Abstract
Mesenchymal stem cell-derived exosomes have emerged as a promising cell-free therapy for tissue engineering. Compared to intact stem cells, exosomes have advantages like low immunogenicity and ability to carry regenerative cargo. This review examined the potential of exosomes to treat defects in skin, bone and cartilage. In preclinical models, exosomes improved wound healing, stimulated bone regeneration, and enabled cartilage repair by transferring proteins, mRNAs and microRNAs. Their effects were elicited by modulating inflammation, angiogenesis, cell proliferation and matrix synthesis. Exosomes represent a promising cell-free therapy for tissue engineering. However, challenges remain regarding scalable isolation, elucidating mechanisms, and translating this approach to human trials. Understanding these challenges will enable the successful clinical translation of exosomes for regenerative medicine applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tissue damage from trauma, disease, aging, or congenital defects presents a major challenge in healthcare. An emerging field called regenerative medicine aims to address this by repairing, replacing, or regenerating damaged tissues and organs. Stem cells sit at the core of many regenerative strategies due to their capacity to self-renew and differentiate into specialized cell types. Of the various stem cell populations, mesenchymal stem cells (MSCs) have emerged as a leading candidate for cell-based therapies. MSCs possess multipotency, allowing their differentiation into mesodermal lineages like bone, fat, and cartilage. Additionally, MSCs demonstrate immunomodulatory effects by suppressing inflammatory responses and releasing trophic factors that stimulate tissue repair [1, 2].
However, clinical use of MSC therapies faces some risks. Intravenous infusion of MSCs has been associated with infusional toxicity. Ectopic tissue formation and tumorigenesis are also concerns if proliferation and differentiation become dysregulated [3, 4]. As an alternative approach, the use of extracellular vesicles (EVs) derived from MSCs is gaining increasing attention. MSCs release EVs such as exosomes and microvesicles, which mediate intercellular communication and mirror many of the therapeutic effects of MSCs themselves. Compared to cell-based treatments, EVs avoid risks like tumor formation [5].
MSC-derived EVs promote tissue repair through several mechanisms. They can transfer regenerative factors and genetic material to recipient cells, reprogramming their behavior. EVs also modulate immune responses and inflammation to create a pro-regenerative microenvironment. Additionally, EVs stimulate resident stem and progenitor cells to enhance endogenous repair processes [6, 7].
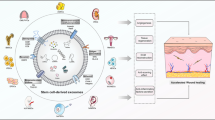
Numerous preclinical studies have shown promising results using MSC-derived EVs to treat skin, bone, and cartilage defects. In models of wound healing, EVs accelerated re-epithelialization, angiogenesis, and collagen remodeling [8]. For bone repair, EVs promoted osteoblast proliferation, differentiation, and mineralization [9]. MSC exosomes enabled cartilage repair by enhancing chondrocyte proliferation and matrix synthesis and by modulating inflammatory pathways. Across these tissues, exosomes elicited regenerative effects by transferring proteins, mRNAs and microRNAs.
This review highlights preclinical animal studies demonstrating the tissue regenerative effects of MSC exosomes. However, challenges remain regarding scalable exosome isolation, customization of exosome cargo, elucidating mechanisms of action, and translating this therapeutic approach to human clinical trials. Understanding the challenges in this field will open up new possibilities for the successful translation of exosomes into clinical applications. Overall, MSC-derived exosomes represent.
a promising cell-free therapy for tissue engineering that warrants further investigation.
MSC-derived EVs/exosomes
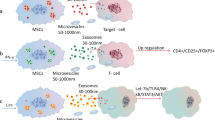
Extracellular vesicles (EVs), a general term encompassing various molecules including exosomes, microvesicles, microparticles, ectosomes, oncosomes, apoptotic bodies etc., are naturally released from all cell types [10]. They are enclosed by a lipid bilayer and cannot replicate [11]. EVs derived from MSCs, including exosomes and shed microvesicles (50–1000 nm diameter), can mimic the biological activity of MSCs by horizontally transferring many functional molecules including mRNAs, miRNAs, proteins and lipids to the cellular microenvironment and target cells, and then mediate restoration of homeostasis and tissue regeneration through various mechanisms [12]. Small EVs (sEVs, diameter 50–200 nm) released from MSCs have emerged as promising therapeutic agents in a wide range of preclinical models. They are considered as major mediators of MSC therapeutic functions [13].
