Abstract
Radopholus similis is a destructive, migratory, and endophytoparasitic nematode. It has two morphologically indistinguishable pathotypes (or physiological races): banana and citrus pathotypes. At present, the only reliable method to differentiate the two pathotypes is testing the infestation and parasitism of nematodes on Citrus spp. via inoculation. However, differences in inoculation methods and conditions adopted by different researchers complicate obtaining consistent results. In this study, the parasitism and pathogenicity of 10 R. similis populations on rough lemon (Citrus limon) seedlings and the tropism and invasion of rough lemon roots were tested. It revealed that populations SWK, GJ, FZ, GZ, DBSR, and YJ were citrus pathotypes, which showed parasitism and pathogenicity on rough lemon and could invade rough lemon roots, whereas populations XIN, ML, HN6, and HL were banana pathotypes, having no parasitism and pathogenicity on rough lemon and they did not invade the rough lemon roots. Four pectate lyase genes (Rs-pel-2, Rs-pel-3, Rs-pel-4, and Rs-pel-5) belonging to the Class III family from these populations were amplified and analysed. The gene Rs-pel-3 could be amplified from six citrus pathotype populations and was stably expressed in the four developmental stages of the nematode, whereas it could not be amplified from the four banana pathotypes. Rs-pel-3 expression may be related to the parasitism and pathogenicity of R. similis on rough lemon. Hence, it can be used as a molecular marker to distinguish between banana and citrus pathotypes and as a target gene for the molecular identification of these two pathotypes.
Key points
• Four pectate lyase genes (Rs-pels) from Radopholus similis were cloned and analysed.
• The expression of Rs-pels is different in two pathotypes of Radopholus similis.
• A molecular identification method for two pathotypes of Radopholus similis using pectate lyase gene Rs-pel-3 as the target gene was established.
Similar content being viewed by others
Introduction
Radopholus similis, with a common name, the burrowing nematode, a migratory endophytoparasitic nematode, is one of the top 10 most damaging plant-parasitic nematodes worldwide (Jones et al. 2013). R. similis has over 350 host plants, including bananas, Citrus spp., and ornamental plants, and is an important factor in reducing the production of bananas and other horticultural crops worldwide; it is listed as a quarantine plant pest in most countries (Xie 2006; Duncan and Mons 2013). Two pathotypes (or physiological races) of R. similis are generally recognised: the banana and citrus pathotypes. The banana pathotype parasitises bananas and many other hosts, but not Citrus spp., whereas the citrus pathotype parasitises both bananas and Citrus spp. (Valette et al. 1998). Therefore, the citrus pathotype has a wider host range and is more harmful than the banana pathotype. The European and Mediterranean plant Protection Organizations and European Union classify banana and citrus pathotypes as A1 and A2 quarantine pests, respectively (EPPO 2008). At present, the mechanisms of host specificity and differences in pathogenicity between these two pathotypes are unclear. Testing the parasitism of nematodes on Citrus spp. is recognised as the only reliable method for distinguishing between these pathotypes.
Pectate lyase (PEL) degrades pectin to produce oligosaccharides via β-trans elimination and exists widely in plants and microorganisms (Chen et al. 2019). Pathogenic microorganisms destroy plant cell walls to facilitate their invasion via secreting PEL to degrade pectin and stimulate plant immune responses (Shevchik et al. 1998; Ferrari et al. 2013). Therefore, PEL plays an important role in the interaction between plant pathogens and their hosts and is an important factor affecting the host range of pathogens (Filho et al. 2016). Currently, plant-parasitic nematodes are the only animals known to produce PEL. The first PEL gene (pel) found in plant-parasitic nematodes was Gr-pel-1 from Globodera rostochiensis (Popeijus et al. 2000). Subsequently, several pel genes were identified and cloned from sedentary parasitic nematodes belonging to the genera Heterodera, Globodera, and Meloidogyne, all of which belong to the Class III PEL family (Deboer et al. 2002; Doyle et al. 2002; Huang et al. 2005; Kudla et al. 2007; Vanholme et al. 2007; Zhuo et al. 2011; Peng et al. 2012, 2016; Li et al. 2017). The PEL of sedentary parasitic plant-parasitic nematodes may play an important role in host specialization and pathogenic differentiation (Huang et al. 2005; Stare et al. 2011; Sabeh et al. 2019; Tian et al. 2019). To date, only three pel genes have been identified and cloned from Bursaphelenchus xylophilus, a migratory parasitic nematode (Kikuchi et al. 2006; Lee et al. 2013); however, the function and mechanism of these genes and their relationship with pathogenic differentiation have not been reported.
