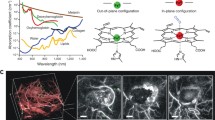
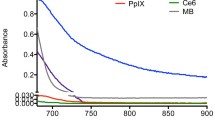
Abstract
Photoacoustic imaging (PAI) is a rapidly emerging modality in biomedical research with the advantages of noncontact operation, high optical resolution, and deep penetration. Great efforts and progress in the development of PAI agents with improved imaging resolution and sensitivity have been made over the past 2 decades. Among them, organic agents are the most promising candidates for preclinical/clinical applications due to their outstanding in vivo properties and facile biofunctionalities. Motivated by the unique properties of aggregation-induced emission (AIE) luminogens (AIEgens), various optical probes have been developed for bioanalyte detection, multimodal bioimaging, photodynamic/photothermal therapy, and imaging-guided therapeutics. In particular, AIE-active contrast agents have been demonstrated in PAI applications with excellent performance in imaging resolution and tissue permeability in vivo. This paper presents a brief overview of recent progress in AIE-based agents in the field of photoacoustic imaging. In particular, we focus on the basic concepts, data sorting and comparison, developing trends, and perspectives of photoacoustic imaging. Through numerous typical examples, the way each system realizes the desired photoacoustic performance in various biomedical applications is clearly illustrated. We believe that AIE-based PAI agents would be promising multifunctional theranostic platforms in clinical fields and will facilitate significant advancements in this research topic.












Similar content being viewed by others
References
Sun Y, Jiang H, O’Neill BE. Photoacoustic imaging: an emerging optical modality in diagnostic and theranostic medicine. J Biosens Bioelectron. 2011;2:3. https://doi.org/10.4172/2155-6210.1000108.
Bell AG. On the production and reproduction of sound by light. American Journal of Science. 1880;s3-20(118):305.
Hosseinaee Z, Le M, Bell K, Reza PH. Towards non-contact photoacoustic imaging [review]. Photoacoustics. 2020;20:100207.
Beard P. Biomedical photoacoustic imaging. Interface Focus. 2011;1(4):602–31.
Karthikesh MS, Yang X. Photoacoustic image-guided interventions. Exp Biol Med. 2019;245(4):153537021988932.
Furdella KJ, Witte RS, Vande Geest JP. Tracking delivery of a drug surrogate in the porcine heart using photoacoustic imaging and spectroscopy. J Biomed Opt. 2017;22(4):41016.
Wang J, Chen F, Arconada-Alvarez SJ, Hartanto J, Yap LP, Park R, Wang F, Vorobyova I, Dagliyan G, Conti PS, et al. A nanoscale tool for photoacoustic-based measurements of clotting time and therapeutic drug monitoring of heparin. Nano Lett. 2016;16(10):6265–71.
Duan Z, Gao YJ, Qiao ZY, Fan G, Liu Y, Zhang D, Wang H. A photoacoustic approach for monitoring the drug release of pH-sensitive poly(β-amino ester)s. J Mater Chem B. 2014;2(37):6271–82.
Cash KJ, Li C, Xia J, Wang LV, Clark HA. Optical drug monitoring: photoacoustic imaging of nanosensors to monitor therapeutic lithium in vivo. ACS Nano. 2015;9(2):1692–8.
Jung HS, Verwilst P, Sharma A, Shin J, Sessler JL, Kim JS. Organic molecule-based photothermal agents: an expanding photothermal therapy universe. Chem Soc Rev. 2018;47(7):2280–97.
Lediju Bell MA, Ostrowski AK, Li K, Kazanzides P, Boctor EM. Localization of transcranial targets for photoacoustic-guided endonasal surgeries. Photoacoustics. 2015;3(2):78–87.
Matthews TP, Zhang C, Yao DK, Maslov K, Wang LV. Label-free photoacoustic microscopy of peripheral nerves. J Biomed Opt. 2014;19(1):16004.
Shubert J, Lediju Bell MA. Photoacoustic imaging of a human vertebra: implications for guiding spinal fusion surgeries. Phys Med Biol. 2018;63(14):144001.
Lediju Bell MA, Shubert J. Photoacoustic-based visual servoing of a needle tip. Sci Rep. 2018;8(1):15519.
Wang H, Liu S, Wang T, Zhang C, Feng T, Tian C. Three-dimensional interventional photoacoustic imaging for biopsy needle guidance with a linear array transducer. J Biophotonics. 2019;12(12):e201900212.