Exosomes are nano-sized membranous vesicles secreted from cell membranes with 5’-nucleotidase activity and physiological function. They are distinguished from other EVs, i.e., shed microvesicles (or ectosomes; 100–1000 nm) and apoptotic bodies (released during apoptosis; 1–5 μm) based on their size, origin (endosomal or plasma membrane), surface markers and cargo [14, 15]. Specifically, exosomes are defined as sEVs of diameter 40–100 nm, density 1.13–1.19 g/ml in sucrose gradient and “cup” or “dish” shaped morphology by electron microscopy analysis. They are released from most cells into the extracellular space after fusion of multivesicular bodies (MVBs) and the plasma membrane [16]. Exosomes are characterized by surface markers (CD9, CD63, CD81) from the tetraspanin family, heat shock proteins (Hsp60, Hsp70, Hsp90), membrane transport proteins (Rab GTPases, annexins), biogenesis-related proteins (ESCRT family, Alix, TSG101), metabolic enzymes (GAPDH, ATPase, PGK1) as well as cytoskeletal proteins, and also lipid rafts [17].
Exosome isolation relies on various methods, each exploiting a particular exosome feature such as density, shape, size and surface proteins. These techniques include techniques based on differential ultracentrifugation, size, immunoaffinity capture, exosome precipitation and microfluidics techniques; however, ultracentrifugation is considered the gold standard method of exosome isolation. Figure 1 shows extracellular vesicle separation by density gradient ultracentrifugation [18].
(1) Extracellular Vesicles (EV) are released by cells during their normal activity. (2) For EV separation, conditioned culture medium is harvested and major contaminants removed by consecutive low speed centrifugations. (3) The cleared supernatant is concentrated by ultracentrifugation, and the resulting 100K pellet is loaded on the bottom of an iodixanol gradient. EVs float upwards and EV-enriched fractions are collected and pelleted. The final sample is rich in EVs and absent in contaminants
Exosomes are released from most cells including MSCs and are present in all biofluids such as blood, urine, saliva, synovial fluid, bile, breast milk, amniotic fluid and semen [19]. Their cargo repertoire can reflect the nature and status of parent cells [13].
MSC-derived exosomes, like exosomes from other cell types, engage in intercellular communication and cell signaling by transferring proteins, mRNAs and regulatory microRNAs (miRNAs), as well as other paracrine mediators like cytokines and growth factors, to recipient cells. Hence, they can mimic and replicate MSC biological functions and can serve as safer, MSC-based, cell-free therapeutic approaches [20]. Therapeutic potential of exosomes released from MSCs derived from various tissues like bone marrow, adipose tissue, umbilical cord or placenta has been demonstrated in various disorders [13]. Here, we will focus on skin, bone and cartilage defects.
MSC exosomes in skin wound healing
Chronic skin wounds represent a major clinical challenge, with substantial impacts on patient health and healthcare costs. Novel regenerative strategies using mesenchymal stem cells (MSCs) or their secreted exosomes show promise in facilitating wound healing through various mechanisms [21, 22].
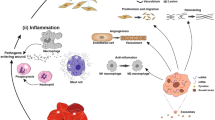
MSC-derived exosomes can modulate inflammatory responses in chronic wounds by transferring regulatory miRNAs to target cells. For example, they can encourage anti-inflammatory M2 macrophage polarization and suppress pro-inflammatory M1 macrophages by shuttling miRNAs like miR-21, miR-146a, and miR-181 to modulate downstream inflammatory genes [6, 7]. This helps resolve prolonged inflammation that prevents healing. One study pretreated MSCs with melatonin to enhance exosome function before collecting and applying the exosomes to diabetic rat wound models [23]. Melatonin-enhanced MSC exosomes strongly suppressed inflammatory cytokines like IL-1β and TNF-α and promoted the anti-inflammatory factor IL-10 compared to non-pretreated exosomes.
Re-epithelialization, driven by epithelial cell proliferation and migration, is another key healing process impaired in chronic wounds that MSC exosomes can stimulate. One experiment applied umbilical cord MSC-derived exosomes to burn wounds in a rat model and found significantly accelerated re-epithelialization and cell proliferation compared to controls [8]. The exosomes activated Wnt/β-catenin signaling and upregulated proliferation/migration markers like CK19 and PCNA. They also inhibited apoptosis under heat stress by activating AKT signaling. In another study, the exosomes suppressed nuclear translocation of pro-apoptotic factors like AIF while upregulating survival proteins like PARP-1 to encourage re-epithelialization [24]. Engineering the exosomes to overexpress specific miRNAs like miR-135a can further enhance their epithelialization effects [25].
