Abstract
The filamentous bacteriophage M13KO7 (M13) is the most used in phage display (PD) technology and, like other phages, has been applied in several areas of medicine, agriculture, and in the food industry. One of the advantages is that they can modulate the immune response in the presence of pathogenic microorganisms, such as bacteria and viruses. This study evaluated the use of phage M13 in the chicken embryos model. We inoculated 13-day-old chicken embryos with Salmonella Pullorum (SP) and then evaluated survival for the presence of phage M13 or E. coli ER2738 (ECR) infected with M13. We found that the ECR bacterium inhibits SP multiplication in 0.32 (M13-infected ECR) or 0.44 log UFC/mL (M13-uninfected ECR) and that the ECR-free phage M13 from the PD library can be used in chicken embryo models. This work provides the use of the chicken embryo as a model to study systemic infection and can be employed as an analysis tool for various peptides that M13 can express from PD selection.
Key points
• SP-infected chicken embryo can be a helpful model of systemic infection for different tests.
• Phage M13 does not lead to embryonic mortality or cause serious injury to embryos.
• Phage M13 from the PD library can be used in chicken embryo model tests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phage display (PD) technology consists of in vitro selection based on the presentation of peptides or proteins exposed on the surface of bacteriophages in the form of fusion proteins (Rahbarnia et al. 2017; Jiang et al. 2022). Bacteriophages are a type of virus that can carry out an infectious process in bacteria, fungi, actinomycetes, or spirochetes (Ge et al. 2020). PD applications are increasing and efficiently employed as phage therapy in veterinary medicine, agriculture, and food safety (Jamal et al. 2019).
Among the different types of bacteriophages used in PD, the most used is the filamentous phage M13KO7 (M13), which receives this name due to its filamentous appearance and dependence on pilus F in the infection process (Ebrahimizadeh and Rajabibazl 2014). Some of its applications are already well described in the literature, such as its use to evaluate antiviral activity (Nakakido et al. 2022) and stimulate the immune system by activating antigen-presenting cells (Dong et al. 2020). Furthermore, it proved that phages and peptides expressed and selected by the PD modulate the immune response against bacterial and viral infections (díaz-Valdés et al. 2011; Van Belleghem et al. 2019).
Given the importance of better understanding infectious processes and the search for the feasibility of experimental models, the chicken embryo (CE) is considered an accessible, inexpensive, and low-maintenance in vivo model. Moreover, it is easy to manipulate and allows a non-invasive follow-up during its development (Rashidi and Sottile 2009). Given all these advantages, this model has recently been used in several areas, such as evaluation of drug toxicity and distribution (Zosen et al. 2021; Ghimire et al. 2022), epigenetics (Bednarczyk et al. 2021), teratology (Wachholz et al. 2021), analysis of snake venom effects (Polláková et al. 2021), and bacterial infections (Li et al. 2019; Kosecka-Strojek et al. 2021).
The CE is a good model for tests with infection since it is possible to determine the pathogenicity of different bacteria (Gibbs et al. 2003; Oh et al. 2012; Blanco et al. 2018; Rezaee et al. 2021). Given the importance of using the PD to select ligands in several processes and the use of chicken embryos as a good study model to understand such mechanisms, this work aims to propose an experimental model for the utilization of phage M13 from the PD library in tests in an experimental model of chicken embryos. It would be helpful to have the chick embryo as an experimental model of pathogen-binding phages or other molecules for disease control.
Materials and methods
This research was performed in the following laboratories of the Federal University of Uberlândia: Poultry Egg Incubation, Nanobiotechnology Luiz Ricardo Goulart Filho, Biochemistry, Laboratory of Infectious Diseases, and Animal Pathology. Project certified by the Ethics and Research with Animals Committee of the Federal University of Uberlândia (Nº 45/2022/CEUA/PROPP/REITO, process Nº23117.043271/2022–61).
Evaluation of the ability of E. coli ER2738 and phage M13 to inhibit S. Pullorum in vitro
We developed a test to understand the S. Pulloruminfection in chicken embryos and then used this bacterium in the control group.
Phage amplification and purification
In this work, we tested the Escherichia coli ER2738 (ECR), the bacteria that amplified phages from the phage display library, and phage M13, the wild-type phage without the peptide insert (also used in the phage display technology as a control). Amplification of wild phage M13 (New England Biolabs) was started by preparing a pre-inoculum containing one colony of ECR (New England Biolabs) at 37 °C in 50 mL of Luria Bertani (LB—Tryptone 10 g/L, yeast extract 5 g/L, NaCl 10 g/L) (Kasvi) culture medium with tetracycline (Sigma Chemical Co., 20 mg/mL) under stirring until reaching OD600 ~ 0.3. After, 10 µL of phage M13 was added and incubated at 37 °C overnight under shaking. Centrifuged the culture at 15,000 × g for 10 min and transferred the supernatant to a tube containing PEG/NaCl (20% polyethylene glycol 8000, Fluka, and 2.5 M NaCl Neon-sterile solution) and incubated at 4 °C overnight. Centrifuged the precipitate for 15 min at 15,000 × g, discarded the supernatant, and resuspended the pellet in PBS. Subsequently, it was centrifuged again for 10 min at 15,000 × g, then we transferred the supernatant to another tube containing PEG/NaCl, incubated for 1 h on ice and centrifuged 10 min at 15,000 × g. At last, resuspended the phage pellet with sterile PBS. After phage amplification, it was filtered on PES membrane with a pore size of 0.22 µm (K18-230, Kasvi) for further use during this work.
