ga-D-pep-PMED were designed based on a 2-methyl-3-biphenyl methanol scaffold and successfully synthesized. Cellular experiments and molecular docking assays were performed to determine their specificity for PD-L1. PD-L1 status was investigated via positron emission tomography (PET) imaging in MC38 tumor models. PET imaging of [68Ga]Ga-D-pep-PMED was performed to noninvasively quantify PD-L1 blocking using an anti-mouse PD-L1 antibody (PD-L1 mAb).
Results
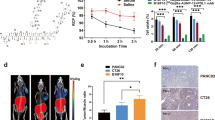
The radiosyntheses of [68Ga]Ga-D-PMED, [68Ga]Ga-D-PEG-PMED, and [68Ga]Ga-D-pep-PMED were achieved with radiochemical yields of 87 ± 6%, 82 ± 4%, and 79 ± 9%, respectively. In vitro competition assays demonstrated their high affinities (the IC50 values of [68Ga]Ga-D-PMED, [68Ga]Ga-D-PEG-PMED, and [68Ga]Ga-D-pep-PMED were 90.66 ± 1.24, 160.8 ± 1.35, and 51.6 ± 1.32 nM, respectively). At 120 min postinjection (p.i.) of the radiotracers, MC38 tumors displayed optimized tumor-to-muscle ratios for all radioligands. Owing to its hydrophilic modification, [68Ga]Ga-D-pep-PMED had the highest target-to-nontarget (T/NT) ratio of approximately 6.2 ± 1.2. Interestingly, the tumor/liver ratio was hardly affected by different concentrations of the inhibitor BMS202. We then evaluated the impacts of dose and time on accessible PD-L1 levels in the tumor during anti-mouse PD-L1 antibody treatment. The tumor uptake of [68Ga]Ga-D-pep-PMED significantly decreased with increasing PD-L1 mAb dose. Moreover, after 8 days of treatment with a single antibody, the uptake of [68Ga]Ga-D-pep-PMED in the tumor significantly increased but remained lower than that in the saline group.
Conclusion
PET imaging with [68Ga]Ga-D-pep-PMED, a small-molecule radiotracer, is a promising tool for evaluating PD-L1 expression and quantifying the target blockade of PD-L1 to assist in the development of effective therapeutic regimens.
Graphical abstract








Similar content being viewed by others
Data availability
All data relevant to the study are included in the article or uploaded as supplementary information.
Abbreviations
- ICIs:
-
Immune checkpoint inhibitors
- ICB:
-
Immune checkpoint blockade
- PD-1:
-
Programmed cell death protein-1
- PD-L1:
-
Programmed death ligand-1
- IHC:
-
Immunohistochemistry
- PET:
-
Positron emission tomography
- mAbs:
-
Monoclonal antibodies
- TACs:
-
Time-activity curves
- NCCN:
-
National Comprehensive Cancer Network
- HPLC:
-
High-performance liquid chromatography
- ROI:
-
Regions of interest
- RCY:
-
Radiochemical yield
- T/NT:
-
Target-to-nontarget
References
Sharma P, Allison JP. Dissecting the mechanisms of immune checkpoint therapy. Nat Rev Immunol. 2020;20:75–6.
Xu W, Atkins MB, McDermott DF. Checkpoint inhibitor immunotherapy in kidney cancer. Nat Rev Urol. 2020;17:137–50.
Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–30.
Ohaegbulam KC, Assal A, Lazar-Molnar E, et al. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med. 2015;21:24–33.
Jin HT, Ahmed R, Okazaki T. Role of PD-1 in regulating T-cell immunity. Curr Top Microbiol Immunol. 2011;350:17–37.
Cha JH, Chan LC, Li CW, et al. Mechanisms controlling PD-L1 expression in cancer. Mol Cell. 2019;76:359–70.
Dermani FK, Samadi P, Rahmani G, et al. PD-1/PD-L1 immune checkpoint: potential target for cancer therapy. J Cell Physiol. 2019;234:1313–25.
Nishino M, Ramaiya NH, Hatabu H, et al. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol. 2017;14:655–68.
Gupta S, Zugazagoitia J, Martinez-Morilla S, et al. Digital quantitative assessment of PD-L1 using digital spatial profiling. Lab Invest. 2020;100:1311–7.
Broos K, Lecocq Q, Raes G, et al. Noninvasive imaging of the PD-1:PD-L1 immune checkpoint: embracing nuclear medicine for the benefit of personalized immunotherapy. Theranostics. 2018;8:3559–70.
Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–74.
Lütje S, Feldmann G, Essler M, et al. Immune checkpoint imaging in oncology: a game changer toward personalized immunotherapy? J Nucl Med. 2020;61:1137–44.
van de Donk PP, Kist de Ruijter L, Lub-de Hooge MN, et al. Molecular imaging biomarkers for immune checkpoint inhibitor therapy. Theranostics. 2020;10:1708–18.
Bensch F, van der Veen EL, Lub-de Hooge MN, et al. 89Zr-atezolizumab imaging as a noninvasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med. 2018;24:1852–8.
Christensen C, Kristensen LK, Alfsen MZ, et al. Quantitative PET imaging of PD-L1 expression in xenograft and syngeneic tumour models using a site-specifically labelled PD-L1 antibody. Eur J Nucl Med Mol Imaging. 2020;47:1302–13.