Witte RS, Karunakaran C, Zuniga AN, Schmitz H, Arif H. Frontiers of cancer imaging and guided therapy using ultrasound, light, and microwaves. Clin Exp Metastasis. 2018;35(5–6):413–8.
Pan D, Kim B, Wang LV, Lanza GM. A brief account of nanoparticle contrast agents for photoacoustic imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5(6):517–43.
Weber J, Beard PC, Bohndiek SE. Contrast agents for molecular photoacoustic imaging. Nat Methods. 2016;13(8):639–50.
Nie L, Chen X. Structural and functional photoacoustic molecular tomography aided by emerging contrast agents. Chem Soc Rev. 2014;43(20):7132–70.
Fan Q, Cheng K, Hu X, Ma X, Zhang R, Yang M, Lu X, Xing L, Huang W, Gambhir SS, et al. Transferring biomarker into molecular probe: melanin nanoparticle as a naturally active platform for multimodality imaging. J Am Chem Soc. 2014;136(43):15185–94.
Sangha GS, Phillips EH, Goergen CJ. In vivo photoacoustic lipid imaging in mice using the second near-infrared window. Biomed Opt Express. 2017;8(2):736–42.
Wang P, Wang P, Wang HW, Cheng JX. Mapping lipid and collagen by multispectral photoacoustic imaging of chemical bond vibration. J Biomed Opt. 2012;17(9):96010–1.
Stoffels I, Morscher S, Helfrich I, Hillen U, Leyh J, Burton NC, Sardella TC, Claussen J, Poeppel TD, Bachmann HS, et al. Metastatic status of sentinel lymph nodes in melanoma determined noninvasively with multispectral optoacoustic imaging. Sci Transl Med. 2015;7(317):317ra199.
Ho CJ, Balasundaram G, Driessen W, McLaren R, Wong CL, Dinish US, Attia AB, Ntziachristos V, Olivo M. Multifunctional photosensitizer-based contrast agents for photoacoustic imaging. Sci Rep. 2014;4:5342.
Wang LV, Yao J. A practical guide to photoacoustic tomography in the life sciences. Nat Methods. 2016;13(8):627–38.
Li JC, Pu KY. Development of organic semiconducting materials for deep-tissue optical imaging, phototherapy and photoactivation. Chem Soc Rev. 2019;48(1):38–71.
Fu QR, Zhu R, Song JB, Yang HH, Chen XY. Photoacoustic imaging: contrast agents and their biomedical applications. Adv Mater. 2019;31(6):e1805875. https://doi.org/10.1002/adma.201805875.
Li W, Chen X. Gold nanoparticles for photoacoustic imaging. Nanomedicine (Lond). 2015;10(2):299–320.
Lin J, Chen X, Huang P. Graphene-based nanomaterials for bioimaging. Adv Drug Deliv Rev. 2016;105(Pt B):242–54.
Chen Y, Tan C, Zhang H, Wang L. Two-dimensional graphene analogues for biomedical applications. Chem Soc Rev. 2015;44(9):2681–701.
Li J, Rao J, Pu K. Recent progress on semiconducting polymer nanoparticles for molecular imaging and cancer phototherapy. Biomaterials. 2018;155:217–35.
Moon GD, Choi SW, Cai X, Li W, Cho EC, Jeong U, Wang LV, Xia Y. A new theranostic system based on gold nanocages and phase-change materials with unique features for photoacoustic imaging and controlled release. J Am Chem Soc. 2011;133(13):4762–5.
Filonov GS, Krumholz A, Xia J, Yao J, Wang LV, Verkhusha VV. Deep-tissue photoacoustic tomography of a genetically encoded near-infrared fluorescent probe. Angew Chem Int Ed Engl. 2012;51(6):1448–51.
Wang X, Ku G, Wegiel MA, Bornhop DJ, Stoica G, Wang LV. Noninvasive photoacoustic angiography of animal brains in vivo with near-infrared light and an optical contrast agent. Opt Lett. 2004;29(7):730–2.
Ou H, Li J, Chen C, Gao H, Xue X, Ding D. Organic/polymer photothermal nanoagents for photoacoustic imaging and photothermal therapy in vivo. Sci China Mater. 2019;62(11):1740–58.
Luu T, Li W, O’Brien-Simpson NM, Hong Y. Recent applications of aggregation induced emission probes for antimicrobial peptide studies. Chemistry An Asian Journal. 2021;16(9):1027–40.