For bacterial inoculum, phage M13-infected and uninfected with ECR streaked on a plate containing LB enriched with IPTG (isopropyl β-d-1-thiogalactopyranoside-Ludwig Biotec) (0.5 mM) + X-gal (5-bromo4-chloro-3-indolyl β-d-galactopyranoside-Ludwig Biotec) (40 µg/mL) and tetracycline (Sigma Chemical Co., 20 mg/mL). After incubating for 24 h at 37 °C, 3 white colonies (not infected with the phage) were diluted in 10 mL of PBS and evaluated on the McFarland scale. In addition, inoculated 3 blue colonies (infected with the phage) into PBS. Both samples went through serial dilutions until it reaches inoculum amount. The exact amount was evaluated and confirmed by titrating the dilutions.
Ability of ECR and M13 to inhibit S. Pullorum
To propose an infection model, we used a SP isolated from free-range chickens by the Laboratory of Infectious Diseases at the Federal University of Uberlândia registered in the National System for the Management of Genetic Heritage and Associated Traditional Knowledge (SPU_SISGEN_28DD3D). The SP was cultured in nutrient agar (Kasvi) at 37 °C for 24 h. Before testing the embryos, we performed an in vitro test to evaluate the interaction between ECR and/or M13 incubated with SP.
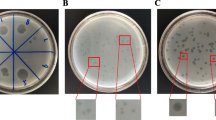
To evaluate whether phage M13 can invade SP or influence its multiplication, incubated 500 µL of SP containing ~ 4.34 log CFU/mL with 500 µL of ECR or ECR infected with M13 (~ 4 log CFU/mL) at room temperature for ~ 20 min. Parallel, inoculated 1 mL of PBS containing 4.34 log CFU/mL of SP with 50 µL 10 log UFP/µL of phage M13 for ~ 20 min at room temperature. After this period, performed serial dilution and plated the samples on LB agar containing 0.5 mM IPTG, 40 µg/mL X-gal, and whether or not containing 20 mg/mL tetracycline. The medium with tetracycline inhibits the growth of SP but does not inhibit that of ECR. Performed SP colony count by the difference between the tetracycline-enriched and non-enriched plates.
Evaluation of the inhibition ability of ECR and M13 on S. Pullorum in a chicken embryo model
Chicken embryos
The eggs line Hy-Line W36 were donated by New World Hatchery (Uberlândia, Brazil). Incubated the eggs in an artificial incubator (Premium Ecological®) at 37 °C, 58% humidity, and turned at a 2-h interval until 13 days of incubation (DI) when the tests started.
Evaluation of the dose and age of SP inoculation in embryos
Since we know that SP leads to high mortality in embryos (Berhanu and Fulasa 2020), we did a pilot test to verify the best age and inoculation dose for them to suffer injury and for mortality to be equal to or lower than 60%. It is essential to evaluate the best age to work with the model. We used embryos at 13 and 14-day-old of incubation and before the experiment, 10 embryos were tested to the presence of SP by microbiology. The embryos were negative to SP and so, we inoculated with 6.13, 4.13, and 2.13 CFU/embryo via allantois (5 chicken embryo/group). The choice of age is because the embryo at this age already has an active immune system (Seto 1981) which facilitates understanding of the response to a challenge. The embryos were monitored daily for viability by ovoscopy. Four days after inoculation, euthanized embryos via cervical dislocation and evaluated macroscopic lesions.
Evaluation of the effects of phage M13 and ECR on the embryo
To verify whether phage M13, phage M13-infected, and phage M13-uninfected ECR can be used on embryos without causing mortality, we performed a test on embryos. For this purpose, we inoculated the 13-day-old embryos via allantois with ~ 2.9 log CFU/embryo of M13-infected or uninfected ECR and 5 and 11 log CFU/embryo of purified phage M13. In parallel, a group of embryos received ~ 2.13 log CFU/embryo of SP, in addition to a negative control group. In each group, there were 5 embryos. The embryos were monitored daily by assessing viability by ovoscopy. After 4 days, euthanized the embryos via cervical dislocation and evaluated macroscopic lesions.
Evaluation of the inhibitory capacity of phage M13 free or infecting ECR on SP infecting chicken embryos
To assess whether phage M13-free or infecting ECR interferes with mortality or injury caused by SP, embryos were infected with ~ 2.13 log CFU/embryo of SP via allantoic fluid at 13 days of incubation. After 1 h, we treated the embryos with ~ 2 log CFU/embryo of ECR, or ~ 2 log CFU/embryo of M13-infected ECR, or 11 log CFU/embryo of the M13 phage. We inserted SP-inoculated and negative control groups. Embryos were evaluated daily for viability by ovoscopy. At 17 days of incubation, we weighed the 21 surviving embryos, collected blood through the allantoic vessel, and performed macro- and microscopic analyses.
Weight of the chicken embryos
Before the inoculation with SP, we numbered the eggs and recorded the weights. Then, at 17 DI, the CE were weighed immediately after collecting blood. As the embryo weight is related to the initial egg weight, we set the initial egg weight to 50 g, according to Ribeiro et al. (2020).
Mortality and macroscopic evaluations
After the determined evaluation time, we checked and counted the embryos that died and determined the date of death according to the degree of development of the embryo. For the animals that were alive, we noted whether the annexes had the presence of circulatory changes, malformation, and/or color changes. We also performed an external evaluation on the embryos and evaluated the internal organs for circulatory changes, malformation, and color changes. We compared the treated groups with their respective control group.