Jung KH, Park JW, Lee JH, et al. 89Zr-labeled anti-PD-L1 antibody PET monitors gemcitabine therapy induced modulation of tumor PD-L1 expression. J Nucl Med. 2021;62:656–64.
Huisman MC, Niemeijer AN, Windhorst AD, et al. Quantification of PD-L1 expression with 18F-BMS-986192 PET/CT in patients with advanced-stage non-small cell lung cancer. J Nucl Med. 2020;61:1455–60.
Nienhuis PH, Antunes IF, Glaudemans A, et al. 18F-BMS986192 PET imaging of PD-L1 in metastatic melanoma patients with brain metastases treated with immune checkpoint inhibitors: a pilot study. J Nucl Med. 2021. J Nucl Med. 2022;63:899–905.
De Silva RA, Kumar D, Lisok A, et al. Peptide-based 68Ga-PET radiotracer for imaging PD-L1 expression in cancer. Mol Pharm. 2018;15:3946–52.
Kumar D, Mishra A, Lisok A, et al. Pharmacodynamic measures within tumors expose differential activity of PD(L)-1 antibody therapeutics. Proc Natl Acad Sci USA. 2021;118: e2107982118.
Zhou X, Jiang J, Yang X, et al. First-in-human evaluation of a PD-L1-binding peptide radiotracer in non-small cell lung cancer patients with PET. J Nucl Med. 2022;63:536–42.
Zhou M, Wang X, Chen B, et al. Preclinical and first-in-human evaluation of F-labeled D-peptide antagonist for PD-L1 status imaging with PET. Eur J Nucl Med Mol Imaging. 2022;49:4312–24.
Awadasseid A, Wu Y, Zhang W. Advance investigation on synthetic small-molecule inhibitors targeting PD-1/PD-L1 signaling pathway. Life Sci. 2021;282: 119813.
Chen T, Li Q, Liu Z, et al. Peptide-based and small synthetic molecule inhibitors on PD-1/PD-L1 pathway: a new choice for immunotherapy? Eur J Med Chem. 2019;161:378–98.
Kumar D, Lisok A, Dahmane E, et al. Peptide-based PET quantifies target engagement of PD-L1 therapeutics. J Clin Invest. 2019;129:616–30.
Bamminger K, Pichler V, Vraka C, et al. On the road towards small-molecule programmed cell death 1 ligand 1 positron emission tomography tracers: a ligand-based drug design approach. Pharmaceuticals (Basel). 2023;16:1051–70.
Meng L, Fang J, Zhao L, et al. Rational design and pharmacomodulation of protein-binding theranostic radioligands for targeting the fibroblast activation protein. J Med Chem. 2022;65:8245–57.
Lin A, Wei T, Meng H, et al. Role of the dynamic tumor microenvironment in controversies regarding immune checkpoint inhibitors for the treatment of non-small cell lung cancer (NSCLC) with EGFR mutations. Mol Cancer. 2019;18:139–47.
Topalian SL, Taube JM, Anders RA, et al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–87.
Rehman JA, Han G, Carvajal-Hausdorf DE, et al. Quantitative and pathologist-read comparison of the heterogeneity of programmed death-ligand 1 (PD-L1) expression in non-small cell lung cancer. Mod Pathol. 2017;30:340–9.
Liu Q, Jiang L, Li K, Li H, Lv G, Lin J, Qiu L. Immuno-PET imaging of 68Ga-labeled nanobody Nb109 for dynamic monitoring the PD-L1 expression in cancers. Cancer Immunol Immunother. 2021;70:1721–33.
Wen X, Shi C, Zhao L, et al. Immuno-SPECT/PET imaging with radioiodinated anti-PD-L1 antibody to evaluate PD-L1 expression in immune-competent murine models and PDX model of lung adenocarcinoma. Nucl Med Biol. 2020;86–87:44–51.
Nedrow JR, Josefsson A, Park S, et al. Imaging of programmed cell death ligand 1: impact of protein concentration on distribution of anti-PD-L1 SPECT agents in an immunocompetent murine model of melanoma. J Nucl Med. 2017;58:1560–6.
Funding
This study was financially supported by the National Natural Science Foundation of China (82372008, 81901805, 21976150), Joint Fund of the National Natural Science Foundation of China—China National Nuclear Corporation for Nuclear Technology Innovation (U1967222), and Shanghai Pujiang Program (22PJ1401700).
Author information
Authors and Affiliations
Contributions
HY, XZ, and JL: conceptualization, data curation, formal analysis, validation, investigation, visualization, methodology, writing—original draft and editing. XW, HL, YL, XW, JF, and QZ: investigation, methodology. JL, XZ, and ZG: conceptualization, resources, supervision, funding acquisition, investigation, writing—review and editing.
Corresponding authors
Ethics declarations
Ethics approval
All animal studies were approved by the Institutional Animal Care and Use Committee of Laboratory Animals Center for Xiamen University. And the protocols were prepared before the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hongzhang Yang, Xinying Zeng, and Jia Liu were the first authors and contributed equally to this work.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, H., Zeng, X., Liu, J. et al. Development of small-molecular-based radiotracers for PET imaging of PD-L1 expression and guiding the PD-L1 therapeutics. Eur J Nucl Med Mol Imaging 51, 1582–1592 (2024). https://doi.org/10.1007/s00259-024-06610-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-024-06610-3