In this study, five pels from R. similis (Rs-pels) were screened and cloned using transcriptome data (Huang et al. 2019), and their basic characteristics were studied using bioinformatics. The pathogenicity and tropism of 10 R. similis populations from different hosts were tested to determine their pathotypes against rough lemon (Citrus limon). Then, the expression of Rs-pels in these populations was analysed to screen the differential pel genes that could potentially distinguish between the banana and citrus pathotypes to establish a molecular identification method for these two pathotypes.
Materials and methods
Ten populations of R. similis and their host sources are shown in Table 1. All populations were identified by the Plant Nematodes Laboratory of South China Agricultural University, propagated and preserved on carrot callus (25°C) according to the method described by Fallas and Sarah (1994). R. similis was isolated from carrot callus by the method of Stoffelen et al. (1999), soaked in 0.2% streptomycin sulfate for 8 hours, and then washed with sterile distilled water for five times, and then the nematode suspensions were obtained for subsequent experiments. The different developmental stages (eggs, juveniles, females, and males) of R. similis were distinguished by morphological characteristics under a Nikon SMZ18 stereomicroscope (Nikon Corporation, Tokyo, Japan). Females and males have vulva and spicule respectively.
The C. limon seeds were donated by Prof. Xiaoling Deng from South China Agricultural University. River sand purchased from Guangzhou Xiankelian Garden Flower Co., Ltd. (Guangzhou, China) was used as the planting medium. A glass tube (3 cm × 15 cm) was filled with dry river sand, accounting for about 1/3 the tube height, and was sterilised at 125 °C 20 p.s.i (about 137.8 kPa) for 1 h; after cooling overnight, it was subjected to secondary sterilisation under the same conditions. After peeling the seed coat, rough lemon seeds were placed in 0.26% sodium hypochlorite solution for 30 min, washed with sterile water four times, laid flat in a Petri dish (d=90 mm) with moist sterilised filter paper at the bottom, and placed in an incubator at 25 ± 1.5 °C for 3–4 days to accelerate germination in the dark.
Sterile distilled water (3 mL) was added to each sand-filled tube to moisten the sand and a 2 cm deep depression was placed in the sand surface centre; a single seed was placed in it, and covered with sterile sand. The tubes were then placed in an incubator at 25 ± 1.5 °C with the light intensity of 3200–4000 l × (9.5 h light and 14.5 h darkness), normal watering management, and humidity maintained at approximately 3% w/w. After 30 days of culture, the rough lemon seedlings were inoculated.
Determining parasitism and pathogenicity of tested nematodes to rough lemon
As per the method described by Kaplan (1994), rough lemon seedlings with essentially the same growth after planting for 30 days were selected and inoculated nematode suspensions by a dropper with an inoculation amount of 200 female nematodes (identified under a Nikon SMZ18 stereomicroscope; Nikon Corporation, Tokyo, Japan) per plant. Inoculation was performed twice at an interval of 5 days, and 100 nematodes were inoculated each time. Each population of R. similis was inoculated for one treatment, and each treatment was repeated five times; non-inoculated seedlings were used as a control treatment. Inoculation tests were repeated twice. The day before inoculation, sterile distilled water was added to each test tube to wet the sand, and the nematode suspension was inoculated at a depth of 1 cm underground. To ensure normal infection of nematodes, plants were not watered for the first three days after inoculation, and routine management was performed. After inoculation with nematodes for 30 days, the inoculated plant root systems were observed and photographed, plant symptoms were recorded, plant height and root weight were measured, and the nematodes were isolated according to Kaplan’s method and counted using a Nikon SMZ18 stereomicroscope (Nikon Corporation, Tokyo, Japan).
Tropism and invasion of nematodes to the rough lemon root system
The root systems of rough lemon seedlings were cut into 1 cm long segments. According to Čepulytė et al. (2018), Pluronic F-127 gel system was prepared to conduct the tropism test of R. similis to the rough lemon root. This gel was liquid at low temperatures (< 15 °C) but solidified into a gel at higher temperatures (> 15 °C). At the temperature of 10 °C, 1 mL of Pluronic F-127 gel was absorbed into a sterile 24-well culture plate. A total of 200 female nematodes were picked up from nematode suspensions by a needle under a Nikon SMZ18 stereomicroscope and added to each well, gently shaken and mixed, and then a fresh rough lemon root segment was placed in each well. The culture plate was sealed and transferred to a dark incubator at 28 °C for culturing. Each nematode population had five wells (five replicates), and the experiment was repeated twice. Nematode tropism was observed under a Nikon SMZ18 stereomicroscope after 2, 4, 6, and 8 h of culturing. The nematode aggregation and invasions around the root incision were photographed and recorded. In addition, a modified sodium chlorate-acid fuchsin staining method (Feng 2001) was used to dye the root segments cultured for 8 h. Stained root tissues were sliced and photographed under a Nikon SMZ18 stereomicroscope.