Histopathological changes
We performed a histopathological analysis of the liver and heart of all live embryos from the positive and negative groups in addition to 5 embryos from the SP-challenged and M13-infected ECR-treated group. The fragments of the liver and heart were fixed in 10% buffered formalin and processed for the preparation of histological slides stained with hematoxylin and eosin (HE) (Behmer and Tolosa 2003).
All slides from liver samples were analyzed by two experienced pathologists without knowledge of the treatment group. After lesions were identified and scored for severity, the slides for the control group were identified and re-evaluated for normality. The control samples were used as a guide for the normal histological appearance and the natural rate of lesion occurrence. All slides were re-examined, in comparison with a normal slide, to ensure accurate recognition and grading of lesions.
All liver slides were examined microscopically for histological evidence of degeneration, inflammation, and circulatory lesions (Molina et al. 2006). Severity scores were based on a scale of 0 to 3, which corresponded to normal, mild, moderate, and severe, respectively.
Hepatic lipidosis was scored as follows: 0, no detectable cytoplasmic vacuolation; 1, scattered individual vacuoles or low numbers of vacuoles within the cytoplasm of some hepatocytes; 2, clusters of vacuoles within the cytoplasm of many hepatocytes; 3, clearing of the cytoplasm because of advanced vacuolation in nearly all hepatocytes. The control samples were used as a guide for the normal histological appearance and the natural rate of lesion occurrence.
ELISA
The levels of Interferon Gamma (IFN-γ), Interleukin-1 beta (IL-1β), and Interleukin 10 (IL-10) in the serum of chicken embryos were measured by enzyme-linked immunosorbent assay (ELISA) technique. High binding plates (Greiner Bio-One) were sensitized with embryo serum diluted (1:1) in 50 mM bicarbonate buffer (pH 8.6) for 1 h at 37 °C. After 3 washes with PBS-T (PBS + Tween 20 at 0.05%), the plates were blocked with 3% BSA in PBS for 1 h at 37 °C. Then, they were rewashed with PBS-T for 4 times. Then, we incubate the plates with the antibodies, rabbit anti-chicken IFN-γ IgG antibody (BioRad), rabbit anti-chicken IL-1β IgG antibody (BioRad) or IL-10 Polyclonal IgG antibody (Thermo), diluted (1:500) in 3% BSA + PBS for 1 h at 37 °C. After 4 washes with PBS-T, all plates were incubated with secondary goat anti-rabbit IgG HRP (Sigma) diluted (1:5000) in 3% BSA + PBS. Following this, washed 4 times with PBS-T, and the binding of the antibody/antigen was detected by adding 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (Thermo Scientific). The reaction was stopped by the addition of 2 Normal (N) H2SO4. Reactivity was determined in a plate reader (Titertek Multiskan Plus, Flow Laboratories, USA) at a wavelength of 450 nm. During the reaction, we used different concentrations of recombinants IFN-γ, IL-1β, and IL-10 proteins (BD Biosciences, San Diego, CA) to construct the standard curve.
Statistical analysis
We performed the Shapiro–Wilk test to evaluate if the data were parametric. The in vitro data were parametric, but the weight data were not. Therefore, we transformed the weight data using a square root transformation to follow a normal distribution and carried out the analyses. Then, we used ANOVA followed by the Tukey test. In mortality analysis, we perform the chi-square test, followed by the binomial between two proportions comparing all groups inoculated with SP. A relative standard curve was constructed from the absorbance values according to the control (recombinant protein IFN-γ, IL-1β, and IL-10). We interpolate the data using Pade (1,1) or hyperbolic approximant. After, the ANOVA test was followed by the Tukey test (p < 0.05) (GraphPad Prism 9.1).
Results
ECR can inhibit SP multiplication in vitro
The presence of the ECR bacterium, both alone and infected with phage M13, significantly decreased the amount of SP. In contrast, we did not observe the same result when only phage M13 was present (Table 1).
The dose of 2 log CFU/embryo of SP leads to a 60% mortality in 13- and 14-day-old embryos
In the animal born, SP causes inflammation and a vertically transmitted disease that leads to lesions and mortality in old embryos. According to the pilot test performed, the embryos inoculated with SP at the lowest dose tested, 2 log CFU/embryo, showed the lowest mortality rate (Fig. 1). And after 4 days of inoculation, the embryos showed macroscopic lesions compared to the negative control, which suggests inflammation, among them thickening and increased redness of blood vessels, and an excess of excreta. Thus, we standardized on using 13-day-old embryos and 2 log CFU for the assays in this study.
Free phage M13 or infecting ECR does not lead to mortality or serious lesions in embryos
From Table 2, phage M13 infecting ECR or free did not cause any death in the embryos. The only lesion found in the embryos treated with free phage or infecting ECR was excess uric acid in the embryos.
ECR can decrease the mortality of embryos challenged with SP
According to Table 3, it was possible to observe that free phage M13 does not reduce the mortality of embryos inoculated with SP. In contrast, when the ECR becomes infected with phage M13, there is a significant reduction in the mortality rate in the face of infection caused by SP, which was present in all groups except the negative controls.
ECR prevents weight loss of SP-treated embryos
According to the graph shown in Fig. 2, it is possible to observe that the weight of embryos tends to decrease due to infection caused by SP. However, in the presence of ECR infected with phage M13, the embryos did not lose weight, and the values were close to those of the negative controls.