Recent work has combined MSC exosomes with biomaterial scaffolds to achieve optimal wound healing results. For example, a chitosan/silk hydrogel sponge loaded with exosomes from gingival MSCs markedly improved re-epithelialization, collagen deposition, angiogenesis, and neurite growth when applied to wounds in a diabetic rat model [26]. Another group loaded umbilical cord MSC exosomes into a Pluronic F-127 hydrogel which extended their release and activity at the wound site, boosting angiogenesis and wound closure rates [23].
Beyond modulating inflammation and epithelialization, MSC exosomes can also enhance two other critical phases of healing: angiogenesis and collagen/ECM remodeling. Multiple studies confirm exosomes derived from induced pluripotent, adipose, or other MSC sources stimulate collagen synthesis and angiogenesis when administered to cutaneous wound models [6, 27]. Specific exosomal miRNAs like miR-125a and lncRNAs like MALAT1 were implicated in inhibiting anti-angiogenic factors and encouraging these repair processes [25, 27].
A recent study by Hoang et al. investigated the regenerative potential of exosomes from various mesenchymal stem cell (MSC) sources on cutaneous wound healing [28]. The researchers isolated exosomes from adipose-derived MSCs (ADMSCs), bone marrow-derived MSCs (BMSCs), and umbilical cord MSCs (UCMSCs) and analyzed their growth factor content. Enzyme-linked immunosorbent assays identified differing levels of factors including platelet-derived growth factor BB (PDGF-BB), fibroblast growth factor 2 (FGF-2), vascular endothelial growth factor A (VEGF-A), and hepatocyte growth factor (HGF) in exosomes from all three MSC types. Notably, transforming growth factor beta (TGF-β) was only detected in exosomes from UCMSCs. Functional assays revealed that exosomes from each source induced proliferation and migration of both dermal fibroblasts and keratinocytes, indicating wound healing promotion potential. However, BMSC-derived exosomes exerted the greatest proliferative and migratory influence specifically on fibroblasts. In contrast, UCMSC-derived exosomes had the strongest effect on keratinocytes. Furthermore, higher doses of exosomes elicited faster cell migration rates overall. In summary, Hoang et al. demonstrated that while exosomes from various MSC origins share regenerative bioactivity, those derived from BMSCs and UCMSCs may be optimal for targeting dermal and epidermal cell populations in cutaneous wound healing. Their distinct growth factor content likely contributes to this source-dependent activity. These findings could inform the development of exosome-based therapeutics tailored to particular wound healing phases and cell types.
Collectively, these findings demonstrate the promise of engineered MSC exosomes, especially when combined with biomaterials, to promote comprehensive regeneration in impaired wounds. Further optimization of exosome production, cargo, and carriers could enable effective clinical translation.
Table 1 shows preclinical studies of mesenchymal stem cell-exosomes in cutaneous wound healing phases.
MSC exosomes in the regeneration of bone defects
Bone defects caused by trauma or disease can be treated surgically using bone grafts. Autografts are considered the gold standard due to their osteoinductive and osteoconductive properties, although they carry infection risk [37,38,39]. Synthetic scaffolds combined with mesenchymal stem cells (MSCs) are a promising alternative [40].
Despite effectiveness of MSC-based tissue engineering for bone regeneration, limitations exist including phenotypic changes during culture, low cell delivery and survival. This has driven interest in exploring cell-free alternatives using exosomes—extracellular vesicles secreted by cells [41].
Exosomes from bone tissue cells (e.g., MSCs, osteoblasts) regulate osteogenesis and bone remodeling. They induce osteogenic differentiation of MSCs by transferring specific miRNAs that modulate target gene expression [42, 43]. Exosomal miRNAs also stimulate osteoblast proliferation and angiogenesis [44]. For example, hypoxic preconditioning of MSCs increases exosomal HIF-1α, enhancing bone regeneration and angiogenesis in a rabbit osteonecrosis model [45].
Immobilizing MSC-derived exosomes on scaffolds enables sustained release, promoting bone regeneration through osteoinduction and MSC recruitment [46]. Combining iPSC-MSC-exosomes with β-TCP scaffolds boosted bone repair in a rat critical defect model by stimulating angiogenesis and osteogenesis [47]. iPSC-MSC-exosomes also prevented steroid-induced bone loss and increase local angiogenesis, probably through activation of the PI3K/Akt pathway in endothelial cells [48].
Additionally, MSC-exosomes have immunomodulatory effects that likely contribute to fracture healing by inhibiting the pro-inflammatory factors TNF-α and IL-1β and increasing the anti-inflammatory factor TGF-β [49].
In summary, substantial evidence indicates exosomes derived from bone tissue cells stimulate osteogenesis, angiogenesis, and bone remodeling to enhance bone defect repair through cell-free approaches. However, therapeutic efficacy may depend on health status of exosome donor cells.
