Abstract
2-Phenylethanol (2-PE) is an aromatic compound with a rose-like fragrance that is widely used in food and other industries. Yeasts have been implicated in the biosynthesis of 2-PE; however, few studies have reported the involvement of filamentous fungi. In this study, 2-PE was detected in Annulohypoxylon stygium mycelia grown in both potato dextrose broth (PDB) and sawdust medium. Among the 27 A. stygium strains investigated in this study, the strain “Jinjiling” (strain S20) showed the highest production of 2-PE. Under optimal culture conditions, the concentration of 2-PE was 2.33 g/L. Each of the key genes in Saccharomyces cerevisiae shikimate and Ehrlich pathways was found to have homologous genes in A. stygium. Upon the addition of L-phenylalanine to the medium, there was an upregulation of all key genes in the Ehrlich pathway of A. stygium, which was consistent with that of S. cerevisiae. A. stygium as an associated fungus provides nutrition for the growth of Tremella fuciformis and most spent composts of T. fuciformis contain pure A. stygium mycelium. Our study on the high-efficiency biosynthesis of 2-PE in A. stygium offers a sustainable solution by utilizing the spent compost of T. fuciformis and provides an alternative option for the production of natural 2-PE.
Key points
• Annulohypoxylon stygium can produce high concentration of 2-phenylethanol.
• The pathways of 2-PE biosynthesis in Annulohypoxylon stygium were analyzed.
• Spent compost of Tremella fuciformis is a potential source for 2-phenylethanol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
2-Phenylethanol (2-PE) is an aromatic alcohol with a rose-like fragrance (Qian et al. 2019). It has been used as a fragrance ingredient in various products, including food, perfumes, and plant preservatives (Chreptowicz et al. 2016; Mo and Sung 2007; Scognamiglio et al. 2012). The majority of 2-PE used in the industry is obtained by chemical synthesis. However, chemical synthesis is limited by toxic compounds and byproducts that are difficult to remove (Hua and Xu 2011; Martínez-Avila et al. 2020). Thus, the microbial biosynthesis process has received considerable attention as an alternative for 2-PE production and is a simpler and more efficient option for purification (Carroll et al. 2016).
The most efficient microorganisms for the production of 2-PE are yeasts, including Saccharomyces cerevisiae (Kim et al. 2014), Kluyveromyces marxianus (Lu et al. 2016), Kluyveromyces lactis (Qian et al. 2019), and Pichia fermentans (Chreptowicz et al. 2018). Yeasts are known to biosynthesize 2-PE via the shikimate or Ehrlich pathways (Wang et al. 2019). The shikimate pathway is a long pathway with multiple branches and various inhibitory feedback mechanisms and results in a low production of 2-PE (Hassing et al. 2019). However, when L-phenylalanine (L-Phe) is rich or serves as the sole nitrogen source, the Ehrlich pathway plays a leading role and produces higher output of 2-PE. The Ehrlich pathway consists of three steps: L-Phe is transformed into phenylpyruvate by a transaminase, then decarboxylated to phenylacetaldehyde by a decarboxylase, and finally reduced to 2-PE by a dehydrogenase (Dickinson et al. 2003). In S. cerevisiae, several genes are involved in the transformation of L-Phe into 2-PE via the Ehrlich pathway, including ARO8, ARO10, and ADH, which encode an aminotransferase, a decarboxylase, and a dehydrogenase, respectively (Dai et al. 2021; Hazelwood et al. 2008). Overexpression of ARO8 and ARO10 leads to an increase in the yield of 2-PE by 37% (Yin et al. 2015), and the co-expression of ARO10 and ADH can significantly improve the yield of 2-PE by 6.5 folds (Shen et al. 2016). Few studies have reported the production of 2-PE by filamentous fungi.
Annulohypoxylon stygium is a white-rot filamentous fungus belonging to the family Xylariaceae. It exhibits a high ability to degrade lignin and carbohydrates (Hsieh et al. 2005; Wingfield et al. 2018). As an associated fungus, A. stygium provides nutrition for the growth and development environments of Tremella fuciformis and is commonly found in natural and artificial cultivation environments (Deng et al. 2016; Liu et al. 2019). More than 500 kt of fresh T. fuciformis were produced in 2020 (Sun 2023), generating more than 800 kt of spent mushroom compost, most of which contains only A. stygium mycelium, just less than 5% being a mixture of the two fungi. A. stygium has also been reported to be a good source of some active metabolites, such as melanin, oxidative stress resistance, and glycohydrolases (Liu et al. 2022; Robl et al. 2015; Wu et al. 2008).
In this study, we used A. stygium as material, detected 2-PE content in its volatile matter spectrum at different culture conditions, screened out the strain with most powerful biosynthesis of 2-PE from our culture collection, optimized medium composition and culture conditions, and analyzed the potential biosynthetic pathway of 2-PE production. Our purpose is to develop an alternative filamentous fungus for high-efficiency biosynthesis of 2-PE.
Materials and methods
Strains and media
The T. fuciformis Tr21 strain, a major cultivar contributing to most of the total production, as well as 27 A. stygium strains (Table 1) were obtained from the Center for Mushroom Germplasm Resources Management and Preservation of Fujian Province (CMMPF, Fujian, China). A. stygium S20 was also deposited in the China Center for Typical Culture Collection (CCTCC) with the accession number CCTCC NO:M 2020504 (Table 1). Potato dextrose broth (PDB) medium: 200 g/L potato infusion, 20 g/L glucose; Potato dextrose agar (PDA) medium: PDB medium plus 20 g/L agar. PDB + L-Phe medium: PDB medium plus 4 g/L L-Phe. Sawdust medium: 78% sawdust, 19.5% wheat bran, 1% sucrose, 1% gypsum, and 0.5% MgSO4.