Weight of surviving embryos challenged with SP and treated or not with ECR infected with M13. NcPBS, negative control group with PBS; NcM13, negative control group with phage M13; SP, group inoculated with SP; SP (ECR + M13), infected by SP and treated with ECR infected with phage M13; M13, phage M13 only. Asterisk (*) shows a statistical difference (p < 0.05). The weights referring to group M13 and group ECR were not inserted in the graph because the embryos died, and the number of surviving embryos was not sufficient for statistical analysis
Surviving embryos inoculated with SP and treated with ECR + M13 have no severe lesions
After 4 days of inoculation, we perform embryo diagnosis on the live embryos. In the group inoculated with SP, the 3 survivors showed excess excreta and thickening and greater redness of blood vessels, and 1 enlarged liver. In the group inoculated with SP and treated with the ECR bacteria infected with M13, only one embryo of the nine survivors had excess excreta. In the negative control group (embryo inoculated with M13), 2 of 4 embryos had excess excreta, while the negative control (inoculated with PBS) had no lesion.
Histopathological changes
We found no histomorphometry changes in the negative control. Granulopoietic cells were present in all livers in connective tissues of hepatic portal spaces. Nevertheless, not among hepatoblasts and not all connective tissue areas in portal spaces were occupied by granulopoiesis foci. We observed mild lipidosis in all livers, even in the controls. In positive control, we found 1 of the 3 live embryos with hemorrhage and congestion in the liver. In group infected by SP and inoculated with M13-infected ECR, 1 of the 4 live embryos presented hemorrhage and congestion in the liver and heart, respectively. We performed only qualitative analysis.
Embryos challenged with SP increase IL-10 secretion 4 days after inoculation, but when treated with ECR + M13, there is no increase in IL-10
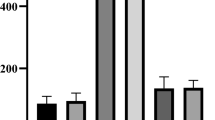
There was no difference between IFN-γ and IL-1β levels and CP or CN (Fig. 3A and B). In contrast, the cytokine IL-10 had a significant decrease and a similar profile to the negative controls (Fig. 3C).
Dosage of inflammatory and anti-inflammatory cytokines in the serum of embryos. Levels (pg/mL) of IFN-γ (A), IL-1β (B), and IL-10 (C) in the serum of embryos. NcPBS, negative control group with PBS; NcM13, negative control group with phage M13; SP, group inoculated with SP; SP (ECR + M13), infected by SP and treated with ECR infected with phage M13; M13, phage M13 only. Asterisk (*) shows a statistical difference (p < 0.05). The cytokine levels referring to group M13 and group ECR were not inserted in the graph because the embryos died, and the number of surviving embryos was not sufficient for statistical analysis
Discussion
In infection tests with SP, it is not interesting to use ECR infected with phage M13
After selecting phage ligands or mimetics by the PD library, the phages can be used as a screening test to choose the best targets by ELISA (da Silva et al. 2010). It can be very useful since peptide synthesis is still costly and time-consuming. There are still no studies of phages from the PD library that use screening tests for infection. It will have many applications because, besides the time and cost issues, the phage being a larger and more stable particle, could hit the target more successfully. To enable the use of phages, we have done an in vitro test inoculating the SP with the free phage or infecting ECR. We noticed a decrease in the amount of SP when inoculated with ECR (Table 1). This fact allows us to propose that in future works, if phages are used, they should be free of ECR so that the bacteria do not interfere with the tests. Although we only tested SP, it is possible that for other gram-negative bacteria, this event could also occur. The interaction between bacteria of different species happens through various mechanisms, such as competition for substrates and production of bacteriocins (Hawlena et al. 2012; Deng and Wang 2016). We cannot conclude what type of mechanism was used by ECR in the inhibition of SP, but other works show that probiotic E. coli strains can inhibit pathogenic bacteria (Setia et al. 2009; Fang et al. 2018; Hrala et al. 2021).
We also observed the in vitro results in tests on an embryonic model. When we challenged embryos with SP and then treated them with phage-infected ECR, it was possible to see a decrease in embryonic mortality. Embryos inoculated with SP showed a 75% mortality, while those inoculated with SP but treated with ECR had a 25% mortality (Table 3). This reduction was due to the presence of the bacteria since embryos challenged with SP and inoculated only with M13 showed no decrease in mortality. Lesions in embryos challenged with SP and treated with ECR infected with M13 were also mild. One embryo of the nine survivors showed increased excreta. However, 1 and 4 embryos in this group presented congestion and bleeding in liver and heart, respectively (Table 4). The weight of surviving embryos and the level of cytokine IL-10 of the SP-challenged group treated with ECR infected with M13 was similar to the negative control showing that the ECR was probably able to control the multiplication of SP in these embryos.
Phage M13 does not lead to embryonic mortality or cause serious injury to embryos
Before we started testing, it was important to know if phage M13 caused damage or death in the embryos. Embryos inoculated with M13 at two doses (5 and 11PFU/embryo), ECR and ECR infected with M13 showed no mortality, and the only macroscopic change observed was excess uric acid (Table 2). Another experiment showed that phage M13 and ECR are harmless to hatchlings (de Almeida Araújo Santos et al. 2022). Our investigation found that tests with the phages can also be performed in chicken embryos as a potential infection model for evaluating PD-selected ligands.