As a recent study shows, BMSC-EXs obtained from type 1 diabetes rat models (dBMSC-EXs) and BMSC-EXs obtained from normal rats (nBMSC-EXs) both promoted the osteogenic differentiation of MSCs and increased the angiogenic activity of endothelial cells, but the therapeutic effect of dBMSCs-EXs was lower than nBMSC-EXs. These results indicate that autologous transplantation of exosomes from donors with chronic underlying diseases may be inappropriate for therapeutic purposes [50].
MSC exosomes in the regeneration of cartilage defects
Cartilage defects arising from injury or osteoarthritis pose a major clinical challenge due to the poor intrinsic capacity of cartilage for self-repair. The avascular and aneural nature of cartilage means damage is not repaired via the typical wound healing mechanisms of most tissues. Furthermore, the low cellularity and lack of stem cell pools in cartilage mean the intrinsic regenerative capacity is limited [51, 52]. Current treatment approaches such as microfracture and autologous chondrocyte implantation have shown some success but are not optimal solutions [53]. Tissue engineering strategies using adult mesenchymal stem cells (MSCs) have emerged as a promising alternative cell source for cartilage repair [54]. MSCs can be isolated from various tissues including bone marrow, adipose tissue, synovium, and others [55]. These cells have chondrogenic potential and secrete tropic factors that can stimulate tissue repair. However, challenges remain with ensuring sufficient cell numbers, retention, and optimal differentiation at defect sites [56].
Recent studies have explored the use of MSC Exosomes as a cell-free therapeutic approach. MSC exosomes have shown preclinical efficacy in stimulating cartilage repair and regeneration through several mechanisms. Firstly, they can enhance chondrocyte migration, proliferation, and matrix synthesis to directly regenerate cartilage [57]. Secondly, they can inhibit inflammatory responses and apoptosis in osteoarthritic chondrocytes [58]. These effects are mediated through specific exosomal cargoes such as microRNAs (miR-140-5p, miR-92a-3p, miR-100-5p) and proteins (TSG-6, PTEN) that modulate signaling pathways including mTOR, Wnt/β-catenin, STAT3, and NF-kB [59].
MSC exosomes have been explored as standalone injections or in combination with biomaterial scaffolds to improve localization and retention [60, 61]. Scaffolds such as hydrogels and 3D-printed matrices provide structural support and can mimic the native cartilage extracellular matrix environment. Sustained release of exosomes from these scaffolds may be beneficial to exert biological effects over longer time periods to achieve functional cartilage repair [62].
Problems with scalability of exosomes
One key challenge is scaling up isolation methods to obtain sufficient exosome quantities for clinical applications. Current benchtop techniques like ultracentrifugation and density gradients have limitations in exosome purity, integrity, and yields beyond small-scale research use [63]. For example, lengthy ultracentrifugation can damage exosomes through high g-forces [64]. Immunoaffinity methods using antibodies to capture exosomes are expensive and suffer from low yields [65]. New scalable methods are needed, such as tangential flow filtration [66], polymeric precipitation [67], microfluidics [68], and hydrostatic dialysis [69]. These can isolate bulk exosomes under mild conditions while maintaining integrity. However, further optimization is required to improve purity and prevent co-isolation of non-exosomal particles [70]. Standardization of techniques suitable for good manufacturing practices is also important for clinical translation [71]. Overall, innovative engineering solutions are still needed to enable scalable, reproducible exosome isolation that retains therapeutic activity.
The benefits of customizing exosome cargo
Exosomes contain various bioactive molecules including proteins, lipids, mRNAs, and microRNAs that contribute to their biological effects. Emerging evidence suggests we can engineer exosomes with specific cargoes to achieve desired outcomes for different regenerative applications.
For instance, overexpressing particular microRNAs in parent mesenchymal stem cells can load enhanced levels of those miRNAs into secreted exosomes [72]. This allowed exosomes to better suppress tumor growth and angiogenesis when engineered with miR-146b [73], or to improve cardiac repair when engineered with miR-21 [74]. Likewise, treating parent cells with drugs or growth factors pre-conditions exosomes with higher concentrations of those agents for more potent effects [75].
Additionally, direct engineering methods to load exogenous cargo like siRNAs, mRNAs, and even drugs are being developed using electroporation, sonication, extrusion, and synthetic liposomes [76]. These customized exosomes retained functionality and successfully delivered cargo to recipient cells.
In summary, the capability to tailor exosome contents for specific therapeutic molecules or miRNAs holds great promise to enhance their natural regenerative properties. This could potentiate their effectiveness for applications in tissue engineering, drug delivery, and more.