Odor chemical determination
As the associated fungus of T. fuciformis Tr21, A. stygium strain S2 was used to detect its odor chemicals in both PDB and sawdust medium. The mycelium was cultivated into the PDB medium for 4 days at 28 °C and 120 rpm, then harvested using the filtering paper method to obtain PDB sample. Sawdust sample was collected from bottom cultivation materials (including only A. stygium mycelia) after T. fuciformis cultivated in the sawdust medium at 23 °C for 34 days and the fruiting bodies being 6–8 cm in diameter. Both PDB and sawdust samples as well as their corresponding un-inoculated media were immediately used to determine their odor chemicals using a TOFMS/GC–MS (Agilent, Santa Clara, CA, USA).
Screening of 2-PE-producing strains
Each of tested A. stygium strains was inoculated into 100 mL PDB + L-Phe medium and incubated in a constant temperature shaker at 28 °C at a shaking speed of 160 rpm for 6 days. The fermentation broth was centrifuged at 4000 rpm for 10 min, and the supernatant was filtered. The contents of L-Phe and 2-PE were determined using high-performance liquid chromatography LC–20AD (HPLC; Shimadzu, Kyoto, Japan) with a C-18 column (5 µm, 250 mm × 4.6 mm; Shimadzu, Shanghai, China). The mobile phase comprised 0.6% acetic acid aqueous solution and methanol, the flow rate was 0.7 mL/min, the temperature was set to 30 °C, and the detection wavelength was 258 nm.
All strains with 2-PE concentration more than 1.00 g/L were selected and evaluated their tolerance ability in the PDA medium. A.stygium mycelium was inoculated onto PDA medium containing 0 g/L (CK), 1 g/L, 2 g/L, and 3 g/L 2-PE, and incubated at 28 °C for 4 days. The strain with the highest tolerance was selected by measuring their mycelial growth rate.
Morphological and ITS analyses
The mycelium of the S20 strain was cultured on PDA medium at 28 °C for 7 days. The mycelial morphology was examined using a bright-field microscope BX63 (Olympus, Tokyo, Japan). Primers ITS1 and ITS4 were used to amplify the ITS region of the S20 strain using a reaction system and operating conditions as described by Liu et al. (2022). The polymerase chain reaction (PCR) products were purified and sequenced by Sangon Biotech Co., Ltd. (Shanghai, China). The obtained ITS sequence (GenBank: PP140388) was used to search for the best hits of known species sequences for species identification using NCBI BLAST.
Optimization of growth media for maximum production of 2-PE
The S20 strain was cultivated into PDB medium at 28 °C for 2 days. Optimization of 2-PE production was achieved in four steps, by evaluating the effects of different carbon sources, L-Phe consumption, and the concentration of potato infusions and MgSO4. Different carbon sources including glucose, maltose, lactose, mannitol, and sucrose were selected for 2-PE production. The S20 strain was incubated in PDB + L-Phe medium containing 20 g/L of the different carbon sources, 4 g/L of L-Phe, 200 g/L of potato infusion, and 0.1 g/L of MgSO4. The optimal carbon source concentrations ranged from 20 to 120 g/L. The L-Phe concentration ranged from 0 to 8 g/L, and the potato infusion content ranged from 200 to 1000 g/L. The MgSO4 concentrations ranged from 0 to 0.4 g/L. HPLC was used to determine 2-PE concentration in the fermentation broth.
Detailed optimization studies of 2-PE production were conducted using orthogonal experiments. Different concentrations of potato infusion, maltose, L-Phe, and MgSO4 were chosen as the factor levels (Table 2). Statistical analyses were carried out using IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp., Armonk, N.Y., USA).
Optimization of culture conditions
The S20 strain was grown for 2 days at 28 °C as described above on fermentation medium with different potato extraction times (5, 10, 15, 20, 25, 30 min), culture temperatures (22, 25, 28, 31, 34 °C), rotation speeds (120,140, 160, 180, 200 rpm), and liquid loading (60, 80, 100, 120, 140 mL); all the other components of the media remained the same. The optimal culture conditions were chosen based on the determination of 2- PE concentration by HPLC.
RNA sequencing and data analyses
The S20 strain was grown in PDB and PDB + L-Phe medium at 25 °C, 150 rpm, for 4 days. Mycelium in both media was collected. Each treatment had three repetitions. Samples were grinded in a mortar with liquid nitrogen. RNA was extracted using the E.Z.N.A. Plant RNA Kit (Omega, Norcross, GA, USA) following the manufacturer’s instructions. RNA sequencing was performed by Novogene Corporation (Beijing, China) to obtain ~ 4 Gb clean data for each sample.
Clean sequencing reads were mapped on the A. stygium genome (GCA_003314315.1) using the STAR alignment software (Dobin et al. 2013). Differential gene expression analysis was performed using StringTie (Pertea et al. 2015) or Cufflinks (Trapnell et al. 2012) packages. Genes were considered to have statistically significant differential expression at p value < 0.05. The raw sequencing data were deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) with the BioProject accession number PRJNA1018449 (https://www.ncbi.nlm.nih.gov/sra/PRJNA1018449).