SP can be a model of infection in chicken embryos
SP is an avian-specific and vertically transmitted bacterium that causes severe injury to embryos, such as skin hemorrhage, subcutaneous edema, and increased mortality (Guo et al. 2017, 2019). Our intention was to find an embryonic period age and infective dose capable of not leading to the death of all embryos. We chose the ages of 13 and 14 days because before 11 days of incubation, there is death of 100% of embryos (data not shown) and because from that age on the embryos already have a more active immunity (Stefaniak et al. 2020). Our results show that embryos inoculated with the 6 and 4 log CFU doses of SP showed a higher mortality rate when compared to the groups inoculated with the dose of 2 log CFU/embryo (Fig. 1). At this dose, the mortality rate was similar, and thus, we decided to use the age of 13 days in the next phase to remove the biological material at 17 days of incubation. The intention was not to pass the age of 18 days of incubation because during this embryonic period, the embryo is already fully developed, becoming similar to an animal in experimental terms (Fonseca et al. 2021).
The decrease in weight of the SP-infected embryos (Fig. 2) and the lesions in the surviving embryos, such as hemorrhage and membrane sticking (Table 2), excess excreta, and hepatomegaly (described in Sect. 3.2.5), shows that these animals, although injured by the infection were able to survive trying to circumvent the inflammation caused by the bacteria. This result, together with the increase in the cytokine IL-10 in the surviving embryos (Fig. 3C), may be an attempt by the embryo’s immune system to modulate the inflammation caused by SP or the initiation of the Th2 type response similar to what occurs with the nascent animal (Tang et al. 2018; Foster et al. 2021). The inflammatory cytokines IFN-γ and IL-1β showed no increase (Fig. 3A and B). It probably happened because these cytokines are released at the onset of inflammation, characterizing the resistance phase of the disease. As the blood collection was 4 days after inoculation, already changed to the induction phase of SP modulation, other cytokines are participating in the process, such as IL-10 (Kogut and Arsenault 2017). This fact reinforces the idea that this cytokine can inhibit the production of inflammatory cytokines (Th1 type) during systemic dissemination to limit the inflammatory response (Rothwell et al. 2004; Tang et al. 2018).
From the histopathological analysis, we observed granulopoietic cells in all livers in connective tissues of hepatic portal spaces. Nevertheless, not among hepatoblasts and not all connective tissue areas in portal spaces were occupied by granulopoiesis foci. Despite the chicken fetal liver is not considered a relevant hematopoietic organ, as is the fetal liver in mammals (Wong and Cavey 1992, 1993), the presence of these granulopoietic foci was considered normal. Granulocytic differentiation in the connective tissue of portal spaces on the 15th day of incubation and onwards was reported by Guedes et al. (2014).
Even without showing inflammatory changes in the heart and liver, the chicks challenge with SP were smaller and depressed, with an increase in the vessels showing that it had a systemic inflammation that did not reach the tissues. In born animals, the histopathological lesions generated by SP are evident (Cheng et al. 2020), and the survival of the embryos in this study combined with the absence of liver and kidney damage (Table 4) together with the increase in serious IL-10 (Fig. 3) shows that embryos surviving the challenge with SP have a better response immune than those who died.
We observed mild lipidosis in all livers, even in the controls. Wong and Cavey (1992) reported that by 14th day of incubation, all hepatoblasts possess lipid and glycogen. The amount of fat in the hepatoblasts was considered at a normal level. The absence of any accompanying cytopathic effects in the liver allows the determination of their individual characteristics, not resulting from drug administration.
We also observed hepatic congestion and hemorrhage in some chicken embryos in the group treated with SP. This event is common in born animal (Shen et al. 2022). Our results indicate that SP is an interesting model of systemic infection in CE, and some embryo can be resistant to the disease progression.
Phage M13 from the PD library can be used in chicken embryo model tests
The PD technology presents numerous advantages in the selection of ligands and structure mimetics of microorganisms, thus allowing both diagnosis and development of molecules for disease control (Sioud 2019). However, depending on the microorganism, the number of clones selected in the PD technology is high, and screening to choose the best ligands is essential. Although phage-ELISA can determine good ligands for diagnostic purposes (da Silva et al. 2010), this technique may not be interesting for understanding the ligand and host relationship, such as the infection and inflammation process. In this sense, cell culture is a useful tool, but considering the chicken embryo a more complex organism that allows the replication of numerous microorganisms such as viruses and bacteria (Farzaneh et al. 2017), this model has several advantages.
The advantages of chicken embryos over hatchlings are mainly related to cost, space, and some ease of handling (Garcia et al. 2021) and are currently accepted by the FDA in testing with some drugs (Kue et al. 2014). Based on the importance of the PD and the embryo as an experimental model, we propose a model of infection and suggest the embryo’s use in testing with the PD. Research with any system, whether organic or inorganic molecules, needs to be well standardized and to present guarantees of harmlessness so that the changes are well known. In this sense, this work clarifies that there are interferences of the ECR on the SP bacteria and that this may occur for other bacteria. Thus, our results show in vitro and in vivo models that in tests with infection, it is important that the M13 amplified in the ECR is purified. Another aspect that warrants the use of phage M13 from the purified PD library in tests with embryos is that M13 does not interfere with bacterial multiplication or the response generated by the embryo. This is seen when embryos inoculated with M13 alone did not lead to embryo mortality (Table 2) and when the mortality rate of the SP-inoculated and M13-treated embryo groups was equal to the SP-only inoculated group (Table 3). Unfortunately, the number of surviving embryos of the group challenged with SP and treated with M13 or ECR, although statistically similar to the group only challenged with SP, did not allow the analysis of the weight and level of cytokines produced as only one or two embryos survived, respectively. One can consider that the number of embryos for the ECR group was low, and a larger quantity is needed for better evaluation. However, the set of results allowed inferring that it is possible to use clones selected by the PD technology in embryo testing since M13 is innocuous and does not interfere with multiplication or bacterial action.