Alam P, Leung NLC, Zhang J, Kwok RTK, Lam JWY, Tang BZ. AIE-based luminescence probes for metal ion detection. Coord Chem Rev. 2021;429:213693.
Wu WB, Li Z. Nanoprobes with aggregation-induced emission for theranostics. Materials Chemistry Frontiers. 2021;5(2):603–26.
Feng GX, Liu B. Multifunctional AIEgens for future theranostics. Small. 2016;12(47):6528–35.
Liu Y, Bhattarai P, Dai Z, Chen X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem Soc Rev. 2019;48(7):2053–108.
Ong SY, Zhang CY, Dong X, Yao SQ. Recent advances in polymeric nanoparticles for enhanced fluorescence and photoacoustic imaging. Angewandte Chemie-International Edition. 2021;60(33):17797–809.
Li C, Liu C, Fan Y, Ma X, Zhan Y, Lu X, Sun Y. Recent development of near-infrared photoacoustic probes based on small-molecule organic dye. RSC Chem Biol. 2021;2(3):743–58.
Li K, Liu B. Polymer-encapsulated organic nanoparticles for fluorescence and photoacoustic imaging. Chem Soc Rev. 2014;43(18):6570–97.
He Z, Ke C, Tang BZ. Journey of aggregation-induced emission research. ACS Omega. 2018;3(3):3267–77.
Ma X, Sun R, Cheng J, Liu J, Gou F, Xiang H, Zhou X. Fluorescence aggregation-caused quenching versus aggregation-induced emission: a visual teaching technology for undergraduate chemistry students. J Chem Educ. 2016;93(2):345–50.
Yang J-S, Swager TM. Fluorescent porous polymer films as TNT chemosensors: electronic and structural effects. J Am Chem Soc. 1998;120(46):11864.
Luo J, Xie Z, Lam JW, Cheng L, Chen H, Qiu C, Kwok HS, Zhan X, Liu Y, Zhu D, et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem Commun (Camb). 2001;18:1740–1.
Leung NLC, Xie N, Yuan W, Liu Y, Wu Q, Peng Q, Miao Q, Lam JWY, Tang BZ. Restriction of intramolecular motions: the general mechanism behind aggregation-induced emission. Chemistry A European Journal. 2014;20(47):15349–53.
Chen Y, Lam JWY, Kwok RTK, Liu B, Tang BZ. Aggregation-induced emission: fundamental understanding and future developments. Mater Horiz. 2019;6(3):428–33.
Zhang JY, Zhang HK, Lam JWY, Tang BZ. Restriction of intramolecular motion(RIM): investigating AIE mechanism from experimental and theoretical studies. Chem Res Chin Univ. 2021;37(1):1–15.
Peng Q, Shuai Z. Molecular mechanism of aggregation-induced emission. Aggregate. 2021;2(5):e91.
Upputuri PK, Pramanik M. Photoacoustic imaging in the second near-infrared window: a review. J Biomed Opt. 2019;24(4):1–20.
Zhang ZJ, Xu WH, Kang MM, Wen HF, Guo H, Zhang PF, Xi L, Li K, Wang L, Wang D, et al. An all-round athlete on the track of phototheranostics: subtly regulating the balance between radiative and nonradiative decays for multimodal imaging-guided synergistic therapy. Adv Mater. 2020;32(36):e2003210. https://doi.org/10.1002/adma.202003210.
Zhao Z, Chen C, Wu W, Wang F, Du L, Zhang X, Xiong Y, He X, Cai Y, Kwok RTK, et al. Highly efficient photothermal nanoagent achieved by harvesting energy via excited-state intramolecular motion within nanoparticles. Nat Commun. 2019;10(1):768.
Li HX, Wen HF, Li J, Huang JC, Wang D, Tang BZ. Doping AIE photothermal molecule into all-fiber aerogel with self-pumping water function for efficiency solar steam generation. ACS Appl Mater Interfaces. 2020;12(23):26033–40.
Xu Z, Tang BZ, Wang Y, Ma DG. Recent advances in high performance blue organic light-emitting diodes based on fluorescence emitters. Journal of Materials Chemistry C. 2020;8(8):2614–42.
Gao M, Tang BZ. Fluorescent sensors based on aggregation-induced emission: recent advances and perspectives. Acs Sensors. 2017;2(10):1382–99.
Geng J, Liao L-D, Qin W, Tang BZ, Thakor N, Liu B. Fluorogens with aggregation induced emission: ideal photoacoustic contrast reagents due to intramolecular rotation. J Nanosci Nanotechnol. 2015;15(2):1864–8.