Determination of biosynthetic pathway of 2-PE in A. stygium
Proteins (Ssy1p, accession number: NP_010444.1, ADH, accession number: NP_014032.1, ARO8, accession number: NP_011313.1 and PDR12, accession number: NP_015267.1) involved in the biosynthetic pathway of 2-PE in S. cerevisiae were downloaded from the NR database of the NCBI website. Local BLASTp was used to align these proteins against the proteomic database of A. stygium to determine their gene analogs. For each gene with multiple analogs, a phylogenetic tree was constructed to determine the closely related analogs using the program Mega 11 (Tamura et al. 2021) with the alignment option of Clustal-W and default parameters. Each candidate gene was further identified using RNA-seq analysis of the samples with or without additional L-Phe.
Results
Determination of odor chemicals
In an initial approach, the odor chemicals produced by the strain S2 in PDB and sawdust media were determined using an TOFMS/GC–MS (Agilent, Santa Clara, CA, USA). A total of 52 and 21 odor chemicals were detected in the inoculated PDB and sawdust media, respectively. In the PDB medium, the top three odor chemicals comprised 4-hepten-3-one, 2-PE, and azulene, with the percentages of 14.7%, 12.6%, and 4.5%, respectively (Fig. 1A). In the sawdust medium, the top three odor chemicals were piperonal, benzaldehyde, and 2-PE, with the percentage of 28.2%, 11.6%, and 4.7%, respectively. 2-PE was undetected in the uninoculated PDB and sawdust media. These results indicated that A. stygium produces large amounts of 2-PE in different media.
Odor chemicals produced by A. stygium (strain S2) in different media. A, C Proportion of odor chemicals emitted from samples in potato dextrose broth (PDB) medium after cultivated 4 days at 28 °C under 120 rpm. B, C Proportion of odor chemicals emitted from samples in sawdust medium (SM) when the fruiting bodies of T. fuciformis reaching 6–8 cm in diameter. CK, un-inoculated media
Best producer strain isolation and identification
Next, a larger collection of A. stygium strains was screened in order to select the best 2-PE producer. After 6 days of culture at 28 °C in PDB medium, all the 27 tested A. stygium strains were found to produce 2-PE. Among them, five strains (strain S12, strain S14, strain S19, strain S20, and strain S26) produced 2-PE at a concentration exceeding 1.00 g/L, while the S20 strain produced up to 1.23 g/L of 2-PE (Fig. 2A, Table 2). After 4 days of culture at 28 °C, the concentration of 2-PE produced by the S20 strain in the PDB medium reached 38 mg/L; however, L-Phe was not detected. In contrast, the 2-PE concentration increased to 300 mg/L in the PDB + L-Phe medium, and the L-Phe concentration decreased from 4.0 to 1.5 g/L (Table 3).
High-yield 2-PE strain screening and identification of A. stygium. A High-yield 2-PE strain screening after cultivated in PDB medium for 6 days at 28 °C. B Test for strain tolerance against 2-PE. CK, PDA medium without 2-PE addition. C Colonial morphology of strain S20. D Phylogenetic tree built by ITS sequences (ITS1-ITS4). Percentage on the right means sequence similarity of each strain compared with S20. A. stygium XH10 (accession number, FJ848859.1); A. stygium XH4 (accession number, FJ848853.1); A. stygium EF2 (accession number, MG881822.1); A. stygium RS15 (accession number, KF612313.1)
During the subsequent tolerance test among the five strains (strain S12, strain S14, strain S19, strain S20, and strain S26), the S20 strain had the highest mycelial growth rate in the PDA medium with the same concentration of 2-PE (Fig. 2B), indicating that S20 had the strongest tolerance against 2-PE. The mycelia of the S20 strain grew densely, emitted a rose smell, and secreted melanin after 7 days of cultivation, which resulted in the entire medium turning black (Fig. 2C). All these are typically morphological and odor characteristics of A. stygium (Deng et al. 2016). PCR with primers ITS1 and ITS4 of the S20 strain generated a single molecule of 873 bp. Sequence alignment and phylogenetic tree showed that the ITS sequence of the S20 strain had the highest similarity with that of A. stygium, showing 100% sequence identity with A. stygium XH4 (accession number, FJ848853.1) and A. stygium XH10 (accession number, FJ848859.1), respectively (Fig. 2D).
Optimization of growth media
To evaluate the 2-PE synthesis capability of the S20 strain, optimal carbon sources were determined. As shown in Fig. 3A, the 2-PE concentration was the highest when maltose was used as the carbon source. When the initial maltose concentration was 60.00 g/L, the 2-PE concentration reached a maximum of 1.33 g/L after 48 h of cultivation (Fig. 3B).
As a nitrogen source, L-Phe plays a crucial role in 2-PE production (De Lima et al. 2018); therefore, it was necessary to explore the optimal addition of L-Phe to the medium. The 2-PE concentration peaked at an L-Phe concentration of 4.00 g/L but decreased at higher L-Phe concentrations (Fig. 3C). The increasing trend of 2-PE production with L-Phe concentration was consistent with the initial maltose concentration on 2-PE production; these results indicated that the concentration of biosynthesis 2- PE in A. stygium was lower with less additions of maltose and L-Phe in the medium.
Different potato infusion content affected 2-PE production. 2-PE production was higher when the potato infusion content was between 200.00 and 600.00 g/L but lower at a higher potato infusion (Fig. 3D). Previous studies have shown that Mg2+ enhanced the activity of dehydrogenase and decarboxylase (Hirano et al. 2007; Hirata et al. 2016). The effect of MgSO4 concentration on 2-PE production is shown in Fig. 2E. 2-PE production was observed to increase until the MgSO4 concentration reached 0.20 g/L.
