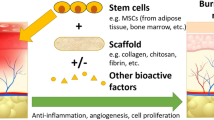
Abstract
Bone has the ability to repair minor injuries through remodeling. However, when the host source of osteoprogenitors is compromised at the defect site, one effective treatment may be cell-based therapy, as it replenishes the area of bone loss with cells possessing osteogenic potential. This review is a concise comparison of different types of stem cells that have the potential to be used in tissue-engineered scaffolds for bone repair. The clinical use of mesenchymal stem or stromal cells isolated from the bone marrow for treating various diseases has been well documented. However, the scarcity of these cells prompts the search for alternative sources of multipotential cells such as amniotic fluid stem cells and umbilical cord perivascular cells. Embryonic stem cells are another controversial source of cells with osteogenic potential. These cells have the ability to differentiate into all cell types of the adult body. Issues such as the use of human embryos and the risk of contamination from animal-derived culture components continue to prevent the therapeutic use of ESCs. As a result, abundant research has been carried out to design defined culture conditions for culturing ESCs, and alternative strategies such as the generation of induced pluripotent stem cells are being developed to eliminate the need for using embryos for cell derivation. In addition to the cell source, the ability to control stem cell differentiation into functional bone and the choice of biomaterial are also paramount objectives that are being examined in research and clinical trials.

Similar content being viewed by others
References
Perry CR. Bone repair techniques, bone grafts, and bone graft substitutes. Clin Orthop Relat Res 1999;360:71–86.
Bruder SP, Fox BS. Tissue engineering of bone—cell based strategies. Clin Orthop Relat Res 1999;367S: S68–S83.
Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284(5411): 143–147.
Horwitz EM, Le Blanc K, Dominici M, et al. Clarification of the nomenclature for MSC: The International Society of Cellular Therapy position statement. Cytotherapy 2005;7: 393–395.
Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8: 315–317.
Fehrer C, Lepperdinger G. Mesenchymal stem cell aging. Exp Gerontol 2005;40: 926–930.
Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med 2004;8(3): 301–316.
Friedenstein AJP-SI, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol 1966;16: 381–390.
Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001;7: 211–228.
Nakahara J, Bruder SP, Haynesworth SE, et al. Bone and cartilage formation in diffusion chambers by subcultured cells derived from the periosteum. Bone 1990;11: 181–188.
De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum 2001;44: 1928–1942.
Jankowski RJ, Deasy BM, Huard J. Muscle-derived stem cells. Gene Ther 2002;9: 642–647.
Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 2003;33: 919–926.
D’Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res 1999;14: 1115–1122.
Bonyadi M, Waldman SD, Liu D, Aubin JE, Grynpas MD, Stanford WL. Mesenchymal precursor self-renewal deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null mice. Proc Natl Acad Sci USA 2003;100(10): 5840–5845.
Rodriguez JP, Montecinos L, Rios S, Reyes P, Martinez J. Mesenchymal stem cells from osteoporotic patients produce a type I collagen-deficient extracellular matrix favoring adipogenic differentiation. J Biol Chem 2000;79(4): 557–565.
Rodriguez JP, Rios S, Fernandez M, Santibanez JF. Differential activation of ERK1,2 MAP kinase signaling pathway in mesenchymal stem cell from control and osteoporotic postmenopausal women. J Biol Chem 2004;92(4): 745–754.
Oreffo RO, Bennett A, Carr AJ, Triffitt JT. Patients with primary osteoarthritis show no change with ageing in the number of osteogenic precursors. Scand J Rheumatol 1998;27(6): 415–424.
Prusa AR, Hengstschlager M. Amniotic fluid cells and human stem cell research: a new connection. Med Sci Monit 2002;8(11): RA253–257.
De Coppi P, Bartsch Jr G, Siddiqui MM, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 2007;25(1): 100–106.
Chiavegato A, Bollini S, Pozzobon M, et al. Human amniotic fluid-derived stem cells are rejected after transplantation in the myocardium of normal, ischemic, immuno-suppressed or immuno-deficient rat. J Mol Cell Cardiol 2007;42(4): 746–759.
Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells 2005;23(2): 220–229.
Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol 2000; 109(1): 235–242.
Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cells in vitro. BMC Cell Biol 2006;7: 14–20.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998;282(5391): 1145–1147.
Hyslop LA, Armstrong L, Stojkovic M, Lako M. Human embryonic stem cells: biology and clinical implications. Expert Rev Mol Med 2005;7(19): 1–21.
O’Shea KS. Embryonic stem cell models of development. Anat Rec 1999;257(1): 32–41.
Cao T, Heng BC, Ye CP, et al. Osteogenic differentiation within intact human embryoid bodies result in a marked increase in osteocalcin secretion after 12 days of in vitro culture, and formation of morphologically distinct nodule-like structures. Tissue Cell 2005;37(4): 325–334.
Karp JM, Ferreira LS, Khademhosseini A, Kwon AH, Yeh J, Langer RS. Cultivation of human embryonic stem cells without the embryoid body step enhances osteogenesis in vitro. Stem Cells 2006;24(4): 835–843.
Ahn SE, Kim S, Park KH, et al. Primary bone-derived cells induce osteogenic differentiation without exogenous factors in human embryonic stem cells. Biochem Biophys Res Commun 2006;340(2): 403–408.
Maniatopoulos C, Sodek J, Melcher AH. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res 1988;254(2): 317–330.
Schnutgen F, Stewart AF, von Melchner H, Anastassiadis K. Engineering embryonic stem cells with recombinase system. Methods Enzymol 2006;420: 100–136.
Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med 2005;11(2): 228–232.
Richards M, Fong CY, Chan WK, Wong PC, Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol 2002;20(9): 933–936.
Ludwig TE, Levenstein ME, Jones JM, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol 2006;24: 185–187.
Chin ACP, Fong WJ, Goh LT, Philp R, Oh SKW, Choo ABH. Identification of proteins from feeder conditioned medium that support human embryonic stem cells. J Biotechnol 2007;130: 320–328.
Gertow K, Wolbank S, Rozell B, et al. Organized development from human embryonic stem cells after injection into immunodeficient mice. Stem Cells Dev 2004;13(4): 421–435.
Kim K, Lerou P, Yabuuchi A, et al. Histocompatible embryonic stem cells by parthenogenesis. Science 2007;315: 482–486.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126(4): 663–676.
Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131(5): 861–872.
Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007;318(5858): 1917–1920.
Bielby RC, Boccaccini AR, Polak JM, Buttery LD. In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tissue Eng 2004;10(9–10): 1518–1525.
O’Flaherty E, Sparrow R, Szer J. Bone marrow stromal function from patients after bone marrow transplantation. Bone Marrow Transplant 1995;15: 207–212.
Galotto M, Berisso G, Delfino L, et al. Stromal damage as consequence of high-dose chemo/radiotherapy in bone marrow transplant recipients. Exp Hematol 1999;27: 1460–1466.
Muschler GF, Nitto H, Matsukura Y, et al. Spine fusion using cell matrix composites enriched in bone marrow-derived cells. Clin Orthop Relat Res 2003;407: 102–118.
Bruder SP, Kraus KH, Goldberg VM, Kadiyala S. The effect of implants loaded with autologous mesenchymal stem cells on healing of canine segmental bone defects. J Bone Joint Surg Am 1998;80: 985–996.
Bruder SP, Kurth AA, Shea M, Hayes WC, Jaiswal N, Kadiyala S. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res 1998;16: 155–162.
Den Boer FC, Wippermann BW, Blokhuis TJ, Patka P, Bakker FC, Haarman HJ. Healing of segmental bone defects with granular porous hydroxyapatite augmented with recombinant human osteogenic protein-1 or autologous bone marrow. J Orthop Res 2003;21(3): 521–528.
Takigami H, Kumagai K, Latson L, et al. Bone formation following OP-1 implantation is improved by addition of autogenous bone marrow cells in a canine femur defect model. J Orthop Res 2007;25(10): 1333–1342.
Horwitz EM, Prockop DJ, Fitzpatrick LA, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 1999;5: 309–313.
Horwitz EM, Gordon PL, Koo WK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA 2002;99(13): 8932–8937.