He XW, Peng C, Qiang SJ, Xiong LH, Zhao Z, Wang ZY, Kwok RTK, Lam JWY, Ma N, Tang BZ. Less is more: silver-AIE core@shell nanoparticles for multimodality cancer imaging and synergistic therapy. Biomaterials. 2020;238:119834. https://doi.org/10.1016/j.biomaterials.2020.119834.
Welsher K, Liu Z, Sherlock SP, Robinson JT, Chen Z, Daranciang D, Dai H. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nat Nanotechnol. 2009;4(11):773–80.
Zha ML, Lin XW, Ni JS, Li YX, Zhang YC, Zhang X, Wang LD, Li K. An ester-substituted semiconducting polymer with efficient nonradiative decay enhances NIR-II photoacoustic performance for monitoring of tumor growth. Angewandte Chemie-International Edition. 2020;59(51):23268–76.
Wang LV, Hu S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science. 2012;335(6075):1458–62.
Erfanzadeh M, Zhu Q. Photoacoustic imaging with low-cost sources; a review. Photoacoustics. 2019;14:1–11.
Wu J, You L, Lan L, Lee HJ, Chaudhry ST, Li R, Cheng JX, Mei J. Semiconducting polymer nanoparticles for centimeters-deep photoacoustic imaging in the second near-infrared window. Adv Mater. 2017;29(41). https://doi.org/10.1002/adma.201703403.
Jiang Y, Upputuri PK, Xie C, Lyu Y, Zhang L, Xiong Q, Pramanik M, Pu K. Broadband absorbing semiconducting polymer nanoparticles for photoacoustic imaging in second near-infrared window. Nano Lett. 2017;17(8):4964–9.
Du WT, Liu XL, Liu LJ, Lam JWY, Tang BZ. Photoresponsive polymers with aggregation-induced emission. Acs Applied Polymer Materials. 2021;3(5):2290–309.
Wang Z, Zhou Y, Xu R, Xu Y, Dang D, Shen Q, Meng L, Tang BZ. Seeing the unseen: AIE luminogens for super-resolution imaging. Coordination Chemistry Reviews. 2022;451:214279.
Kim D, Kim J, Park YI, Lee N, Hyeon T. Recent development of inorganic nanoparticles for biomedical imaging. ACS Cent Sci. 2018;4(3):324–36.
Chen Y-S, Zhao Y, Beinat C, Zlitni A, Hsu E-C, Chen D-H, Achterberg F, Wang H, Stoyanova T, Dionne J, et al. Ultra-high-frequency radio-frequency acoustic molecular imaging with saline nanodroplets in living subjects. Nat Nanotechnol. 2021;16(6):717–24.
Attia ABE, Balasundaram G, Moothanchery M, Dinish US, Bi R, Ntziachristos V, Olivo M. A review of clinical photoacoustic imaging: current and future trends. Photoacoustics. 2019;16:100144.
Wang Z, Kumar UP, Zhen X, Zhang R, Jiang Y, Ai X, Zhang Z, Ming H, Meng Z, Lu Y. pH-sensitive and biodegradable charge-transfer nanocomplex for second near-infrared photoacoustic tumor imaging. Nano Res. 2019:1–7. https://doi.org/10.1007/s12274-018-2175-9.
Liu SJ, Li YY, Kwok RTK, Lam JWY, Tang BZ. Structural and process controls of AIEgens for NIR-II theranostics. Chem Sci. 2021;12(10):3427–36.
Xu YZ, Li CB, Xu RH, Zhang N, Wang Z, Jing XN, Yang ZW, Dang DF, Zhang PF, Meng LJ. Tuning molecular aggregation to achieve highly bright AIE dots for NIR-II fluorescence imaging and NIR-I photoacoustic imaging. Chem Sci. 2020;11(31):8157–66.
Xu YZ, Dang DF, Zhu HR, Jing XA, Zhu X, Zhang N, Li CB, Zhao YZ, Zhang PF, Yang ZW, et al. Boosting the AIEgen-based photo-theranostic platform by balancing radiative decay and non-radiative decay. Materials Chemistry Frontiers. 2021;5(11):4182–92.
Soto AM, Longo G, Montévil M, Sonnenschein C. The biological default state of cell proliferation with variation and motility, a fundamental principle for a theory of organisms. Prog Biophys Mol Biol. 2016;122(1):16–23.