Based on the single-factor experiments, crucial experimental parameters affecting 2-PE production were optimized, including potato infusion content and maltose, L-Phe, and MgSO4 concentrations (Table 2). According to the orthogonal test, it can be concluded that the primary and secondary factors affecting 2-PE production were L-Phe concentration and potato infusion. The optimal potato infusion, maltose, L-Phe, and MgSO4 concentration were set at 600.00 g/L, 40.00 g/L, 6.00 g/L, and 0.30 g/L, respectively (Table 4). The 2-PE concentration measured using this formula was 2.33 g/L.
Optimization of culture conditions
To improve the concentration of 2-PE, culture conditions were optimized based on the defined medium. As shown in Fig. 4A, the 2-PE concentration increased with increasing soaking time of potatoes. The 2-PE concentration was highest when the soaking time reached 30 min.
The optimization of temperature was evaluated every two centigrade from 22 to 34 °C (Fig. 4B). The 2-PE concentration increased as the temperature increased and then decreased at higher temperatures. The results showed that the 2-PE concentration highest value at 28 °C which is consistent with the optimal growth temperature range of A. stygium (Mu 2012).
Dissolved oxygen was also a factor that affected the 2-PE concentration of the S20 strain. The rotational speed and loading volume are shown in Fig. 4C and D, respectively. The results showed that the S20 strain inoculated into 100 mL PDB medium with vigorous agitation at 160 rpm was best.
Analyses of the 2-PE synthesis pathway
To date, no studies have reported the ability of A. stygium to produce 2-PE. The genes involved in the production of 2-PE and metabolism of L-Phe in S. cerevisiae were retrieved. These genes were then compared with the complete genome sequence of A. stygium using Blast analysis (https://blast.ncbi.nlm.nih.gov/Blast.cgi), which could help speculate on the biosynthesis pathway of 2-PE in A. stygium.
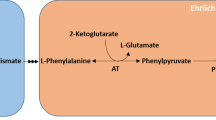
In S. cerevisiae, nine genes (ARO3, ARO1, ARO2, ARO7, PHA2, ARO8, ARO10, ADH, and PDR12) and the Ssy1p membrane protein (encoded by the SSY1 gene) have been identified to be involved in 2-PE synthesis (Delaney et al. 2023; Hazelwood et al. 2008; Larroude et al. 2021; Zhu et al. 2021). The first five genes (ARO3, ARO1, ARO2, ARO7, and PHA2) play important roles in the pathway and are primarily responsible for the conversion of glucose to phenylpyruvate. Phenylpyruvate is then converted to 2-PE via a series of reactions. Each of these genes had at least one homologue in the S20 strain genome. ARO3 and ARO1 had two homologues each in the S20 strain. ARO2, ARO7, and PHA2 genes each had one homologue in the S20 strain genome (Table 5). The last four genes (ARO8, ARO10, ADH, and PDR12) encode relevant enzymes in the Ehrlich pathway that catalyze the conversion of L-Phe to 2-PE. The SSY1 and PDR12 genes had nine and six homologues in the S20 strain genome (accession number, JBAIZS000000000), respectively. ARO8, ARO10, and ADH had four, two, and seven homologues in the S20 strain genome, respectively.
Ssy1p is a membrane protein with a function to import L-Phe from the extracellular environment (Delaney et al. 2023). It is encoded by the member of the family of amino acid transporter genes, SSY1 (Didion et al. 1998). Nine genes in the S20 strain genome were detected by doing tBLASTx searches the S. cerevisiae genome. According to the phylogenetic tree, the products of the genes TJAS01-V10044110, TJAS01-V10088760, and TJAS01-V10068300 had a close genetic relationship with Ssy1p of S. cerevisiae (Fig. 5A). When 4 g/L of L-Phe was added to the PDB medium, the gene expression level of TJAS01-V10088760 was significantly upregulated, whereas those of TJAS01-V10044110 and TJAS01-V10068300 were significantly downregulated or remained unchanged, respectively (Table 5). Changes in gene expression levels of TJAS01-V10088760 were compared to the gene function of SSY1. As a result, TJAS01-V10088760 was most likely to be a SSY1 candidate in the S20 strain. TJAS01-V10005660, TJAS01-V10069960, TJAS01-V10028140, and TJAS01-V10054500 were most likely to be ARO8, ARO10, ADH, and PDR12, respectively, in the Ehrlich pathway (Fig. 5B). In addition, upon the addition of L-phenylalanine to the medium, the expression levels of genes in the Ehrlich pathway were higher than those in the shikimate pathway, suggesting that the synthesis of 2-PE through the Ehrlich pathway was higher than that through the shikimate pathway in the S20 strain (Fig. 5B). The 2-PE synthesis pathway in the S20 strain was thus similar to that in S. cerevisiae (Dai et al. 2021; Hazelwood et al. 2008).