Cloning of pel genes from R. similis
A total of 20,000 mixed-stage nematodes of the GJ population were isolated and collected from a carrot callus in which R. similis was cultured. The total RNA of the nematodes was extracted by a Trizol Regent (Invitrogen, Carlsbad, California, USA). The 5' RACE and the 3' RACE cDNA templates for RACE amplification were synthesized by the SMART RACE cDNA amplification kit (Clontech, Takara Biotechnology (Dalian) Co., Ltd., Dalian, China), respectively. The cDNA template for the open reading frames (ORF) sequence amplification was synthesized by One-step gDNA Removal and cDNA synthesis SuperMix Kit (Transgen, Beijing, China). The total genomic DNA of R. similis was extracted by HiPure Mollusc DNA Kit (Magen Co., Ltd., Guangzhou, China).
Screening and blasting of the transcriptome data of R. similis (Huang et al. 2019) were performed to obtain the suspected pels sequences. Primers for RACE amplification of pels were designed according to those sequences (Supplementary Information). The 5' and the 3' terminal fragments of pels were amplified using these primers, with the 5' RACE cDNA and the 3' RACE cDNA serving as templates for amplification, respectively. The amplified fragments were sequenced and spliced together by Sangon Biotech Co., ltd (Shanghai, China). The ORFs of Rs-pels (Rs-pel-1, Rs-pel-2, Rs-pel-3, Rs-pel-4, Rs-pel-5) were predicted on an ORF finder website (http://www.ncbi.nlm.nih.gov/gorf/orfig.cgi), and a pair of full-length primers were designed for each Rs-pel (Supplemental Material, Table S1) by Primer Premier 6 software (PREMIER Biosoft International, Palo Alto, CA, USA). The ORF and genomic DNA sequences of Rs-pels were amplified using full-length primers, with the cDNA and genomic DNA serving as templates for amplification, respectively.
The similarity of Rs-PELs was compared to the NCBI database using the BlastX tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Signalp-4.0 (http://www.cbs.dtu.dk/services/signalp-4.0/) and TMHMMServer v.2.0 (https://services.healthtech.dtu.dk/services/TMHMM-2.0/) were used to predict the signal peptide (SP) and transmembrane domains of Rs-PELs, respectively. Multiple sequence alignments of Rs-PEL and other PELs from other nematodes were performed using Mega 6.0 (http://www.megasoftware.net/), and a phylogenetic tree was constructed using the maximum-likelihood method.
Expression determination of Rs-pels in R. similis populations
Using Rs-pels as the target, the DNA of a single nematode from each population was extracted for polymerase chain reaction (PCR) detection, and the expression of Rs-pels in different populations was analysed and determined. The target genes were selected for identification. To analyse the stable expression of the target gene, DNA from four developmental stages (eggs, juveniles, females, and males) was used as a template for PCR amplification to establish a method for rapid identification of the pathotypes of R. similis. The PCR amplification of single nematode DNA was performed as described by Xu et al. (2016).
Data processing and analysis
The SPSS 14.0 Software (SPSS Inc., Chicago, IL, USA) and Excel 2013 Software (Microsoft Inc., Washington, D.C.) were used for statistical analyses. One-way analysis of variance (ANOVA) was employed for the analysis of the mean and standard error, and Duncan’s Multiple Ranger Test (DMRT) was used for multiple comparisons. The significance of the difference was analysed at the level of p=0.05. Preliminary analyses showed that there was no significant difference (p>0.05) between the data from two runs of each experiment using dependent-sample t tests and that allowed data from two runs to be combined for analyses.
Results
Parasitism and pathogenicity of 10 R. similis populations to rough lemon
Thirty days after inoculation, the root systems of rough lemons inoculated with the SWK, GJ, FZ, GZ, YJ, and DBSR populations were weak and had evident brown spots (Fig. 1). Plant height, root length, and root weight of the six treatments were significantly lower than those of the control (p<0.05) and the number of nematodes in the root and rhizosphere soils was significantly higher than those in the control (p<0.05). However, the average nematode number in the roots of the XIN, ML, HN6, and HL inoculation treatments was only 1–2, and the nematode number in the rhizosphere soil was less than 10, which was not significantly different from that of the control (p>0.05). The root length difference between these four treatments was also not significant, and the root length and weight of XIN and HN6 inoculation treatments were not significantly different from those of the control (p>0.05) (Table 2). Therefore, SWK, GJ, FZ, GZ, YJ, and DBSR populations could parasitise rough lemons and had clear pathogenicity, whereas XIN, ML, HN6, and HL populations did not parasitise rough lemons.