Meir R, Motiei M, Popovtzer R. Gold nanoparticles for in vivo cell tracking. Nanomedicine. 2014;9(13):2059–69.
Xu W, Dong S, Han Y, Li S, Liu Y. Hydrogels as antibacterial biomaterials. Curr Pharm Des. 2018;24(8):843–54.
Zhang M, Wang Z, Huang P, Jiang G, Xu C, Zhang W, Guo R, Li W, Zhang X. Real-time and noninvasive tracking of injectable hydrogel degradation using functionalized AIE nanoparticles. Nanophotonics. 2020;9(7):2063–75.
Langen KJ, Galldiks N, Hattingen E, Shah NJ. Advances in neuro-oncology imaging. Nat Rev Neurol. 2017;13(5):279–89.
Yang X, Chen YH, Xia F, Sawan M. Photoacoustic imaging for monitoring of stroke diseases: a review. Photoacoustics. 2021;23:100287.
Sheng Z, Guo B, Hu D, Xu S, Wu W, Liew WH, Yao K, Jiang J, Liu C Zheng H, Liu B. Bright aggregation-induced-emission dots for targeted synergetic NIR-II fluorescence and NIR-I Photoacoustic imaging of orthotopic brain tumors. Adv Mater. 2018:e1800766. https://doi.org/10.1002/adma.201800766.
Zhang Y, Jeon M, Rich LJ, Hong H, Geng J, Zhang Y, Shi S, Barnhart TE, Alexandridis P, Huizinga JD, et al. Non-invasive multimodal functional imaging of the intestine with frozen micellar naphthalocyanines. Nat Nanotechnol. 2014;9(8):631–8.
Jiang Z, Sun B, Wang Y, Gao H, Ren H, Zhang H, Lu T, Ren X, Wei W, Wang X, Zhang L, Li J, Ding D, Lovell JF, Zhang Y. Surfactant-stripped micelles with aggregation-induced enhanced emission for bimodal gut imaging in vivo and microbiota tagging ex vivo. Adv Healthc Mater. 2021;10(24):e2100356. https://doi.org/10.1002/adhm.202100356.
Hu F, Qi G. Kenry, Mao D, Zhou S, Wu M, Wu W, Liu B: Visualization and insitu ablation of intracellular bacterial pathogens through metabolic labeling. Angew Chem Int Ed. 2020;59(24):9288–92.
Hu XM, Li ZX, Lin RH, Shan JQ, Yu QW, Wang RX, Liao LS, Yan WT, Wang Z, Shang L, et al. Guidelines for regulated cell death assays: a systematic summary, a categorical comparison, a prospective. Front Cell Dev Biol. 2021;9:634690.
Zhang WW, Ding XY, Cheng H, Yin CY, Yan J, Mou ZP, Wang WY, Cui DX, Fan CD, Sun DD. Dual-targeted gold nanoprism for recognition of early apoptosis, dual-model imaging and precise cancer photothermal therapy. Theranostics. 2019;9(19):5610–25.
Joseph JP, Harishankar MK, Pillai AA, Devi A. Hypoxia induced EMT: a review on the mechanism of tumor progression and metastasis in OSCC. Oral Oncol. 2018;80:23–32.
Lee JW, Ko J, Ju C, Eltzschig HK. Hypoxia signaling in human diseases and therapeutic targets. Exp Mol Med. 2019;51(6):1–13. https://doi.org/10.1038/s12276-019-0235-1.
Boedtkjer E, Pedersen SF. The acidic tumor microenvironment as a driver of cancer. Annu Rev Physiol. 2020;82:103–26. https://doi.org/10.1146/annurev-physiol-021119-034627.
Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discovery. 2011;10(10):767–77.
Tejero J, Shiva S, Gladwin MT. Sources of vascular nitric oxide and reactive oxygen species and their regulation. Physiol Rev. 2019;99(1):311–79.
Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452(7187):580–9.
Chong WK, Papadopoulou V, Dayton PA. Imaging with ultrasound contrast agents: current status and future. Abdom Radiol (NY). 2018;43(4):762–72.
Kobayashi H, Ogawa M, Alford R, Choyke PL, Urano Y. New strategies for fluorescent probe design in medical diagnostic imaging. Chem Rev. 2010;110(5):2620–40.