Prediction of key genes and pathway for biosynthesis 2-PE in A. stygium. A Phylogenetic trees constructed by protein sequences corresponding to Ssy1p (accession number, NP_010444.1; upper left), ADH (accession number, NP_014032.1; upper right), ARO8 (accession number, NP_011313.1; low left), and PDR12 (accession number, NP_015267.1; low right) of S. cerevisiae. Percentage on the right means protein similarity of each gene compared with A. stygium homologues. B Possible pathway of biosynthesis 2-PE in A. stygium. PEP, phosphoenolpyruvate; E4P, erythrose-4-phosphate; DAHP, 3-deoxy-D-arabinoheptulosonate; SHK, shikimate; CHR, chorismic acid
Discussion
Currently, studies on the biosynthesis of 2-PE have focused primarily on yeasts and few on filamentous fungi, such as some species of Aspergillus (Vázquez et al. 2022). Based on GC–MS analysis, Wani et al. (2010) found that Aspergillus niger JUBT 3 M was able to produce 2-PE at a lower concentration than many of the tested yeasts. After optimizing the culture conditions, Etschmann et al. (2015) used A. niger DSM 821 to produce 2-PE at concentrations as high as 1.43 g/L. Under the optimal glucose and L-Phe concentration, the 2-PE concentration of A. oryzae RIB40 may reach up to 0.1 g/L (Masuo et al. 2015). In the present study, 2-PE was detected as one of the main components emitted from the A. stygium mycelia grown in bothPDB and sawdust media. Among 27 A. stygium isolates screened in this study, the strain “Jinjiling” (strain S20) showed the highest production of 2-PE and had the strongest tolerance of the product. By optimizing the initial concentrations of maltose, L-Phe, potato infusion, and MgSO4, the concentration of 2-PE could be as high as 2.33 g/L, which is a value relatively higher than those among other filamentous fungi reported (Etschmann et al. 2015; Masuo et al. 2015). As a result, A. stygium has great potential as a filamentous candidate for the high production of 2-PE.
In addition to S. cerevisiae (Dai et al. 2021; Zhu et al. 2021), many other yeast species, such as P. fermentans (Fan et al. 2020; Mierzejewska et al. 2019), K.marxianus (De Lima et al. 2018; Etschmann and Schrader 2006; Li et al. 2021), Yarrowia lipolytica (Gu et al. 2020a, b), Zygosaccharomyces rouxii (Dai et al. 2020), and Metschnikowia pulcherrima (Chantasuban et al. 2018; Zhu et al. 2022), have been reported to synthesize 2-PE from glucose through the shikimate pathway and from L-Phe through the Ehrlich pathway.
The production of 2-PE through the Ehrlich pathway in Aspergillus sp. is not as widely studied as in yeasts (Etschmann et al. 2015). A. niger CMICC 298302 was firstly reported in filamentous fungi to produce 2-PE using L-Phe as the nitrogen source (Lomascolo et al. 2001). Ashbya gossypii is a filamentous ascomycete that harbors genes for aromatic amino acid catabolism (ARO8a, ARO8b, ARO10, and ARO80) and produces high concentrations of 2-PE. Deletion of these genes, except for AgARO8a, strongly impairs the production of 2-PE, indicating that the Ehrlich pathway plays an important role in 2-PE production (Ravasio et al. 2014). Both Ehrlich and PEA (phenylethyl alcohol) pathways were responsible for the synthesis of 2-PE in A. oryzae RIB40. In the PEA pathway, phenylalanine was decarboxylated to phenylethylamine, which was then oxidatively deaminated to form phenylacetaldehyde, and subsequently dehydrogenated to yield 2-PE (Tieman et al. 2006). However, when L-Phe was added in the culture medium, the isolate synthesized 2-PE primarily via the Ehrlich pathway (Masuo et al. 2015). Genome comparisons revealed that each key gene in the S. cerevisiae shikimate and Ehrlich pathways had one or more homologous genes in A. stygium. When L-Phe was added to the PDB medium, the amount of 2-PE produced by A. stygium increased rapidly, which was consistent with that produced by S. cerevisiae (Dai et al. 2021). Gene expression levels of candidates in the Ehrlich pathway were upregulated when L-Phe was added to the medium. Based on the above evidence, it is speculated that A. stygium has a synthetic pathway similar to that of S. cerevisiae for the production of 2-PE. More evidence, such as gene knockout, is essential to further confirm the shikimate and Ehrlich pathways and their corresponding genes in A. stygium.
Each year, agro-food industries produce large quantities of residues, and their utilization as a cheap substrate for the production of 2-PE has many advantages, such as abundant substrate resources, reduced cost, and environmental friendliness (Mitri et al. 2022). Many studies have tested various agro-industrial waste and by-products for raw materials to produce 2-PE, including whey (Chreptowicz et al. 2018), grape must (Garavaglia et al. 2007), corn stover (Mierzejewska et al. 2019), sugar beet molasses (Martínez-Avila et al. 2018; Martínez et al. 2018b; Zhan et al. 2020), sugarcane bagasse (Martínez-Avila et al. 2018, 2020; Martínez et al. 2018a), tobacco (Wang et al. 2013), and cassava wastewater (Oliveira et al. 2015). Most spent compost of T. fuciformis substrate contains only A. stygium and no other microorganisms (Liu et al. 2019) and is considered an ideal material for direct extraction of 2-PE. T. fuciformis has been cultivated in Northeast Asia since the 1960s (Sun 2023). In 2020, more than 500 kt of fresh T. fuciformis was produced (Sun 2023), generating more than 800 kt of substrate waste. The mycelia of A. stygium spread over the entire substrate and provide nutrition for T. fuciformis (Deng et al. 2018). However, the growth of T. fuciformis mycelia is limited to the region (20–30 mm in diameter) near the inoculation area (our unpublished observations). As a result, approximately 95% of T. fuciformis substrate contains only A. stygium and no other microorganisms and is considered an ideal material for direct extraction of 2-PE. Further studies are necessary to construct a cultivation system of T. fuciformis, including optical strains, culture medium, and cultivation conditions, to obtain high yield of T. fuciformis and good 2-PE production at the same time. Our study on high-efficiency 2-PE biosynthesis in A. stygium not only makes it possible to reuse the spent compost substrate of T. fuciformis cultivation but also provides an alternative option for the production of natural 2-PE.