Engineered exosomes loaded with specific cargo
Several recent studies have engineered exosomes with particular proteins, mRNAs, or microRNAs as designer nanotherapeutics. For example, MSCs overexpressing miR-375 were shown to package the miRNA into secreted exosomes, which improved their efficacy in cardiac repair models [77]. In another study, MSC exosomes were loaded with exogenous siRNA against a target gene using electroporation, leading to successful siRNA delivery and gene silencing [78].
Additionally, directly packaging chemotherapeutic drugs into tumor cell-derived exosomes allowed targeted drug delivery to cancer cells in vivo [79]. The exosomes provided a protective, natural delivery vehicle. In a tissue engineering application, hypoxic culture conditions increased growth factor levels like VEGF in MSC-exosomes, enhancing their angiogenesis-promoting capacity [80].
These examples demonstrate the potential of developing engineered designer exosomes as advanced regenerative therapies. Loading exosomes with specific therapeutic cargoes could amplify their natural bioactive properties. Further optimization of cargo packaging approaches will support clinical translation of this innovative technology. Discussion of these engineered exosome studies provides important context on the future directions and possibilities in this rapidly evolving field.
Exosome storage
Recent studies have shown lyophilization (freeze-drying) can significantly extend exosome shelf life compared to liquid storage. For example, lyophilized MSC exosomes retained structural integrity and biological activity for at least 6 months when stored at -20°C [81]. Refrigerated storage at 4°C in PBS maintains exosome quality for up to 7 days, while freezing at -20°C or -80°C can preserve intact exosomes for at least 90 days [82]. In contrast, living MSC potency declines within 1–3 days after refrigeration and 7–14 days frozen [83].
Additionally, exosomes tolerate freeze–thaw cycles better than cells. Repeated thawing and re-freezing decreased exosome particle count by just 13% compared to 61% loss of cell viability [84]. These emerging stability studies demonstrate the promise of exosome therapies to overcome limitations in cell product storage and shelf life. We can incorporate this data to highlight the improved stability of exosomes versus cells.
Conclusions
Mesenchymal stem cell (MSC) derived exosomes have emerged as a promising new paradigm for regenerative medicine. Extensive preclinical evidence demonstrates their ability to enhance tissue repair and regeneration across diverse models of skin, bone, and cartilage defects. The therapeutic effects are mediated through transfer of bioactive proteins, mRNAs, and miRNAs that modulate cellular behaviors and microenvironmental factors to stimulate healing processes.
Exosomes overcome limitations of cell-based therapies, providing similar benefits in a safer, more stable extracellular vesicle format. They appear inherently immunomodulatory and lack risks like ectopic tissue formation. Exosomes can be isolated from various MSC sources such as bone marrow, adipose, and synovium. Their cargo can also be engineered for enhanced regenerative bioactivity.
However, challenges remain in translating these preclinical findings to clinical applications. Further optimization is needed in scalable production, cargo enhancement, controlled biodistribution and release kinetics, as well as mechanistic understanding. Combining exosomes with biomaterial scaffolds appears promising to improve localization and retention. Larger studies directly comparing exosomes to native MSCs will better define their advantages.
Overall, MSC-derived exosomes represent a novel nanotherapeutic strategy with significant potential but remains early in the developmental pipeline. Continued multidisciplinary research to address translational barriers will accelerate the impact of this innovative nanomedicine approach on regenerative therapies.
Availability of data and materials
Not applicable.
References
Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–84.
Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103(11):1204–19.
Lalu MM, McIntyre L, Pugliese C, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS ONE. 2012;7(10): e47559.
Volkmer E, Kallukalam BC, Maertz J, et al. Hypoxic preconditioning of human mesenchymal stem cells overcomes hypoxia-induced inhibition of osteogenic differentiation. Tissue Eng Part A. 2010;16(1):153–64.
Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2015;35(4):851–8.
Zhang B, Wang M, Gong A, et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2015;33(7):2158–68.
Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49.
Hu L, Wang J, Zhou X, Xiong Z, Zhao J, Yu R, Huang F, Zhang H, Chen L. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep. 2016;6:32993.
Qin Y, Wang L, Gao Z, Chen G, Zhang C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci Rep. 2016;6:21961.
Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8(4):307.
Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, Nolte-’t Hoen EN, Piper MG, Sivaraman S, Skog J, Théry C, Wauben MH, Hochberg F. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;27:2.
Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;15(9):19.
Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017;35(4):851–8.
Edgar JR. Q&A: What are exosomes, exactly? BMC Biol. 2016;13(14):46.