Fang HY, Stangl S, Marcazzan S, Carvalho MJB, Baumeister T, Anand A, Strangmann J, Huspenina JS, Wang TC, Schmid RM, Feith M, Friess H, Ntziachristos V, Multhoff G, Gorpas D, Quante M. Targeted Hsp70 fluorescence molecular endoscopy detects dysplasia in Barrett’s esophagus. Eur J Nucl Med Mol Imaging. 2021. https://doi.org/10.1007/s00259-021-05582-y.
Lucero MY, East AK, Reinhardt CJ, Sedgwick AC, Su S, Lee MC, Chan J. Development of NIR-II photoacoustic probes tailored for deep-tissue sensing of nitric oxide. J Am Chem Soc. 2021;143(18):7196–202.
Zhang J, He B, Hu Y, Alam P, Zhang H, Lam JWY, Tang BZ. Stimuli-responsive AIEgens. Adv Mater. 2021;33(32):2008071.
Liang J, Tang B, Liu B. Specific light-up bioprobes based on AIEgen conjugates. Chem Soc Rev. 2015;44(10):2798–811.
Kobayashi H, Choyke PL. Target-cancer-cell-specific activatable fluorescence imaging probes: rational design and in vivo applications. Acc Chem Res. 2011;44(2):83–90.
Li M, Li H, Wu Q, Niu N, Huang J, Zhang L, Li Y, Wang D, Tang BZ. Hypoxia-activated probe for NIR fluorescence and photoacoustic dual-mode tumor imaging. iScience. 2021;24(3):102261.
Xu L, Sun L, Zeng F, Wu S. Activatable fluorescent probe based on aggregation-induced emission for detecting hypoxia-related pathological conditions. Anal Chim Acta. 2020;1125:152–61.
Vordermark D. Hypoxia-specific targets in cancer therapy: role of splice variants. BMC Med. 2010;8:45.
Wouters BG, Weppler SA, Koritzinsky M, Landuyt W, Nuyts S, Theys J, Chiu RK, Lambin P. Hypoxia as a target for combined modality treatments. Eur J Cancer. 2002;38(2):240–57.
Boddu RS, Perumal O, Divakar K. Microbial nitroreductases: a versatile tool for biomedical and environmental applications. Biotechnol Appl Biochem. 2021;68(6):1518–30. https://doi.org/10.1002/bab.2073.
Akiva E, Copp JN, Tokuriki N, Babbitt PC. Evolutionary and molecular foundations of multiple contemporary functions of the nitroreductase superfamily. Proc Natl Acad Sci U S A. 2017;114(45):E9549-e9558.
Searle PF, Chen MJ, Hu LQ, Race PR, Lovering AL, Grove JI, Guise C, Jaberipour M, James ND, Mautner V, et al. Nitroreductase: a prodrug-activating enzyme for cancer gene therapy. Clin Exp Pharmacol Physiol. 2004;31(11):811–6.
de Garibay GR, Jalon EGD, Stigen E, Lund KB, Popa M, Davidson B, Safont MM, Rygh CB, Espedal H, Barrett TM, et al. Repurposing F-18-FMISO as a PET tracer for translational imaging of nitroreductase-based gene directed enzyme prodrug therapy. Theranostics. 2021;11(12):6044–57.
Qiao J, Wang M, Cui M, Fang Y, Li H, Zheng C, Li Z, Xu Y, Hua H, Li D. Small-molecule probes for fluorescent detection of cellular hypoxia-related nitroreductase. J Pharm Biomed Anal. 2021;203:114199.
Ouyang J, Sun LH, Zeng Z, Zeng C, Zeng F, Wu SZ. Nanoaggregate probe for breast cancer metastasis through multispectral optoacoustic tomography and aggregation-induced NIR-I/II fluorescence imaging. Angewandte Chemie-International Edition. 2020;59(25):10111–21.
Sun L, Ouyang J, Ma Y, Zeng Z, Zeng C, Zeng F, Wu S. An activatable probe with aggregation-induced emission for detecting and imaging herbal medicine induced liver injury with optoacoustic imaging and NIR-II fluorescence imaging. Adv Healthc Mater. 2021;10(24):e2100867. https://doi.org/10.1002/adhm.202100867.
Wang XZ, Xue RF, Zhang SY, Zheng YT, Zhang LY, Jiang ZZ. Activation of natural killer T cells contributes to triptolide-induced liver injury in mice. Acta Pharmacol Sin. 2018;39(12):1847–54.
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–5.