Le Blanc K, Gotherstrom C, Ringden O, et al. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation 2005;79(11): 1607–1614.
Dallari D, Savarino L, Stagni C, et al. Enhanced tibial osteotomy healing with use of bone grafts supplemented with platelet gel or platelet gel and bone marrow stromal cells. J Bone Joint Surg Am 2007;89: 2413–2420.
Quarto R, Mastrogiacomo M, Cancedda R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med 2001;344(5): 385–386.
Kneser U, Schaefer DJ, Polykandriotis E, Horch RE. Tissue engineering of bone: the reconstructive surgeon’s point of view. J Cell Mol Med 2006;10(1): 7–19.
Boden SD. The ABCs of BMPs. Orthop Nurs 2005;24(1):49–52.
Gazit D, Turgeman G, Kelley P, et al. Engineered pluripotent mesenchymal cells integrate and differentiate in regenerating bone: a novel cell-mediated gene therapy. J Gen Med 1999;1(2): 121–133.
Lieberman JR, Daluiski A, Stevenson S, et al. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am 1999;81(7): 905–917.
Wang JC, Kanim LE, Yoo S, Campbell PA, Berk AJ, Lieberman JR. Effect of regional gene therapy with bone morphogenetic protein-2-producing bone marrow cells on spinal fusion in rats. J Bone Joint Surg Am 2003;85-A(5): 905–911.
Dragoo JL, Choi JY, Lieberman JR, et al. Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res 2003;21(4): 622–629.
Dragoo JL, Lieberman JR, Lee RS, et al. Tissue-engineered bone from BMP-2 transduced stem cells derived from human fat. Plast Reconstr Surg 2005;115(6): 1665–1673.
Tu Q, Valverde P, Chen J. Osterix enhances proliferation and osteogenic potential of bone marrow stromal cells. Biochem Biophys Res Commun 2006;341(4): 1257–1265.
Wu L, Wu Y, Lin Y, et al. Osteogenic differentiation of adipose derived stem cells promoted by overexpression of osterix. Mol Cell Biochem 2007;301(1–2): 83–92.
Tu Q, Valverde P, Li S, Zhang J, Yang P, Chen J. Osterix overexpression in mesenchymal stem cells stimulates healing of critical-sized defects in murine calvarial bone. Tissue Eng 2007;13(10): 2431–2440.
Zhang X, Yang M, Lin L, et al. Runx2 overexpression enhances osteoblastic differentiation and mineralization in adipose-derived stem cells in vitro and in vivo. Calcif Tissue Int 2006;79(3): 169–178.
Temenoff JS, Mikos AG. Review: tissue engineering for regeneration of articular cartilage. Biomaterials 2000;21: 431–440.
Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am 1993;75: 532–553.
Redman SN, Oldfield SF, Archer CW. Current strategies for articular cartilage repair. Eur Cells Mater 2005;9: 23–32.
Mano JF, Reis RL. Osteochondral defects: present situation and tissue engineering approaches. J Tissue Eng Regen Med 2007;1: 261–273.
Ahmed N, Stanford WL, Kandel RR. Mesenchymal stem and progenitor cells for cartilage repair. Skeletal Radiol 2007;36(10): 909–912.
Horwitz EM, Prockop DJ, Gordon PL, et al. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood 2001;97(5): 1227–1231.
Kitoh H, Kitakoji T, Tsuchiya H, Mitsuyama H, Nakamura H, Katoh M, Ishiguro N. Transplantation of marrow-derived mesenchymal stem cells and platelet-rich plasma during distraction osteogenesis—a preliminary result of three cases. Bone 2004;35(4): 892–898.
Marcacci M, Kon E, Moukhachev V, Lavroukov A, Kutepov S, Quarto R, Mastrogiacomo M, Cancedda R. Tissue engineeringstem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng 2007;13(5): 947–955.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at https://doi.org/10.1007/s00256-008-0476-x
Rights and permissions
About this article
Cite this article
Waese, E.Y.L., Kandel, R.R. & Stanford, W.L. Application of stem cells in bone repair. Skeletal Radiol 37, 601–608 (2008). https://doi.org/10.1007/s00256-007-0438-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-007-0438-8