Chicken embryo may be a potential alternative for studying and selecting ligand-binding peptides from M13 phages selected from the PD library. The SP-infected chicken embryo can be a helpful model of systemic infection for different tests.
Data availability
All data will be available to the reviewer or editor upon request.
References
Bednarczyk M, Dunislawska A, Stadnicka K, Grochowska E (2021) Chicken embryo as a model in epigenetic research. Poult Sci 100:101164. https://doi.org/10.1016/j.psj.2021.101164
Behmer OA, Tolosa EMC (2003) Manual de técnicas para histologia normal e patológica. Man técnicas para Histol Norm e patológica 331–331
Berhanu G, Fulasa A (2020) Pullorum disease and fowl typhoid in poultry: a review. Br J Poult Sci 9:48–56. https://doi.org/10.5829/idosi.bjps.2020.48.56
Blanco AE, Cavero D, Icken W, Voss M, Schmutz M, Preisinger R, Sharifi AR (2018) Genetic approach to select against embryo mortality caused by Enterococcus faecalis infection in laying hens. Poult Sci 97:4177–4186. https://doi.org/10.3382/ps/pey310
Cheng Y, Sihua Z, Lu Q, Zhang W, Wen G, Luo Q, Shao H, Zhang T (2020) Evaluation of young chickens challenged with aerosolized Salmonella Pullorum. Avian Pathol 49:507–514. https://doi.org/10.1080/03079457.2020.1783433
da Silva RV, Manhani MN, Cardoso R, Vieira CU, Goulart LR, Costa-Cruz JM (2010) Selection of high affinity peptide ligands for detection of circulating antibodies in neurocysticercosis. Immunol Lett 129:94–99. https://doi.org/10.1016/j.imlet.2010.01.008
de Almeida Araújo Santos F, Valadares Junior EC, Goulart LR, Nunes PLF, Mendonça EP, Girão LVC, da Hora AS, Ferreira TB, Bastos LM, Medeiros-Ronchi AA, Fonseca BB (2022) Alternative use of phage display: phage M13 can remain viable in the intestines of poultry without causing damage. AMB Express 12. https://doi.org/10.1186/s13568-022-01407-9
de Ribeiro LNM, de Paula E, Rossi DA, Monteiro GP, Júnior ECV, Silva RR, Franco R, Espíndola FS, Goulart LR, Fonseca BB (2020) Hybrid pectin-liposome formulation against multi-resistant bacterial strains. Pharmaceutics 12:1–15. https://doi.org/10.3390/pharmaceutics12080769
Deng YJ, Wang SY (2016) Synergistic growth in bacteria depends on substrate complexity. J Microbiol 54:23–30. https://doi.org/10.1007/s12275-016-5461-9
díaz-Valdés N, Manterola L, Belsúe V, Riezu-Boj JI, Larrea E, Echeverria I, Llópiz D, López-Sagaseta J, Lerat H, Pawlotsky JM, Prieto J, Lasarte JJ, Borrás-Cuesta F, Sarobe P (2011) Improved dendritic cell-based immunization against hepatitis C virus using peptide inhibitors of interleukin 10. Hepatology 53:23–31. https://doi.org/10.1002/hep.23980
Dong X, Pan P, Zheng DW, Bao P, Zeng X, Zhang XZ (2020) Bioinorganic hybrid bacteriophage for modulation of intestinal microbiota to rem u el tumor-immune microenvironment against colorectal cancer. Sci Adv 6. https://doi.org/10.1126/sciadv.aay1497
Ebrahimizadeh W, Rajabibazl M (2014) Bacteriophage vehicles for phage display: biology, mechanism, and application. Curr Microbiol 69:109–120. https://doi.org/10.1007/s00284-014-0557-0
Fang K, Jin X, Hong SH (2018) Probiotic Escherichia coli inhibits biofilm formation of pathogenic E. coli via extracellular activity of DegP. Sci Rep 8:1–12. https://doi.org/10.1038/s41598-018-23180-1
Farzaneh M, Hassani SN, Mozdziak P, Baharvand H (2017) Avian embryos and related cell lines: a convenient platform for recombinant proteins and vaccine production. Biotechnol J 12:1–10. https://doi.org/10.1002/biot.201600598
Fonseca BB, da Silva MV, de Morais Ribeiro LN (2021) The chicken embryo as an in vivo experimental model for drug testing: advantages and limitations. Lab Anim (NY) 50:138–139. https://doi.org/10.1038/s41684-021-00774-3
Foster N, Tang Y, Berchieri A, Geng S, Jiao X, Barrow P (2021) Revisiting persistent Salmonella infection and the carrier state: what do we know? Pathogens 10. https://doi.org/10.3390/pathogens10101299
Garcia P, Wang Y, Viallet J, Macek Jilkova Z (2021) The chicken embryo model: a novel and relevant model for immune-based studies. Front Immunol 12:1–16. https://doi.org/10.3389/fimmu.2021.791081
Ge H, Hu M, Zhao G, Du Y, Xu N, Chen X, Jiao X (2020) The “fighting wisdom and bravery” of tailed phage and host in the process of adsorption. Microbiol Res 230. https://doi.org/10.1016/j.micres.2019.126344
Ghimire S, Zhang X, Zhang J, Wu C (2022) Use of chicken embryo model in toxicity studies of endocrine-disrupting chemicals and nanoparticles. Chem Res Toxicol 7:17703–17712. https://doi.org/10.1021/acs.chemrestox.1c00399