Symptoms of Citrus limon infected with 200 female nematodes of Radopholus similis per plant for 30 days. CK: non-inoculated rough lemon seedling and root system; XIN, ML, SWK, HN6, HL, GJ, FZ, GZ, YJ and DBSR: the rough lemon seedlings and root systems were inoculated with the different populations of R. similis, which originated from Zingiber officinale Roscoe (XIN), Crataegus pinnatifida (ML), Chrysalidocarpus lutescens (SWK), Musa AAA Giant Cavendish cv.Baxi (HN6), Maranta arundinacea (HL), Citrus reticulata (GJ), Anthurium andraeanum ‘Pink Champion’ (FZ), Anthurium andraeanum Linden (GZ), Curcuma longa (YJ), and Anubias nana (DBSR), respectively
Tropism and invasion of 10 R. similis populations to the root system of rough lemon
The tropism test of 10 R. similis populations to the rough lemon root system showed that the nematodes of all populations were randomly and uniformly dispersed in the gel when the root segment was placed in the well, and the nematodes started to migrate and aggregate to the root segment after 2 h and 4 h. The nematodes of the SWK, GJ, FZ, GZ, DBSR, and YJ populations were observed to invade the root from the wound position after 6 h, whereas the nematodes of the XIN, ML, HN6, and HL populations were still around the root and did not invade it (Fig. 2A). The root tissues treated for 8 h were dyed with modified sodium chlorate-acid fuchsin. Under the stereomicroscope, many nematodes of SWK, GJ, FZ, GZ, DBSR, and YJ populations were observed in the root segments, but only one or no nematodes of the XIN, ML, HN6, and HL populations were found in the root segments (Fig. 2B). The results showed that all the tested populations tended towards the rough lemon root; however, the SWK, GJ, FZ, GZ, DBSR, and YJ populations could invade the rough lemon root, whereas the XIN, ML, HN6, and HL populations did not invade the rough lemon root.
Tropism of 10 populations of Radopholus similis to the Citrus limon root. A: Tropism of R. similis to the root of C. limon in Pluronic F-127 gel evenly mixed with 200 female nematodes 6 h after assay initiation; B: Fuchsin staining of C. limon roots 8 h after assay initiation by infection with 200 female nematodes; XIN, ML, SWK, HN6, HL, GJ, FZ, GZ, YJ, and DBSR: the C. limon roots were infected with populations of R. similis, which originated from Zingiber officinale Roscoe (XIN), Crataegus pinnatifida (ML), Musa AAA Giant Cavendish cv.Baxi (HN6), Maranta arundinacea (HL), Chrysalidocarpus lutescens (SWK), Citrus reticulata (GJ), Anthurium andraeanum ‘Pink Champion’ (FZ), Anthurium andraeanum Linden (GZ), Curcuma longa (YJ), and Anubias nana (DBSR), respectively; scale bar = 500 μm
Cloning and analysis of genes of the pel family in R. similis
Five transcripts of suspected pels were obtained via screening and blasting the transcriptome data of the GJ population of R. similis. The ORF and DNA genomic sequences of these genes were obtained using PCR amplification (Fig. 3).
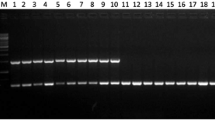
PCR amplification of five pectate lyase genes from Radopholus similis (Rs-pels). A: Amplification of the ORF sequences of Rs-pels; B: Amplification of the genomic DNA sequences of Rs-pels. M:DS2000 marker (GDSBio Co., Ltd., Guangzhou China); 1–5: products of Rs-pel-1, Rs-pel-2, Rs-pel-3, Rs-pel-4, Rs-pel-5 amplification respectively
After sequencing these fragments, a BLAST comparison confirmed that the deduced proteins of all five genes had conserved domains of the PEL family, and these five pels were named Rs-pel-1, Rs-pel-2, Rs-pel-3, Rs-pel-4, and Rs-pel-5. Their ORFs were 843, 819, 873, 771, and 810 bp long, and the number of encoded amino acids (aa) were 280, 272, 290, 256, and 269 aa, respectively (Table 3). ClustalX was used to compare the amino acid sequences of these five Rs-PELs with known Class III PEL sequences, and Rs-PEL-2, Rs-PEL-3, Rs-PEL-4, and Rs-PEL-5 were confirmed to have four conserved regions unique to the Class III PEL family (Fig. 4) (Shevchik et al. 1997), whereas Rs-PEL-1 did not have a conserved region unique to Class III PEL.