In this study, 2-PE was detected in A. stygium mycelial growth medium. The strain “Jinjiling” (strain S20) produced the highest 2-PE concentration and had the strongest tolerance of the product. By optimizing the initial concentrations of maltose, L-Phe, potato infusion, and MgSO4, the concentration of 2-PE was as high as 2.33 g/L. The pathway for the biosynthesis of 2-PE in A. stygium was similar to that in S. cerevisiae. In summary, A. stygium has great potential to utilize substrate waste of T. fuciformis cultivation to produce high yields of 2-PE.
Data availability
The datasets generated during the study are available from the corresponding author on reasonable request. Illumina sequencing data for six RNA sequencing have been deposited in the NCBI with the accession number PRJNA1018449.
References
Carroll AL, Desai SH, Atsumi S (2016) Microbial production of scent and flavor compounds. Curr Opin Biotechnol 37:8–15
Chantasuban T, Santomauro F, Gore-Lloyd D, Parsons S, Henk D, Scott RJ, Chuck C (2018) Elevated production of the aromatic fragrance molecule, 2-phenylethanol, using Metschnikowia pulcherrima through both de novo and ex novo conversion in batch and continuous modes. J Chem Technol Biotechnol 93(8):2118–2130
Chreptowicz K, Wielechowska M, Główczyk-Zubek J, Rybak E, Mierzejewska J (2016) Production of natural 2-phenylethanol: from biotransformation to purified product. Food Bioprod Process 100:275–281
Chreptowicz K, Sternicka M, Kowalska P, Mierzejewska J (2018) Screening of yeasts for the production of 2-phenylethanol (rose aroma) in organic waste-based media. Lett Appl Microbiol 66(2):153–160
Dai J, Li K, Song N, Yao W, Xia H, Yang Q, Zhang X, Li X, Wang Z, Yao L (2020) Zygosaccharomyces rouxii, an aromatic yeast isolated from chili sauce, is able to biosynthesize 2-phenylethanol via the shikimate or Ehrlich pathways. Front Microbiol 11:597454
Dai J, Xia H, Yang C, Chen X (2021) Sensing, uptake and catabolism of L-phenylalanine during 2-phenylethanol biosynthesis via the Ehrlich pathway in Saccharomyces cerevisiae. Front Microbiol 12:601963
De Lima LA, Diniz RHS, De Queiroz MV, Fietto LG, Da Silveira WB (2018) Screening of yeasts isolated from Brazilian environments for the 2-phenylethanol (2-PE) production. Biotechnol Bioprocess Eng 23:326–332
Delaney C, Short B, Rajendran R, Kean R, Burgess K, Williams C, Munro CA, Ramage G (2023) An integrated transcriptomic and metabolomic approach to investigate the heterogeneous Candida albicans biofilm phenotype. Biofilm 5:100112
Deng Y, van Peer AF, Lan F-S, Wang Q-F, Jiang Y, Lian L-D, Lu D-M, Xie B (2016) Morphological and molecular analysis identifies the associated fungus (“ Xianghui”) of the medicinal white jelly mushroom, Tremella fuciformis, as Annulohypoxylon stygium. Int J Med Mushrooms 18(3):253–260
Deng Y, Hsiang T, Li S, Lin L, Wang Q, Chen Q, Xie B, Ming R (2018) Comparison of the mitochondrial genome sequences of six Annulohypoxylon stygium isolates suggests short fragment insertions as a potential factor leading to larger genomic size. Front Microbiol 9:398624
Dickinson JR, Salgado LEJ, Hewlins MJ (2003) The catabolism of amino acids to long chain and complex alcohols in Saccharomyces cerevisiae. J Biol Chem 278(10):8028–8034
Didion T, Regenberg B, Jørgensen MU, Kielland-Brandt MC, Andersen HA (1998) The permease homologue Ssy1p controls the expression of amino acid and peptide transporter genes in Saccharomyces cerevisiae. Mol Microbiol 27(3):643–650
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29(1):15–21. https://doi.org/10.1093/bioinformatics/bts635
Etschmann M, Schrader J (2006) An aqueous–organic two-phase bioprocess for efficient production of the natural aroma chemicals 2-phenylethanol and 2-phenylethylacetate with yeast. Appl Microbiol Biotechnol 71:440–443
Etschmann M, Huth I, Walisko R, Schuster J, Krull R, Holtmann D, Wittmann C, Schrader J (2015) Improving 2-phenylethanol and 6-pentyl-α-pyrone production with fungi by microparticle-enhanced cultivation (MPEC). Yeast 32(1):145–157
Fan G, Cheng L, Fu Z, Sun B, Teng C, Jiang X, Li X (2020) Screening of yeasts isolated from Baijiu environments for 2-phenylethanol production and optimization of production conditions. 3 Biotech 10:1–17
Garavaglia J, Flôres SH, Pizzolato TM, Peralba MdC, Ayub MAZ (2007) Bioconversion of L-phenylalanine into 2-phenylethanol by Kluyveromyces marxianus in grape must cultures. World J Microbiol Biotechnol 23:1273–1279
Gu Y, Ma J, Zhu Y, Ding X, Xu P (2020a) Engineering Yarrowia lipolytica as a chassis for de novo synthesis of five aromatic-derived natural products and chemicals. ACS Synth Biol 9(8):2096–2106
Gu Y, Ma J, Zhu Y, Xu P (2020b) Refactoring Ehrlich pathway for high-yield 2-phenylethanol production in Yarrowia lipolytica. ACS Synth Biol 9(3):623–633
