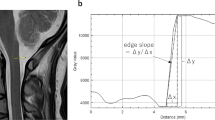
Abstract
Objectives
To validate the subjective image quality and lesion detectability of deep learning-accelerated Dixon (DL-Dixon) imaging of the cervical spine compared with routine Dixon imaging.
Materials and methods
A total of 50 patients underwent sagittal routine Dixon and DL-Dixon imaging of the cervical spine. Acquisition parameters were compared and non-uniformity (NU) values were calculated. Two radiologists independently assessed the two imaging methods for subjective image quality and lesion detectability. Interreader and intermethod agreements were estimated with weighted kappa values.
Results
Compared with the routine Dixon imaging, the DL-Dixon imaging reduced the acquisition time by 23.76%. The NU value is slightly higher in DL-Dixon imaging (p value: 0.015). DL-Dixon imaging showed superior visibility of all four anatomical structures (spinal cord, disc margin, dorsal root ganglion, and facet joint) for both readers (p value: < 0.001 ~ 0.002). The motion artifact scores were slightly higher in the DL-Dixon images than in routine Dixon images (p value = 0.785). Intermethod agreements were almost perfect for disc herniation, facet osteoarthritis, uncovertebral arthritis, central canal stenosis (κ range: 0.830 ~ 0.980, all p values < 0.001) and substantial to almost perfect for foraminal stenosis (κ = 0.955, 0.705 for each reader). There was an improvement in the interreader agreement of foraminal stenosis by DL-Dixon images, from moderate to substantial agreement.
Conclusion
The DLR sequence can substantially decrease the acquisition time of the Dixon sequence with subjective image quality at least as good as the conventional sequence. And no significant differences in lesion detectability were observed between the two sequence types.





Similar content being viewed by others
References
Herrmann J, Koerzdoerfer G, Nickel D, Mostapha M, Nadar M, Gassenmaier S, et al. Feasibility and implementation of a deep learning MR reconstruction for TSE sequences in musculoskeletal imaging. Diagnostics. 2021;11(8):1484.
Bash S, Tanenbaum LN. Deep learning: promising to revolutionize image reconstruction. Appl Radiol. 2021;50:32–7.
Gassenmaier S, Afat S, Nickel D, Mostapha M, Herrmann J, Othman AE. Deep learning–accelerated T2-weighted imaging of the prostate: reduction of acquisition time and improvement of image quality. Eur J Radiol. 2021;137:109600.
Hammernik K, Knoll F, Sodickson D, Pock T. Learning a variational model for compressed sensing MRI reconstruction. Proceedings of the International Society of Magnetic Resonance in Medicine (ISMRM); 2016:2016
Schlemper J, Caballero J, Hajnal JV, Price A, Rueckert D. A deep cascade of convolutional neural networks for MR image reconstruction. Information Processing in Medical Imaging: 25th International Conference, IPMI 2017, Boone, NC, USA, June 25-30, 2017, Proceedings 25; 2017: Springer; 2017. p. 647–658
Lee D, Lee J, Ko J, Yoon J, Ryu K, Nam Y. Deep learning in MR image processing. Investig Magn Reson Imaging. 2019;23(2):81–99.
Liang D, Cheng J, Ke Z, Ying L. Deep MRI reconstruction: unrolled optimization algorithms meet neural networks. arXiv preprint arXiv:190711711. 2019.
Hahn S, Yi J, Lee H-J, Lee Y, Lim Y-J, Bang J-Y, et al. Image quality and diagnostic performance of accelerated shoulder MRI with deep learning–based reconstruction. Am J Roentgenol. 2022;218(3):506–16.
Bash S, Johnson B, Gibbs W, Zhang T, Shankaranarayanan A, Tanenbaum L. Deep learning image processing enables 40% faster spinal MR scans which match or exceed quality of standard of care. Clin Neuroradiol. 2022;32(1):197–203.
Kashiwagi N, Tanaka H, Yamashita Y, Takahashi H, Kassai Y, Fujiwara M, et al. Applicability of deep learning-based reconstruction trained by brain and knee 3T MRI to lumbar 1.5 T MRI. Acta Radiologica Open. 2021;10(6):20584601211023940.
Yasaka K, Tanishima T, Ohtake Y, Tajima T, Akai H, Ohtomo K, et al. Deep learning reconstruction for 1.5 T cervical spine MRI: effect on interobserver agreement in the evaluation of degenerative changes. Eur Radiol. 2022;32(9):6118–25.
Ragoschke-Schumm A, Schmidt P, Schumm J, Reimann G, Mentzel H-J, Kaiser WA, et al. Decreased CSF-flow artefacts in T2 imaging of the cervical spine with periodically rotated overlapping parallel lines with enhanced reconstruction (PROPELLER/BLADE). Neuroradiol. 2011;53(1):13–8.
Noebauer-Huhmann I-M, Glaser C, Dietrich O, Wallner C-P, Klinger W, Imhof H, et al. MR imaging of the cervical spine: assessment of image quality with parallel imaging compared to non-accelerated MR measurements. Eur Radiol. 2007;17(5):1147–55.
Kang Y, Lee JW, Koh YH, Hur S, Kim SJ, Chai JW, et al. New MRI grading system for the cervical canal stenosis. Am J Roentgenol. 2011;197(1):W134–40.
Park HJ, Kim S, Lee S, Park N, Chung E, Rho M, et al. A practical MRI grading system for cervical foraminal stenosis based on oblique sagittal images. Br J Radiol. 2013;86(1025):20120515.
Cohen J. Weighted kappa: nominal scale agreement provision for scaled disagreement or partial credit. Psychol Bull. 1968;70(4):213.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;159–74.
Guerini H, Omoumi P, Guichoux F, Vuillemin V, Morvan G, Zins M, et al. Fat suppression with Dixon techniques in musculoskeletal magnetic resonance imaging: a pictorial review. Seminars in musculoskeletal radiology; 2015: Thieme Medical Publishers; 2015. p. 335–347
Funding
This work was supported by 2022 Inje University Busan Paik Hospital Research Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Seo, G., Lee, S.J., Park, D.H. et al. Image quality and lesion detectability of deep learning-accelerated T2-weighted Dixon imaging of the cervical spine. Skeletal Radiol 52, 2451–2459 (2023). https://doi.org/10.1007/s00256-023-04364-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-023-04364-x