Predicting the conserved regions of pectate lyases of the Class III PEL family from Radophulus similis. Regions I–IV indicate conserved regions characteristic of the Class III pectate lyase family (Shevchik et al.1997). Highly conserved charged residues are indicated with asterisks (*), RS_PEL2 to 5 indicate the amino acid sequences of Rs-PEL-2 to Rs-PEL-5 from R. similis, the species of the bacterium or the fungus, the gene, the amino acid size, and the accession number of the aligned sequences are: F_sol_PelA = Fusarium solani f. sp. pisi, PelA, 242 aa (M94692.1); F_sol_PelB = F. solani f. sp. pisi, PelB, 242 aa (U13051); F_sol_PelC = F. solani f. sp. pisi, PelC, 215 aa (U13049); F_sol_PelD = F. solani f. sp. pisi, PelD, 233 aa (U13050); E_car_PelB = Erwinia carotovora, PelB, 347 aa (X79232)
Sequence analysis of the five Rs-PELs predicted that none of them had a transmembrane domain. Among them, Rs-PEL-2, Rs-PEL-4, and Rs-PEL-5 contained signal peptides, whereas Rs-PEL-1 and Rs-PEL-3 did not have signal peptide sequences (Table 3).
Amino acid sequences of the five Rs-PELs were blasted against those of other plant-parasitic nematodes, bacteria, and fungi, and a phylogenetic tree was constructed using the maximum likelihood method in MEGA software (Fig. 5). The results showed that PELs from fungi and bacteria clustered into a large category, whereas those from plant-parasitic nematodes clustered independently into another large category. The Rs-PELs could be divided into three categories: first, Rs-PEL-3 and the PELs from four species of Meloidogyne were clustered into a branch; second, Rs-PEL-2 and Rs-PEL-5 were clustered with the PELs from two species of Meloidogyne and species of Heterodera and Globodera into a branch; third, Rs-PEL-1 and Rs-PEL-4 were clustered with the PELs from the nematodes of Heterodera, Globodera, and Aphelenchida into one branch.
Maximum-likelihood phylogenetic trees of pectate lyases of Radopholus similis (Rs-PELs) and other organisms. Phylogenetic tree for proteins with conserved domains of pectate lyases from cyst nematodes, root-knot nematodes, Aphelenchus, Bursaphelenchus, bacteria, and fungi generated by MEGA6.0. The Rs-PELs amino acid sequences were marked in bold, and each sequence was followed by its accession number in GenBank
Expression of PEL genes in 10 populations of R. similis
Single nematode DNA from 10 populations was used as the template, and sequence amplification of four Rs-pels of the class III PEL family, i.e., Rs-pel-2, Rs-pel-3, Rs-pel-4, and Rs-pel-5, was performed. The results showed that Rs-pel-2, Rs-pel-4, and Rs-pel-5 could be amplified from the SWK, GJ, FZ, GZ, DBSR, and YJ populations that parasitised rough lemon, and the XIN, ML, HN6, and HL populations that did not parasitise rough lemon. However, the gene Rs-pel-3 could only be amplified from the six populations parasitizing rough lemon, showed stable expression in four developmental stages of these populations, and could not be amplified from the four non-parasitized rough lemon populations (Fig. 6A). Therefore, Rs-pel-3 could be used as a target gene to identify the banana and citrus pathotypes of R. similis and could be identified at all developmental stages in the citrus pathotype (Fig. 6B).
DNA amplification of pectate lyase genes of Radopholus similis (Rs-pels) in 10 populations. A: DNA amplification of Rs-pels in 10 populations of R. similis; B: DNA amplification of Rs-pel-3 in different populations and developmental stages of R. similis; XIN, ML, SWK, HN6, HL, GJ, FZ, GZ, YJ, and DBSR: single nematode DNA from populations of R. similis, which originated from Zingiber officinale Roscoe (XIN), Crataegus pinnatifida (ML), Musa AAA Giant Cavendish cv.Baxi (HN6), Maranta arundinacea (HL), Chrysalidocarpus lutescens (SWK), Citrus reticulata (GJ), Anthurium andraeanum ‘Pink Champion’ (FZ), Anthurium andraeanum Linden (GZ), Curcuma longa (YJ), and Anubias nana (DBSR), respectively; M: DS2000 marker (GDSBio Co., Ltd., Guangzhou China); Fe: female; Ma: male; J2: second stage juvenile; Eg: egg
Discussion
Two pathotypes, the banana and citrus pathotypes of R. similis are generally believed to exist with different host ranges and be morphologically indistinguishable. In this study, the parasitism and pathogenicity of 10 R. similis populations to rough lemon were determined using the method described by Kaplan et al. (1994) and the Pluronic F-127 gel system (Čepulytė et al. 2018; Wang et al. 2009). The results showed that six populations could parasitise rough lemon, whereas four populations could not. Four Rs-pels of the Class III PEL family (Rs-pel-2, Rs-pel-3, Rs-pel-4, Rs-pel-5) could be amplified from six populations of parasitised rough lemon, whereas only three Rs-pel of the Class III PEL family (Rs-pel-2, Rs-pel-4, Rs-pel-5) could be amplified from four populations of non-parasitised rough lemon, and Rs-pel-3 was stably expressed in four developmental stages of populations of parasitised rough lemon. Rs-pel-3 was proven to be used as a target gene to distinguish the banana and citrus pathotypes of R. similis.