Hassing E-J, de Groot PA, Marquenie VR, Pronk JT, Daran J-MG (2019) Connecting central carbon and aromatic amino acid metabolisms to improve de novo 2-phenylethanol production in Saccharomyces cerevisiae. Metab Eng 56:165–180
Hazelwood LA, Daran J-M, Van Maris AJ, Pronk JT, Dickinson JR (2008) The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism. Appl Environ Microbiol 74(8):2259–2266
Hirano J-i, Miyamoto K, Ohta H (2007) Purification and characterization of aldehyde dehydrogenase with a broad substrate specificity originated from 2-phenylethanol-assimilating Brevibacterium sp. KU1309. Appl Microbiol Biotechnol 76:357–363
Hirata H, Ohnishi T, Watanabe N (2016) Biosynthesis of floral scent 2-phenylethanol in rose flowers. Biosci Biotechnol Biochem 80(10):1865–1873
Hsieh H-M, Ju Y-M, Rogers JD (2005) Molecular phylogeny of Hypoxylon and closely related genera. Mycologia 97(4):844–865
Hua D, Xu P (2011) Recent advances in biotechnological production of 2-phenylethanol. Biotechnol Adv 29(6):654–660
Kim B, Cho BR, Hahn JS (2014) Metabolic engineering of Saccharomyces cerevisiae for the production of 2-phenylethanol via Ehrlich pathway. Biotechnol Bioeng 111(1):115–124
Larroude M, Nicaud JM, Rossignol T (2021) Yarrowia lipolytica chassis strains engineered to produce aromatic amino acids via the shikimate pathway. Microb Biotechnol 14(6):2420–2434
Li M, Lang X, Moran Cabrera M, De Keyser S, Sun X, Da Silva N, Wheeldon I (2021) CRISPR-mediated multigene integration enables shikimate pathway refactoring for enhanced 2-phenylethanol biosynthesis in Kluyveromyces marxianus. Biotechnol Biofuels 14:1–15
Liu D, Pujiana D, Wang Y, Zhang Z, Zheng L, Chen L, Ma A (2019) Comparative transcriptomic analysis identified differentially expressed genes and pathways involved in the interaction between Tremella fuciformis and Annulohypoxylon stygium. Antonie Van Leeuwenhoek 112(11):1675–1689. https://doi.org/10.1007/s10482-019-01294-4
Liu D, Sun X, Yan B, Ma A (2022) Alternative oxidase is involved in oxidative stress resistance and melanin synthesis in Annulohypoxylon stygium, a companion fungus of Tremella fuciformis. Antonie Van Leeuwenhoek 115(3):365–374
Lomascolo A, Lesage-Meessen L, Haon M, Navarro D, Antona C, Faulds C, Marcel A (2001) Evaluation of the potential of Aspergillus niger species for the bioconversion of L-phenylalanine into 2-phenylethanol. World J Microbiol Biotechnol 17:99–102
Lu X, Wang Y, Zong H, Ji H, Zhuge B, Dong Z (2016) Bioconversion of L-phenylalanine to 2-phenylethanol by the novel stress-tolerant yeast Candida glycerinogenes WL2002-5. Bioengineered 7(6):418–423
Martínez O, Sánchez A, Font X, Barrena R (2018a) Bioproduction of 2-phenylethanol and 2-phenethyl acetate by Kluyveromyces marxianus through the solid-state fermentation of sugarcane bagasse. Appl Microbiol Biotechnol 102:4703–4716
Martínez O, Sánchez A, Font X, Barrena R (2018b) Enhancing the bioproduction of value-added aroma compounds via solid-state fermentation of sugarcane bagasse and sugar beet molasses: operational strategies and scaling-up of the process. Bioresour Technol 263:136–144
Martínez-Avila O, Sánchez A, Font X, Barrena R (2018) Bioprocesses for 2-phenylethanol and 2-phenylethyl acetate production: current state and perspectives. Appl Microbiol Biotechnol 102(23):9991–10004
Martínez-Avila O, Sánchez A, Font X, Barrena R (2020) 2-phenylethanol (rose aroma) production potential of an isolated Pichia kudriavzevii through solid-state fermentation. Process Biochem 93:94–103
Masuo S, Osada L, Zhou S, Fujita T, Takaya N (2015) Aspergillus oryzae pathways that convert phenylalanine into the flavor volatile 2-phenylethanol. Fungal Genet Biol 77:22–30
Mierzejewska J, Dąbkowska K, Chreptowicz K, Sokołowska A (2019) Hydrolyzed corn stover as a promising feedstock for 2-phenylethanol production by nonconventional yeast. J Chem Technol Biotechnol 94(3):777–784
Mitri S, Koubaa M, Maroun RG, Rossignol T, Nicaud J-M, Louka N (2022) Bioproduction of 2-phenylethanol through yeast fermentation on synthetic media and on agro-industrial waste and by-products: a review. Foods 11(1):109
Mo EK, Sung CK (2007) Phenylethyl alcohol (PEA) application slows fungal growth and maintains aroma in strawberry. Postharvest Biol Technol 45(2):234–239
Mu Y (2012) Molecular identification based on partial β-tubulin gene sequences and biological characters of Annulohypoxylon spp. paired cultivated Tremella fuciformis Berk. Master Thesis, Sichuan Agricultural University
Oliveira SM, Gomes SD, Sene L, Christ D, Piechontcoski J (2015) Production of natural aroma by yeast in wastewater of cassava starch industry. Eng Agric 35:721–732
Pertea M, Pertea GM, Antonescu CM, Chang T-C, Mendell JT, Salzberg SL (2015) StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol 33(3):290–295