Carlos-Reyes A, Muñiz-Lino MA, Romero-Garcia S, López-Camarillo C, Hernández-de la Cruz ON. Biological adaptations of tumor cells to radiation therapy. Front Oncol. 2021;11:718636. https://doi.org/10.3389/fonc.2021.718636.
Gu G, Dustin D, Fuqua SA. Targeted therapy for breast cancer and molecular mechanisms of resistance to treatment. Curr Opin Pharmacol. 2016;31:97–103.
Cai Y, Si W, Huang W, Chen P, Shao J, Dong X. Organic dye based nanoparticles for cancer phototheranostics. Small. 2018;14(25):e1704247.
Chen C, Ou H, Liu R, Ding D. Regulating the photophysical property of organic/polymer optical agents for promoted cancer phototheranostics. Adv Mater. 2020;32(3):e1806331.
Dai H, Shen Q, Shao J, Wang W, Gao F, Dong X. Small molecular NIR-II fluorophores for cancer phototheranostics. Innovation (N Y). 2021;2(1):100082.
Wang S, Wang X, Yu L, Sun M. Progress and trends of photodynamic therapy: from traditional photosensitizers to AIE-based photosensitizers. Photodiagnosis and Photodynamic Therapy. 2021;34:102254.
Xiao Y-F, Chen W-C, Chen J-X, Lu G, Tian S, Cui X, Zhang Z, Chen H, Wan Y, Li S, et al. Amplifying free radical generation of AIE photosensitizer with small singlet–triplet splitting for hypoxia-overcoming photodynamic therapy. ACS Appl Mater Interfaces. 2022;14(4):5112–21.
He Z, Tian S, Gao Y, Meng F, Luo L. Luminescent AIE dots for anticancer photodynamic therapy. Front Chem. 2021;9:672917.
Qi J, Ou H, Liu Q, Ding D. Gathering brings strength: how organic aggregates boost disease phototheranostics. Aggregate. 2021;2(1):95–113.
Schaafsma BE, Mieog JS, Hutteman M, van der Vorst JR, Kuppen PJ, Löwik CW, Frangioni JV, van de Velde CJ, Vahrmeijer AL. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J Surg Oncol. 2011;104(3):323–32.
Hou Y-J, Yang X-X, Liu R-Q, Zhao D, Guo C-X, Zhu A-C, Wen M-N, Liu Z, Qu G-F, Meng H-X. Pathological mechanism of photodynamic therapy and photothermal therapy based on nanoparticles. Int J Nanomedicine. 2020;15:6827–38.
Wei Y, Wang Z, Yang J, Xu R, Deng H, Ma S, Fang T, Zhang J, Shen Q. Reactive oxygen species / photothermal therapy dual-triggered biomimetic gold nanocages nanoplatform for combination cancer therapy via ferroptosis and tumor-associated macrophage repolarization mechanism. J Colloid Interface Sci. 2022;606(Pt 2):1950–65.
Li H, Kim H, Han J, Nguyen V-N, Peng X, Yoon J. Activity-based smart AIEgens for detection, bioimaging, and therapeutics: recent progress and outlook. Aggregate. 2021;2(4):e51.
Li X, Zhang D, Yin C, Lu G, Wan Y, Huang Z, Tan J, Li S, Luo J, Lee C-S. A diradicaloid small molecular nanotheranostic with strong near-infrared absorbance for effective cancer photoacoustic imaging and photothermal therapy. ACS Appl Mater Interfaces. 2021;13(14):15983–91.
Xu W, Wang D, Tang BZ. NIR-II AIEgens: a win-win integration towards bioapplications. Angew Chem Int Ed Engl. 2021;60(14):7476–87.
Xu Y, Zhang Y, Li J, An J, Li C, Bai S, Sharma A, Deng G, Kim JS, Sun Y. NIR-II emissive multifunctional AIEgen with single laser-activated synergistic photodynamic/photothermal therapy of cancers and pathogens. Biomaterials. 2020;259:120315. https://doi.org/10.1016/j.biomaterials.2020.120315.
Punnoose J, Nachman H, Ashkenazi S. Oxygen imaging for non-invasive metastasis detection. Sensors. 2022;22(1):237.
Kwak YH, Lee YJ, Lee JY, Nam EJ, Kim S, Kim YT, Kim SW. Indocyanine green fluorescent image-guided inguinal sentinel lymph node biopsy in vulvar cancer. Obstet Gynecol Sci. 2021. https://doi.org/10.5468/ogs.21335.
Chow S, Karam A. Role of sentinel lymph node biopsy for gynecologic cancers. Curr Opin Obstet Gynecol. 2022;34(1):15–9.