Matsumoto A, Takahashi Y, Chang HY, Wu YW, Yamamoto A, Ishihama Y, Takakura Y. Blood concentrations of small extracellular vesicles are determined by a balance between abundant secretion and rapid clearance. J Extracell Vesicles. 2019;9(1):1696517.
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750.
Witwer KW, Van Balkom BWM, Bruno S, Choo A, Dominici M, Gimona M, Hill AF, De Kleijn D, Koh M, Lai RC, Mitsialis SA, Ortiz LA, Rohde E, Asada T, Toh WS, Weiss DJ, Zheng L, Giebel B, Lim SK. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles. 2019;8(1):1609206.
Pavani KC, Hendrix A, Van Den Broeck W, Couck L, Szymanska K, Lin X, De Koster J, Van Soom A, Leemans B. Isolation and characterization of functionally active extracellular vesicles from culture medium conditioned by bovine embryos in vitro. Int J Mol Sci. 2019;20(1):38.
Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6(4):287–96.
Lai RC, Yeo RW, Tan KH, Lim SK. Exosomes for drug delivery—a novel application for the mesenchymal stem cell. Biotechnol Adv. 2013;31(5):543–51.
Sen CK. Human wounds and its burden: an updated compendium of estimates. Adv Wound Care (New Rochelle). 2019;8(2):39–48.
EL Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347–57.
Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh WS. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27.
Zhao B, Zhang Y, Han S, Zhang W, Zhou Q, Guan H, Liu J, Shi J, Su L, Hu D. Exosomes derived from human amniotic epithelial cells accelerate wound healing and inhibit scar formation. J Mol Histol. 2017;48(2):121–32.
Fang S, Xu C, Zhang Y, Xue C, Yang C, Bi H, Qian X, Wu M, Ji K, Zhao Y, Wang Y, Liu H, Xing X. Umbilical cord-derived mesenchymal stem cell-derived exosomal MicroRNAs suppress myofibroblast differentiation by inhibiting the transforming growth factor-β/SMAD2 pathway during wound healing. Stem Cells Transl Med. 2016;5(10):1425–39.
Tutuianu R, Rosca AM, Iacomi DM, Simionescu M, Titorencu I. Human mesenchymal stromal cell-derived exosomes promote in vitro wound healing by modulating the biological properties of skin keratinocytes and fibroblasts and stimulating angiogenesis. Int J Mol Sci. 2021;22(12):6239.
Liang X, Zhang L, Wang S, Han Q, Zhao RC. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci. 2016;129(11):2182–9.
Hoang DTN, Nguyen THP, Quynh Do TT, et al. Comparative characterization of exosomes secreted from various mesenchymal stem cells and their effects on cutaneous would healing. Stem Cell Res Ther. 2021;12(1):232.
Hu JC, Zheng CX, Sui BD, Liu WJ, Jin Y. Mesenchymal stem cell-derived exosomes: a novel and potential remedy for cutaneous wound healing and regeneration. World J Stem Cells. 2022;14(5):318–29.
Ti D, Hao H, Tong C, Liu J, Dong L, Zheng J, Zhao Y, Liu H, Fu X, Han W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015;13:308.
He X, Dong Z, Cao Y, Wang H, Liu S, Liao L, Jin Y, Yuan L, Li B. MSC-derived exosome promotes m2 polarization and enhances cutaneous wound healing. Stem Cells Int. 2019:7132708.
Li X, Liu L, Yang J, Yu Y, Chai J, Wang L, Ma L, Yin H. Exosome derived from human umbilical cord mesenchymal stem cell mediates MiR-181c attenuating burn-induced excessive inflammation. EBioMedicine. 2016;8:72–82.
Su N, Hao Y, Wang F, Hou W, Chen H, Luo Y. Mesenchymal stromal exosome-functionalized scaffolds induce innate and adaptive immunomodulatory responses toward tissue repair. Sci Adv. 2021;7(20):eabf7207.
Zhang W, Bai X, Zhao B, Li Y, Zhang Y, Li Z, Wang X, Luo L, Han F, Zhang J, Han S, Cai W, Su L, Tao K, Shi J, Hu D. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp Cell Res. 2018;370:333–42.
Wang L, Hu L, Zhou X, Xiong Z, Zhang C, Shehada HMA, Hu B, Song J, Chen L. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci Rep. 2017;7:13321.
Zhang Y, Yan J, Liu Y, Chen Z, Li X, Tang L, Li J, Duan M, Zhang G. Human amniotic fluid stem cell-derived exosomes as a novel cell-free therapy for cutaneous regeneration. Front Cell Dev Biol. 2021;9: 685873.
Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36(3):S20–7.
Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;329:300–9.
Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity. A Stat Eval Spine. 1995;20(9):1055–60.
Petite H, Viateau V, Bensaïd W, Meunier A, de Pollak C, Bourguignon M, et al. Tissue-engineered bone regeneration. Nat Biotechnol. 2000;18(9):959–63.
Li D, Liu J, Guo B, Liang C, Dang L, Lu C, et al. Osteoinductive silk fibroin/titanium dioxide/hydroxyapatite hybrid scaffold for bone tissue engineering. ACS Appl Mater Interfaces. 2016;8(18):11452–9.
Xie H, Wang Z, Zhang L, Lei Q, Zhao A, Wang H, et al. Extracellular vesicle-functionalized decalcified bone matrix scaffolds with enhanced pro-angiogenic and pro-bone regeneration activities. Sci Rep. 2017;7:45622.
Zhang S, Chu WC, Lai RC, Lim SK, Hui JHP, Toh WS. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27.
Eskildsen T, Taipaleenmäki H, Stenvang J, Abdallah BM, Ditzel N, Nossent AY, et al. MicroRNA-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo. Proc Natl Acad Sci USA. 2011;108(15):6139–44.
Kim JH, Kim DK, Lee OJ, Ju HW, Lee JM, Moon BM, Park HJ, Kim DW, Lee JH, Park CH. Osteoinductive silk fibroin/titanium dioxide/hydroxyapatite hybrid scaffold for bone tissue engineering. Int J Biol Macromol. 2016;82:160–7.
Liu A, Lin D, Zhao H, Chen L, Cai B, Lin K, Shen SG. Optimized BMSC-derived osteoinductive exosomes immobilized in hierarchical scaffold via lyophilization for bone repair through Bmpr2/Acvr2b competitive receptor-activated Smad pathway. Biomaterials. 2021;272: 120718.
Qi X, Zhang J, Yuan H, Xu Z, Li Q, Niu X, Hu B, Wang Y, Li X. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int J Biol Sci. 2016;12(7):836–49.
Guo SC, Tao SC, Yin WJ, Qi X, Yuan T, Zhang CQ. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics. 2017Jan 1;7(1):81–96.
Zhang S, Chu WC, Lai RC, Lim SK, Hui JHP, Toh WS. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016Dec;24(12):2135–40.
Zhu Y, Jia Y, Wang Y, Xu J, Chai Y. Impaired bone regenerative effect of exosomes derived from bone marrow mesenchymal stem cells in type 1 diabetes. Stem Cells Transl Med. 2019;8(6):593–605.
Oryan A, Sahvieh S. Effectiveness of chitosan scaffold in skin, bone and cartilage healing. Int J Biol Macromol. 2017;104(Pt A):1003–11.
Mao AS, Mooney DJ. Regenerative medicine: current therapies and future directions. PNAS. 2015;112(47):14452–9.
Mistry H, Connock M, Pink J, Shyangdan D, Clar C, Royle P, et al. Autologous chondrocyte implantation in the knee: systematic review and economic evaluation. Health Technol Assess (Rockv). 2017;21:6.
Bijukumar DR, McGeehan C, Mathew MT. Regenerative medicine strategies in biomedical implants. Curr Osteoporos Rep. 2018;16(3):236–45.
Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35(2): e00191.
Phan J, Kumar P, Hao D, Gao K, Farmer D, Wang A. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J Extracell Vesicles. 2018;7(1):1522236.
Toh WS, Lai RC, Hui JHP, Lim SK. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin Cell Dev Biol. 2017;67:56–64.
Liu SL, Sun P, Li Y, Liu SS, Lu Y. Exosomes as critical mediators of cell-to-cell communication in cancer pathogenesis and their potential clinical application. Transl Cancer Res. 2019;8(1):298–311.
Jia Y, Chen Y, Wang Q, Jayasinghe U, Luo X, Wei Q, Wang J, Xiong H, Chen C, Xu B, Hu W, Wang L, Zhao W, Zhou J, et al. Exosome: emerging biomarker in breast cancer. Oncotarget. 2017;8(25):41717–33.
Liu X, Yanga Y, Li Y, Niu X, Zhao B, Wang Y, Bao C, Xie Z, Lin Q, Zhu L. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale. 2017;9(13):4430–8.
Yamashita T, Takahashi Y, Takakura Y. Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biomed Res Int. 2018;41(6):835–42.
Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214–22.
Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed Res Int. 2018;2018:8545347.