Qian X, Yan W, Zhang W, Dong W, Ma J, Ochsenreither K, Jiang M, Xin F (2019) Current status and perspectives of 2-phenylethanol production through biological processes. Crit Rev Biotechnol 39(2):235–248
Ravasio D, Wendland J, Walther A (2014) Major contribution of the Ehrlich pathway for 2-phenylethanol/rose flavor production in Ashbya gossypii. FEMS Yeast Res 14(6):833–844
Robl D, dos Santos CP, Büchli F, da Silva Lima DJ, da Silva DP, Squina FM, Pimentel IC, Padilla G, da Cruz Pradella JG (2015) Enhancing of sugar cane bagasse hydrolysis by Annulohypoxylon stygium glycohydrolases. Bioresour Technol 177:247–254
Scognamiglio J, Jones L, Letizia C, Api A (2012) Fragrance material review on phenylethyl alcohol. Food Chem Toxicol 50:S224–S239
Shen L, Nishimura Y, Matsuda F, Ishii J, Kondo A (2016) Overexpressing enzymes of the Ehrlich pathway and deleting genes of the competing pathway in Saccharomyces cerevisiae for increasing 2-phenylethanol production from glucose. J Biosci Bioeng 122(1):34–39
Sun S (2023) Blue book on the development of China’s Tremella fuciformis industry. China Agricultural Science and Technology Press, Beijing
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38(7):3022–3027
Tieman D, Taylor M, Schauer N, Fernie AR, Hanson AD, Klee HJ (2006) Tomato aromatic amino acid decarboxylases participate in synthesis of the flavor volatiles 2-phenylethanol and 2-phenylacetaldehyde. Proc Natl Acad Sci 103(21):8287–8292
Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L (2012) Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7(3):562–578
Vázquez MB, Matencio A, Bianchinotti MV, García-Carmona F, López-Nicolás JM (2022) Enhanced production of 2-phenylethanol by salicylic acid and cyclodextrins in cell suspension cultures of the unexplored filamentous fungus Monochaetinula geoffroeana. J Sci Food Agric 102(4):1609–1618
Wang Q, Song Y, Jin Y, Liu H, Zhang H, Sun Y, Liu G (2013) Biosynthesis of 2-phenylethanol using tobacco waste as feedstock. Biocatal Biotransform 31(6):292–298
Wang Y, Zhang H, Lu X, Zong H, Zhuge B (2019) Advances in 2-phenylethanol production from engineered microorganisms. Biotechnol Adv 37(3):403–409
Wani MA, Sanjana K, Kumar DM, Lal DK (2010) GC–MS analysis reveals production of 2–phenylethanol from Aspergillus niger endophytic in rose. J Basic Microbiol 50(1):110–114
Wingfield BD, Bills GF, Dong Y, Huang W, Nel WJ, Swalarsk-Parry BS, Vaghefi N, Wilken PM, An Z, De Beer ZW (2018) Draft genome sequence of Annulohypoxylon stygium, Aspergillus mulundensis, Berkeleyomyces basicola (syn. Thielaviopsis basicola), Ceratocystis smalleyi, two Cercospora beticola strains, Coleophoma cylindrospora, Fusarium fracticaudum, Phialophora cf. hyalina, and Morchella septimelata. IMA Fungus 9(1):199–223
Wu Y, Shan L, Yang S, Ma A (2008) Identification and antioxidant activity of melanin isolated from Hypoxylon archeri, a companion fungus of Tremella fuciformis. J Basic Microbiol 48(3):217–221
Yin S, Zhou H, Xiao X, Lang T, Liang J, Wang C (2015) Improving 2-phenylethanol production via Ehrlich pathway using genetic engineered Saccharomyces cerevisiae strains. Curr Microbiol 70:762–767
Zhan Y, Zhou M, Wang H, Chen L, Li Z, Cai D, Wen Z, Ma X, Chen S (2020) Efficient synthesis of 2-phenylethanol from L-phenylalanine by engineered Bacillus licheniformis using molasses as carbon source. Appl Microbiol Biotechnol 104:7507–7520
Zhu L, Wang J, Xu S, Shi G (2021) Improved aromatic alcohol production by strengthening the shikimate pathway in Saccharomyces cerevisiae. Process Biochem 103:18–30
Zhu W, Zhang W, Qin T, Liao J, Zhang X (2022) Effects of purified β-glucosidases from Issatchenkia terricola, Pichia kudriavzevii, Metschnikowia pulcherrima on the flavor complexity and typicality of wines. J Fungi 8(10):1057
Funding
This work was supported by Fujian Provincial Department of Science and Technology funding project (No. 2021L3006) and Fujian provincial major national R&D project (No. 2002NZ029015).
Author information
Authors and Affiliations
Contributions
Conceptualization, QWT, LZY, and JXZ; methodology, QWT, LZY, and JXZ; formal analysis, JXZ, YZ; writing—original draft, QWT, LZY; Writing—review & editing, YJJ, XRL, and YJD.
Corresponding authors
Ethics declarations
Ethics approval
This article does not contain any studies with animals performed by any of the authors.
Competing interests
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tong, Q., Yang, L., Zhang, J. et al. Comprehensive investigations of 2-phenylethanol production by the filamentous fungus Annulohypoxylon stygium. Appl Microbiol Biotechnol 108, 374 (2024). https://doi.org/10.1007/s00253-024-13226-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-024-13226-y