Kaplan and Opperman (1997) conducted parasitism and pathogenicity experiments on R. similis in citrus using the method described by Kaplan et al. (1994). They reported that if fewer than 10 nematodes were isolated from the rhizosphere (including in root and rhizosphere soil), the population would not parasitise citrus, whereas if more than 100 nematodes were isolated from the rhizosphere, the population would parasitise citrus (Kaplan and Opperman 1997). However, the parasitism of a population of 10–100 nematodes isolated from the rhizosphere has not been clearly defined by Kaplan and Opperman (1997). Goo and Sipes (1997) proposed that if low numbers of nematodes were recovered from the root, it suggested a low level of nematode reproduction might have occurred on the plant, indicating it was a poor host; if a few nematodes were recovered from the media but not from the root, it suggested that these nematodes might have been survivors from the inoculation, so the plant was considered a non-host or a very poor host. In this study, 30 days after rough lemons were inoculated with SWK, GJ, FZ, GZ, DBSR, and YJ populations of R. similis nematodes were isolated from each inoculated root system. The average number of nematodes in the roots was 8–20, and the total number of nematodes isolated from the rhizosphere of each inoculation treatment was more than 20. The roots of rough lemons inoculated with these six populations showed clear symptoms of damage, and the average nematode number in the root and rhizosphere soil was significantly higher than that of the control treatment and the other four populations. However, 30 days after rough lemon inoculation with XIN, ML, HN6, and HL populations, only 1–5 nematodes were isolated from seven inoculated root systems, and no nematodes were isolated from the other three inoculated root systems. The average nematode number in the roots was only one or two, and the total number of nematodes isolated from the rhizosphere was less than 10 in each inoculation treatment, both of which were not significantly different from those of the control. The roots of rough lemons inoculated with these four populations did not show symptoms of damage (Fig. 1).
In addition, the nematodes of SWK, GJ, FZ, GZ, DBSR, and YJ populations were observed to be attracted to and invaded the rough lemon roots, whereas the nematodes of XIN, ML, HN6, and HL populations were only attracted to but did not invade the rough lemon roots in the Pluronic F-127 gel system (Fig. 2). Plant root exudates can lead to tropism of plant-parasitic nematodes in both host and non-host plants (Sasakicrawley 2013; Hu et al. 2017); however, they can only induce nematodes to infect host plants and not non-host plants. Therefore, we considered that rough lemon was a host plant for the SWK, GJ, FZ, GZ, DBSR, and YJ populations, but not for the XIN, ML, HN6, and HL populations. This indicated that the SWK, GJ, FZ, GZ, DBSR, and YJ populations belonged to the citrus pathotype (or citrus race), whereas the XIN, ML, HN6, and HL populations belonged to the banana pathotype (or banana race).
Many studies have shown that pel is an important factor affecting host range. Different types of PELs recognise different sequences of methylated and unmethylated galacturonate sites with different chemical and enzyme properties (Herron et al. 2000; Filho et al. 2016), which may result in different pathogen host ranges. In this study, five pels from R. similis (Rs-pel-1, Rs-pel-2, Rs-pel--3, Rs-pel-4, Rs-pel-5) were screened and cloned based on R. similis transcriptome data, four of which belonged to the Class III family (Rs-pel-2, Rs-pel-3, Rs-pel-4, Rs-pel-5). The amplification from genomic DNA and analysing gene expression of pels in the 10 R. similis populations indicated that the gene Rs-pel-3 could be amplified from six populations that parasitised rough lemon (SWK, GJ, FZ, GZ, DBSR, and YJ), but could not be amplified from four populations that did not parasitise rough lemon (XIN, ML, HN6, and HL). Therefore, the gene Rs-pel-3 was absent in the genomes of non-citrus parasitic populations, and the expression of Rs-pel-3 may be related to R. similis parasitism in Citrus spp.. Reportedly, the difference in pel expression in sedentary plant-parasitic nematodes is associated with their host range (Stare et al. 2011; Sabeh et al. 2019; Tian et al. 2019). Stare et al. (2011) reported that the difference in sequence polymorphism and gene copy of the pel-2 gene from Globodera was associated with the variation in the host range of G. rostochiensis, G. pallida, and G. tabacum, while Sabeh et al. (2019) considered that the difference expression of pel-1 was related to the host range of G. rostochiensis, G. pallida, G. tabacum, and G. mexicana. Tian et al. (2019) reported differences in pel expression between tobacco and soybean pathotypes of Heterodera glycines. This study revealed the relationship between the gene Rs-pel-3 and host specialisation of R. similis and confirmed the difference between the two R. similis pathotypes at the molecular level. It is the first time that the difference in pel expression from migratory plant-parasitic nematodes may also be related to their host range.