Cai X, Liu J, Liew WH, Duan Y, Geng J, Thakor N, Yao K, Liao L-D, Liu B. Organic molecules with propeller structures for efficient photoacoustic imaging and photothermal ablation of cancer cells. Materials Chemistry Frontiers. 2017;1(8):1556–62.
Wang D, Dong H, Li M, Cao Y, Yang F, Zhang K, Dai W, Wang C, Zhang X. Erythrocyte-cancer hybrid membrane camouflaged hollow copper sulfide nanoparticles for prolonged circulation life and homotypic-targeting photothermal/chemotherapy of melanoma. ACS Nano. 2018;12(6):5241–52.
Chen W, Ouyang J, Liu H, Chen M, Zeng K, Sheng J, Liu Z, Han Y, Wang L, Li J, et al. Black phosphorus nanosheet-based drug delivery system for synergistic photodynamic/photothermal/chemotherapy of cancer. Adv Mater. 2017;29(5):1603864.
Ma N, Zhang M-K, Wang X-S, Zhang L, Feng J, Zhang X-Z. NIR light-triggered degradable MoTe2 nanosheets for combined photothermal and chemotherapy of cancer. Adv Func Mater. 2018;28(31):1801139.
Xiao YF, An FF, Chen JX, Yu J, Tao WW, Yu Z, Ting R, Lee CS, Zhang XH. The nanoassembly of an intrinsically cytotoxic near-infrared dye for multifunctionally synergistic theranostics. Small. 2019;15(38):e1903121. https://doi.org/10.1002/smll.201903121.
Antaris AL, Chen H, Cheng K, Sun Y, Hong G, Qu C, Diao S, Deng Z, Hu X, Zhang B, et al. A small-molecule dye for NIR-II imaging. Nat Mater. 2016;15(2):235–42.
Xu WH, Zhang ZJ, Kang MM, Guo H, Li YM, Wen HF, Lee MMS, Wang ZY, Kwok RTK, Lam JWY, et al. Making the best use of excited-state energy: multimodality theranostic systems based on second near-infrared (NIR-II) aggregation-induced emission luminogens (AIEgens). Acs Materials Letters. 2020;2(8):1033–40.
Liu LQ, Wang X, Wang LJ, Guo LQ, Li YB, Bai B, Fu F, Lu HG, Zhao XW. One-for-all phototheranostic agent based on aggregation-induced emission characteristics for multimodal imaging-guided synergistic photodynamic/photothermal cancer therapy. ACS Appl Mater Interfaces. 2021;13(17):19668–78.
Wen H, Zhang Z, Kang M, Li H, Xu W, Guo H, Li Y, Tan Y, Wen Z, Wu Q, Huang J, Xi L, Li K, Wang L, Wang D, Tang BZ. One-for-all phototheranostics: Single component AIE dots as multi-modality theranostic agent for fluorescence-photoacoustic imaging-guided synergistic cancer therapy. Biomaterials. 2021;274:120892. https://doi.org/10.1016/j.biomaterials.2021.120892.
Acknowledgements
This project was financially supported by the Research Grants Council of Hong Kong (16306620, N-HKUST609/19, and C6014-20W), the National Natural Science Foundation of China (21788102), the Innovation and Technology Commission (ITC-CNERC14SC01 and ITCPD/17-9), the General Program of Shenzhen Science and Technology Innovation Commission (JCYJ20190807144209381), the Shenzhen Key Medical Discipline Construction Fund (No. SZXK015) and the National Natural Youth Foundation of China (81901771).
Author information
Authors and Affiliations
Contributions
Pei Li and Ryan Tsz Kin Kwok had the idea for the article. Pei Li performed article search and selection, analyzed the data, and drafted the manuscript. Yang Li gave support during study search and selection. Jacky Wing Yip Lam, Cun Chuan Wang, Li Gang Xia, and Ben Zhong Tang conceptualized the study and coordinated the study workflow. Xue Wen He and Ryan Tsz Kin Kwok critically reviewed the manuscript. All the authors reviewed the final version of the text.
Corresponding authors
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Preclinical Imaging.
Rights and permissions
About this article
Cite this article
Li, P., He, X., Li, Y. et al. Recent advances in aggregation-induced emission luminogens in photoacoustic imaging. Eur J Nucl Med Mol Imaging 49, 2560–2583 (2022). https://doi.org/10.1007/s00259-022-05726-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05726-8