Jeppesen DK, Hvam ML, Primdahl-Bengtson B, et al. Comparative analysis of discrete exosome fractions obtained by differential centrifugation. J Extracell Vesicles. 2014;6(3):25011.
He M, Crow J, Roth M, Zeng Y, Godwin AK. Integrated immunoisolation and protein analysis of circulating exosomes using microfluidic technology. Lab Chip. 2014;14(19):3773–80.
Watson DC, Yung BC, Bergamaschi C, et al. Scalable, cGMP-compatible purification of extracellular vesicles carrying bioactive human heterodimeric IL-15/lactadherin complexes. J Extracell Vesicles. 2018;7(1):1442088.
Shin H, Han C, Labuz JM, et al. High-yield isolation of extracellular vesicles using aqueous two-phase system. Sci Rep. 2015;14(5):13103.
Wu M, Ouyang Y, Wang Z, et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc Natl Acad Sci U S A. 2017;114(40):10584–10589. https://doi.org/10.1073/pnas.1709210114. Epub 2017 Sep 18. Erratum in: Proc Natl Acad Sci U S A. 2020 Nov 10;117(45):28525.
Baranyai T, Herczeg K, Onódi Z, et al. Isolation of exosomes from blood plasma: qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS ONE. 2015;10(12): e0145686.
Welton JL, Webber JP, Botos LA, Jones M, Clayton A. Ready-made chromatography columns for extracellular vesicle isolation from plasma. J Extracell Vesicles. 2015;26(4):27269.
Lötvall J, Hill AF, Hochberg F, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;22(3):26913.
Ohno S, Takanashi M, Sudo K, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21(1):185–91.
Katakowski M, Buller B, Wang X, Rogers T, Chopp M. Functional microRNA is transferred between glioma cells. Cancer Res. 2010;70(21):8259–63.
Qiao L, Hu S, Liu S, Zhang H, Ma H, Huang K, Li Z, Su T, Vandergriff A, Tang J, Allen T, Dinh PU, Cores J, Yin Q, Li Y, Cheng K. microRNA-21-5p dysregulation in exosomes derived from heart failure patients impairs regenerative potential. J Clin Invest. 2019;129(6):2237–50. https://doi.org/10.1172/JCI123135.PMID:31033484;PMCID:PMC6546482.
Piffoux M, Silva AKA, Wilhelm C, Gazeau F, Tareste D. Modification of extracellular vesicles by fusion with liposomes for the design of personalized biogenic drug delivery systems. ACS Nano. 2018;12(7):6830–42.
Lamichhane TN, Raiker RS, Jay SM. Exogenous DNA loading into extracellular vesicles via electroporation is size-dependent and enables limited gene delivery. Mol Pharm. 2015;12(10):3650–7.
Xiao J, Pan Y, Li XH, Yang XY, Feng YL, Tan HH, Jiang L, Feng J, Yu XY. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis. 2016;7(6): e2277.
Wahlgren J, De L Karlson T, Brisslert M, Vaziri Sani F, Telemo E, Sunnerhagen P, Valadi H. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40(17):e130.
Pascucci L, Coccè V, Bonomi A, et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release. 2014;28(192):262–70.
Anderson JD, Johansson HJ, Graham CS, et al. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-kappab signaling. Stem Cells. 2016;34(3):601–13.
Maas SLN, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27(3):172–88.
Saury C, Lardenois A, Schleder C, Leroux I, Lieubeau B, David L, Charrier M, Guével L, Viau S, Delorme B, Rouger K. Human serum and platelet lysate are appropriate xeno-free alternatives for clinical-grade production of human MuStem cell batches. Stem Cell Res Ther. 2018;9(1):128.
Galipeau J, Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824–33.
Watson DC, Yung BC, Bergamaschi C, Chowdhury B, Bear J, Stellas D, Morales-Kastresana A, Jones JC, Felber BK, Chen X, Pavlakis GN. Scalable, cGMP-compatible purification of extracellular vesicles carrying bioactive human heterodimeric IL-15/lactadherin complexes. J Extracell Vesicles. 2018;7(1):1442088.
Funding
This work was supported by “Excellence Initiative—Research University” Emerging Fields: “Applying New Technologies and Artificial Intelligence in Oncology”.
Author information
Authors and Affiliations
Contributions
S.R. wrote the main manuscript text and prepared figure and table.
Corresponding author
Ethics declarations
Conflict of interest
The author has no relevant financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Roszkowski, S. Therapeutic potential of mesenchymal stem cell-derived exosomes for regenerative medicine applications. Clin Exp Med 24, 46 (2024). https://doi.org/10.1007/s10238-023-01282-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10238-023-01282-z