Moreover, this study confirmed that the gene Rs-pel-3 was stably expressed in eggs, juveniles, females, and males of six populations of R. similis parasitising rough lemon. Therefore, Rs-pel-3 can not only be used as a molecular marker to distinguish citrus from banana pathotypes of R. similis, but also as a target gene to develop molecular identification methods for the quick and accurate identification of R. similis pathotypes.
References
Čepulytė R, Danquah WB, Bruening G, Williamson VM (2018) Potent attractant for root-knot nematodes in exudates from seedling root tips of two host species. Sci Rep 8:10847
Chen LT, Wang HT, Han JL, Luan Y (2019) Research progress and perspective of plant pectin lysase. J South China Agri Univ 40:71–77 (in Chinese)
De Boer JM, McDermott JP, Davis EL, Hussey RS, Popeijus H, Smant G, Baum TJ (2002) Cloning of a putative pectate lyase gene expressed in the subventral esophageal glands of Heterodera glycines. J Nematol 34:9–11
Doyle EA, Lambert KN (2002) Cloning and characterization of an esophageal-gland- specific pectate lyase from the root-knot nematode Meloidogyne javanica. Mol Plant Microbe In 15:549–556
Duncan LW, Moens M (2013) Migratory endoparasitic nematodes. In: Perry RN, Moens M (eds) Plant nematology Ed. 2. CABI, Wallingford, UK, pp 144-178.
European and Mediterranean Plant Protection Organization (2008) Radopholus similis. EPPO Bulletin 3:374–378
Fallas GA, Sarah JL (1994) Effect of storage temperature on the in vitro reproduction of Rahodpholus similis. Nematropica 24:175–177
Feng ZX (2001) Plant nematology. China Agriculture Press, Beijing (in Chinese)
Ferrari S, Savatin DV, Sicilia F, Gramegna G, Cervone F, De Lorenzo G (2013) Oligogalacturonides: plant damage-associated molecular patterns and regulators of growth and development. Front Plant Sci 4:1–9
Filho RM, Martins LSS (2016) In silico comparative analysis of tylenchid nematode pectate lyases. Genet Mol Res 15:1–13
Goo MYC, Sipes BS (1997) Host preference of Radopholus citrophilus from hawaiian Anthurium among selected tropical ornamentals. Hortscience 32:1237–1123
Herron SR, Benen JAE, Scavetta RD, Visser J, Jurnak F (2000) Structure and function of pectic enzymes: virulence factors of plant pathogens. Proc Natl Acad Sci 97:8762–8769
Hu Y, You J, Li C, Williamson VM, Wang C (2017) Ethylene response pathway modulates attractiveness of plant roots to soybean cyst nematode Heterodera glycines. Sci Rep 7:41282
Huang G, Dong R, Allen R, Davis EL, Baum TJ, Hussey RS (2005) Developmental expression and molecular analysis of two Meloidogyne incognita pectate lyase genes. Int J Parasitol 35:685–692
Huang X, Xu CL, Yang SH, Li JY, Wang HL, Zhang ZX, Chen C, Xie H (2019) Life-stage specific transcriptomes of a migratory endoparasitic plant nematode, Radopholus similis elucidate a different parasitic and life strategy of plant parasitic nematodes. Sci Rep 9:1–11
Jones JT, Haegeman A, Danchin EG, Gaur HS, Helder J, Jones MGK, Kikuchi T, Manzanilla-López R, Palomares-Rius JE, Wesemael WML, Perry RN (2013) Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol 14:946–961
Kaplan DT (1994) An assay to estimate citrus rootstock resistance to burrowing nematodes. Proc Fla State Hort Soc 107:85–89
Kaplan DT, Opperman CH (1997) Genome similarity implies that citrus-parasitic burrowing nematodes do not represent a unique species. J Nematol 29:430
Kikuchi T, Shibuya H, Aikawa T, Jones JT (2006) Cloning and characterization of pectate lyases expressed in the esophageal gland of the pine wood nematode Bursaphelenchus xylophilus. Mol Plant Microbe In 19:280–287
Kudla U, Milac AL, Qin L, Overmars H, Roze E, Holterman M, Petrescu AJ, Goverse A, Bakker J, Helder J, Smant G (2007) Structural and functional characterization of a novel, host penetration-related pectate lyase from the potato cyst nematode Globodera rostochiensis. Mol Plant Pathol 8:293–305
Lee DW, Kang JS, Jung CS, Han HR, Moon YS, Park SJ, Lee SH, Koh YH (2013) Identification and biochemical analysis of a novel pectate lyase 3 gene in Bursaphelenchus xylophilus. J Asia-Pac Entomol 16:335–342