Gibbs PS, Maurer JJ, Nolan LK, Wooley RE (2003) Prediction of chicken embryo lethality with the avian Escherichia coli traits complement resistance, Colicin V production, and presence of the increased serum survival gene cluster (iss). Avian Dis 47:370–379. https://doi.org/10.1637/0005-2086(2003)047[0370:POCELW]2.0.CO;2
Guedes PT, de Oliveira BCEPD, de Manso PPA, Caputo LFG, Cotta-Pereira G, Pelajo-Machado M (2014) Histological analyses demonstrate the temporary contribution of yolk sac, liver, and bone marrow to hematopoiesis during chicken development. PLoS One 9:e90975. https://doi.org/10.1371/journal.pone.0090975
Guo R, Li Z, Jiao Y, Geng S, Pan Z, Chen X, Li Q, Jiao X (2017) O-polysaccharide is important for Salmonella Pullorum survival in egg albumen, and virulence and colonization in chicken embryos. Avian Pathol 46:535–540. https://doi.org/10.1080/03079457.2017.1324197
Guo R, Li Z, Zhou X, Huang C, Hu Y, Geng S, Chen X, Li Q, Pan Z, Jiao X (2019) Induction of arthritis in chickens by infection with novel virulent Salmonella Pullorum strains. Vet Microbiol 228:165–172. https://doi.org/10.1016/j.vetmic.2018.11.032
Hawlena H, Bashey F, Lively CM (2012) Bacteriocin-mediated interactions within and between coexisting species. Ecol Evol 2:2521–2526. https://doi.org/10.1002/ece3.354
Hrala M, Bosák J, Micenková L, Křenová J, Lexa M, Pirková V, Tomáštíková Z, Koláčková I, Šmajs D (2021) Escherichia coli strains producing selected bacteriocins inhibit porcine enterotoxigenic Escherichia coli (ETEC) under both in vitro and in vivo conditions. Appl Environ Microbiol 87. https://doi.org/10.1128/AEM.03121-20
Jamal M, Bukhari SMAUS, Andleeb S, Ali M, Raza S, Nawaz MA, Hussain T, Rahman SU, Shah SSA (2019) Bacteriophages: an overview of the control strategies against multiple bacterial infections in different fields. J Basic Microbiol 59:123–133. https://doi.org/10.1002/jobm.201800412
Jiang H, Li Y, Cosnier S, Yang M, Sun W, Mao C (2022) Exploring phage engineering to advance nanobiotechnology. Materials Today Nano 19. https://doi.org/10.1016/j.mtnano.2022.100229
Kogut MH, Arsenault RJ (2017) Immunometabolic phenotype alterations associated with the induction of disease tolerance and persistent asymptomatic infection of Salmonella in the chicken intestine. Front Immunol 8:1–7. https://doi.org/10.3389/fimmu.2017.00372
Kosecka-Strojek M, Trzeciak J, Homa J, Trzeciak K, Władyka B, Trela M, Międzobrodzki J, Lis MW (2021) Effect of Staphylococcus aureus infection on the heat stress protein 70 (HSP70) level in chicken embryo tissues. Poult Sci 100. https://doi.org/10.1016/j.psj.2021.101119
Kue CS, Tan KY, Lam ML, Lee HB (2014) Chick embryo chorioallantoic membrane (CAM): an alternative predictive model in acute toxicological studies for anti-cancer drugs. Exp Anim 64:129–138. https://doi.org/10.1538/expanim.14-0059
Li Q, Li Y, Xia J, Wang X, Yin K, Hu Y, Yin C, Liu Z, Jiao X (2019) Virulence of Salmonella enterica serovar Pullorum isolates compared using cell-based and chicken embryo infection models. Poult Sci 98:1488–1493. https://doi.org/10.3382/ps/pey482
Molina ED, Balander R, Fitzgerald SD, Giesy JP, Kannan K, Mitchell R, Bursian SJ (2006) Effects of air cell injection of perfluorooctane sulfonate before incubation on development of the white leghorn chicken (Gallus domesticus) embryo. Environ Toxicol Chem an Int J 25:227–232. https://doi.org/10.1897/04-414R.1
Nakakido M, Tanaka N, Shimojo A, Miyamae N, Tsumoto K (2022) Development of a high-throughput method to screen novel antiviral materials. PLoS One 17:1–8. https://doi.org/10.1371/journal.pone.0266474
Oh JY, Kang MS, Yoon H, Choi HW, An BK, Shin EG, Kim YJ, Kim MJ, Kwon JH, Kwon YK (2012) The embryo lethality of Escherichia coli isolates and its relationship to the presence of virulence-associated genes. Poult Sci 91:370–375. https://doi.org/10.3382/ps.2011-01807
Polláková M, Petrilla V, Andrejčáková Z, Petrillová M, Sopková D, Petrovová E (2021) Spitting cobras: experimental assay employing the model of chicken embryo and the chick chorioallantoic membrane for imaging and evaluation of effects of venom from African and Asian species (Naja ashei, Naja nigricollis, Naja siamensis, Naja sumatrana). Toxicon 189:79–90. https://doi.org/10.1016/j.toxicon.2020.10.025
Rahbarnia L, Farajnia S, Babaei H, Majidi J, Veisi K, Ahmadzadeh V, Akbari B (2017) Evolution of phage display technology: from discovery to application. J Drug Target 25:216–224. https://doi.org/10.1080/1061186X.2016.1258570