Li X, Gu XC, Long HB, Peng H, Huang WK, Peng DL (2017) Identification and expression analysis of a new pectate lyase gene Ha-pel-1 from Heterodera avenae. Sci Agri Sin 50:3723–3732 (in Chinese)
Peng H, Peng DL, Huang WK, He WT, Hu XQ (2012) Molecular cloning and analysis of a novel pectate lyase gene Hg-pel-5 from soybean cyst nematode. Sci Agri Sin 45:854–866 (in Chinese)
Peng H, Cui JK, Long HB, Huang WK, Kong LA, Liu SM, He WT, Hu XQ, Peng DL (2016) Novel pectate lyase genes of Heterodera glycines play key roles in the early stage of parasitism. PloS One 11:1–18
Popeijus H, Overmars H, Jones J, Blok V, Goverse A, Helder J, Schots A, Bakker J, Smant G (2000) Degradation of plant cell walls by a nematode. Nature 406:36–37
Sabeh M, Lord E, Grenier É, St-Arnaud M, Mimee B (2019) What determines host specificity in hyperspecialized plant parasitic nematodes? BMC Genomics 20:1–13
Sasakicrawley A (2013) Signalling and behaviour of Globodera pallida in the rhizosphere of the trap crop Solanum sisymbriifolium. University of Plymouth, Plymouth, England
Shevchik VE, Robert-Baudouy J, Hugouvieux-Cotte-Pattat N (1997) Pectate lyase PelI of Erwinia chrysanthemi 3937 belongs to a new family. J Bacteriol 179:7321–7330
Shevchik VE, Boccara M, Vedel R, Hugouvieux-Cotte-Pattat N (1998) Processing of the pectate lyase PelI by extracellular proteases of Erwinia chrysanthemi 3937. Mol Microbiol 29:1459–1469
Stare BG, Fouville D, Širca S, Gallot A, Urek G, Grenier E (2011) Molecular variability and evolution of the pectate lyase (pel-2) Parasitism gene in cyst nematodes parasitizing different Solanaceous plants. J Mol Evol 72:169–181
Stoffelen R, Jimenez MI, Dierckxsens C, Tam VTT, Swennen R, De WD (1999) Effect of time and inoculum density on the reproductive fitness of Pratylenchus coffeae and Radopholus similis populations on carrot disks. Nematology 7:243–250
Tian ZL, Shi HL, Maria M, Zheng JW (2019) Pectate lyase is a factor in the adaptability for Heterodera glycines infecting tobacco. J Integr Agr 18:618–626
Valette C, Andary C, Geiger JP, Sarah JL, Nicole M (1998) Histochemical and cytochemical investigations of phenols in roots of banana infected by the burrowing nematode Radopholus similis. Phytopathology 88:1141–1148
Vanholme B, Thuyne WVAN, Vanhouteghem K, Meutter JDE, Cannoot B, Gheysen G (2007) Molecular characterization and functional importance of pectate lyase secreted by the cyst nematode Heterodera schachtii. Mol Plant Pathol 8:267–278
Wang C, Bruening G, Williamson VM (2009) Application of Pluronic gel to the study of root-knot nematode behavior. Nematology 11:453–464
Xie H (2006) Detection and epidemic prevention control of Radopholus similis. Plant Quarantine 20:321–324
Xu CL, Zhao CB, Ding S, Zhang JF, Xie H (2016) A modified crude DNA preparation for direct PCR reaction of single plant-parasitic nematodes. Nematology 18:625–628
Zhuo K, Chi YL, Hu LL, Luo M, Liao JL (2011) Cloning and RNA interference analysis of a pectate lyase gene of Meloidogyne enterolobii. Acta Phytopathol Sin 41:473–481
Acknowledgments
HX and CX conceived and designed the research. SY, SY, QL, YL, XH, and CC conducted experiments. SY analyzed data. SY and HX wrote the manuscript. All authors read and approved the manuscript.
Funding
This study was funded by the Special Provincial Project of Rural Revitalization Strategy in Guangdong Province (2022-92) and the Guangdong Basic and Applied Basic Research Foundation (2021A1515011273).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
No specific permissions were required for the nematodes used in this study, and these nematodes were plant pests and not protected by the government.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 186 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, S., Yang, S., Li, Q. et al. Pectate lyase genes from Radopholus similis and their application in pathotype identification. Appl Microbiol Biotechnol 108, 298 (2024). https://doi.org/10.1007/s00253-024-13124-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-024-13124-3