Rashidi H, Sottile V (2009) The chick embryo: hatching a model for contemporary biomedical research. BioEssays 31:459–465. https://doi.org/10.1002/bies.200800168
Rezaee MS, Liebhart D, Hess C, Hess M, Paudel S (2021) Bacterial infection in chicken embryos and consequences of yolk sac constitution for embryo survival. Vet Pathol 58:71–79. https://doi.org/10.1177/0300985820960127
Rothwell L, Young JR, Zoorob R, Whittaker CA, Hesketh P, Archer A, Smith AL, Kaiser P (2004) Cloning and characterization of chicken IL-10 and its role in the immune response to Eimeria maxima. J Immunol 173:2675–2682. https://doi.org/10.4049/jimmunol.173.4.2675
Setia A, Bhandari SK, House JD, Nyachoti CM, Krause DO (2009) Development and in vitro evaluation of an Escherichia coli probiotic able to inhibit the growth of pathogenic Escherichia coli K88. J Anim Sci 87:2005–2012. https://doi.org/10.2527/jas.2008-1400
Seto F (1981) Early development of the avian immune system. Poult Sci 60:1981–1995. https://doi.org/10.3382/ps.0601981
Shen X, Zhang A, Gu J, Zhao R, Pan X, Dai Y, Yin L, Zhang Q, Hu X, Wang H, Zhang D (2022) Evaluating Salmonella pullorum dissemination and shedding patterns and antibody production in infected chickens. BMC Vet Res 18:240. https://doi.org/10.1186/s12917-022-03335-z
Sioud M (2019) Phage display libraries: from binders to targeted drug delivery and human therapeutics. Mol Biotechnol 61:286–303. https://doi.org/10.1007/s12033-019-00156-8
Stefaniak T, Madej JP, Graczyk S, Siwek M, Łukaszewicz E, Kowalczyk A, Sieńczyk M, Maiorano G, Bednarczyk M (2020) Impact of prebiotics and synbiotics administered in ovo on the immune response against experimental antigens in chicken broilers. Animals 10:1–15. https://doi.org/10.3390/ani10040643
Tang Y, Foster N, Jones MA, Barrow PA (2018) Model of persistent Salmonella infection: Salmonella enterica serovar Pullorum modulates the immune response of the chicken from a Th17-type response towards a Th2-type response response. Infect Immun 86. https://doi.org/10.1128/IAI.00307-18
Van Belleghem JD, Dąbrowska K, Vaneechoutte M, Barr JJ, Bollyky PL (2019) Interactions between bacteriophage, bacteria, and the mammalian immune system. Viruses 11. https://doi.org/10.3390/v11010010
Wachholz GE, Rengel BD, Vargesson N, Fraga LR (2021) From the farm to the lab: how chicken embryos contribute to the field of teratology. Front Genet 12:1–11. https://doi.org/10.3389/fgene.2021.666726
Wong GK, Cavey MJ (1992) Development of the liver in the chicken embryo. I. Hepatic Cords Sinusoids Anat Rec 234:555–567. https://doi.org/10.1002/ar.1092340411
Wong GK, Cavey MJ (1993) Development of the liver in the chicken embryo. II. Erythropoietic Granulopoietic Cells Anat Rec 235:131–143. https://doi.org/10.1002/ar.1092350114
Zosen D, Hadera MG, Lumor JS, Andersen JM, Paulsen RE (2021) Chicken embryo as animal model to study drug distribution to the developing brain. J Pharmacol Toxicol Methods 112:0–4. https://doi.org/10.1016/j.vascn.2021.107105
Acknowledgements
The authors thank Luiz Ricardo Goulart Filho for idealizing and designing this study. Your departure left us with a vast sadness, but your brilliance and generosity reached all who had the honor to learn from you. You live within us.
We thank New World Hatchery (Hy-Line Company), which never spared efforts to support our research by donating chicken eggs and embryos.
Funding
This study was funded by the INCT in Theranostics and Nanobiotechnology (INCT-TeraNano, CNPq Process nº 403193/2022–2 e FAPEMIG Process nº CBB—APQ-03613–17). Research Project MCTI/ CNPq nº 28/2018.
Author information
Authors and Affiliations
Contributions
BBF and LRG conceived and designed the experiments; JBS, SS, ERV, HOAS, FAAS, ACR, AAMR, and BBF performed the experiments; JBS and BBF analyzed the data; LMB contributed reagents/materials/analysis tools; JBS, HOAS, and BBF wrote the manuscript and all authors revised. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The work was approved by the Ethics Committee on Animal Use (CEUA) of the Federal University of Uberlândia, protocol number 008/21 and 45/2022/CEUA/PROPP/REITO.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Luiz Ricardo Goulart is deceased before publication of this work was completed.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Souza, J.B., Sommerfeld, S., Almeida-Souza, H.O. et al. A new standardization for the use of chicken embryo: selection of target from the phage display library and infection. Appl Microbiol Biotechnol 108, 412 (2024). https://doi.org/10.1007/s00253-024-13227-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-024-13227-x