Abstract
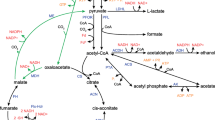
Shewanella oneidensis MR-1 is a potent hydrogen producer in the deficiency of exogenous electron acceptors. The electron transfer pathway for hydrogen production remains unclear, although enzymes for hydrogen production have been identified in S. oneidensis MR-1. In this study, we investigated the electron transfer pathway from formate to hydrogen, given that formate is commonly a key chemical for bacterial hydrogen production. We revealed that two formate dehydrogenases FdhA1B1C1 and FdhA2B2C2, rather than FdnGHI, played a dominant role in formate-driven hydrogen production. Menaquinone was indispensable for the electron transfer from formate to hydrogen, which excluded the presence of formate hydrogen-lyase in S. oneidensis MR-1. A previously proposed formate dehydrogenase subunit HydC was identified as a menaquinone-binding subunit of [FeFe] hydrogenase HydAB, and the hydABC operon is conserved in bacteria living in diverse environments. A formate exporter FocA and transcriptional regulator FhlA were identified for their effect on formate metabolism and hydrogen production. FhlA positively affected the metabolism of formate and hydrogen by regulating the expression of fdhA2B2C2, fdnGHI, focA, and dld-II. Overall, the electron transfer pathway deciphered in this work will facilitate the improvement of biohydrogen production by S. oneidensis MR-1.
Key Points • The electron transfer pathway from formate to hydrogen in MR-1 is deciphered. • Menaquinone is indispensable for hydrogen production. • A cytochrome b subunit transfers electrons from menaquinone to [FeFe] hydrogenase. |








Similar content being viewed by others
References
Bagos PG, Nikolaou EP, Liakopoulos TD, Tsirigos KD (2010) Combined prediction of Tat and Sec signal peptides with hidden Markov models. Bioinformatics 26(22):2811–2817
Beyer L, Doberenz C, Falke D, Hunger D, Suppmann B, Sawers RG (2013) Coordination of FocA and pyruvate formate-lyase synthesis in Escherichia coli demonstrates preferential translocation of formate over other mixed-acid fermentation products. J Bacteriol 195(7):1428–1435
Dehio C, Meyer M (1997) Maintenance of broad-host-range incompatibility group P and group Q plasmids and transposition of Tn5 in Bartonella henselae following conjugal plasmid transfer from Escherichia coli. J Bacteriol 179(2):538–540
Edwards RA, Keller LH, Schifferli DM (1998) Improved allelic exchange vectors and their use to analyze 987P fimbria gene expression. Gene 207(2):149–157
Gorkiewicz G, Feierl G, Zechner R, Zechner EL (2002) Transmission of Campylobacter hyointestinalis from a pig to a human. J Clin Microbiol 40(7):2601–2605
Hong YG, Guo J, Sun GP (2008) Identification of an uptake hydrogenase for hydrogen-dependent dissimilatory azoreduction by Shewanella decolorationis S12. Appl Microbiol Biotechnol 80(3):517–524
Hunt KA, Flynn JM, Naranjo B, Shikhare ID, Gralnick JA (2010) Substrate-level phosphorylation is the primary source of energy conservation during anaerobic respiration of Shewanella oneidensis strain MR-1. J Bacteriol 192(13):3345–3351
Jormakka M, Tornroth S, Byrne B, Iwata S (2002) Molecular basis of proton motive force generation: structure of formate dehydrogenase-N. Science 295(5561):1863–1868
Jormakka M, Byrne B, Iwata S (2003) Formate dehydrogenase--a versatile enzyme in changing environments. Curr Opin Struct Biol 13(4):418–423
Kaakoush NO, Castano-Rodriguez N, Mitchell HM, Man SM (2015) Global epidemiology of Campylobacter infection. Clin Microbiol Rev 28(3):687–720
Kamei K, Hatanaka N, Asakura M, Somroop S, Samosornsuk W, Hinenoya A, Misawa N, Nakagawa S, Yamasaki S (2015) Campylobacter hyointestinalis isolated from pigs produces multiple variants of biologically active cytolethal distending toxin. Infect Immun 83(11):4304–4313
Kane AL, Brutinel ED, Joo H, Maysonet R, VanDrisse CM, Kotloski NJ, Gralnick JA (2016) Formate metabolism in Shewanella oneidensis generates proton motive force and prevents growth without an electron acceptor. J Bacteriol 198(8):1337–1346
Kanehisa M (2000) Post-genome informatics. Oxford University Press, Oxford
Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM 2nd, Peterson KM (1995) Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166(1):175–176
Kreuzer HW, Hill EA, Moran JJ, Bartholomew RA, Yang H, Hegg EL (2014) Contributions of the NiFe -and FeFe -hydrogenase to H2 production in Shewanella oneidensis MR-1 as revealed by isotope ratio analysis of evolved H2. FEMS Microbiol Lett 352(1):18–24
Le Laz S, Kpebe A, Lorquin J, Brugna M, Rousset M (2014) H2-dependent azoreduction by Shewanella oneidensis MR-1: involvement of secreted flavins and both [Ni–Fe] and [Fe–Fe] hydrogenases. Appl Microbiol Biotechnol 98(6):2699–2707
McDowall JS, Murphy BJ, Haumann M, Palmer T, Armstrong FA, Sargent F (2014) Bacterial formate hydrogenlyase complex. Proc Natl Acad Sci U S A 111(38):3948–3956
Meshulam-Simon G, Behrens S, Choo AD, Spormann AM (2007) Hydrogen metabolism in Shewanella oneidensis MR-1. Appl Environ Microbiol 73(4):1153–1165
Myers CR, Nealson KH (1988) Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 240(4857):1319–1321
Nealson KH, Scott J (2006) Ecophysiology of the genus Shewanella. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E (eds) The Prokaryotes. Springer, New York, pp 1133–1151
Nealson KH, Moser DP, Saffarini DA (1995) Anaerobic electron acceptor chemotaxis in Shewanella putrefaciens. Appl Environ Microbiol 61(4):1551–1554
Ng CK, Tan TKC, Song H, Cao B (2013) Reductive formation of palladium nanoparticles by Shewanella oneidensis: role of outer membrane cytochromes and hydrogenases. RSC Adv 3(44):22498–22503
Nicolet Y, Piras C, Legrand P, Hatchikian CE, Fontecilla-Camps JC (1999) Desulfovibrio desulfuricans iron hydrogenase: the structure shows unusual coordination to an active site Fe binuclear center. Structure 7:13–13
Pinchuk GE, Geydebrekht OV, Hill EA, Reed JL, Konopka AE, Beliaev AS, Fredrickson JK (2011) Pyruvate and lactate metabolism by Shewanella oneidensis MR-1 under fermentation, oxygen limitation, and fumarate respiration conditions. Appl Environ Microbiol 77(23):8234–8240
Rossmann R, Sawers G, Bock A (1991) Mechanism of regulation of the formate-hydrogenlyase pathway by oxygen, nitrate, and pH: definition of the formate regulon. Mol Microbiol 5(11):2807–2814
Schlensog V, Lutz S, Bock A (1994) Purification and DNA-binding properties of FHLA, the transcriptional activator of the formate hydrogenlyase system from Escherichia coli. J Biol Chem 269(30):19590–19596
Self WT, Hasona A, Shanmugam KT (2001) N-terminal truncations in the FhlA protein result in formate- and MoeA-independent expression of the hyc (formate hydrogenlyase) operon of Escherichia coli. Microbiology 147(11):3093–3104
Shi L, Belchik SM, Plymale AE, Heald S, Dohnalkova AC, Sybirna K, Bottin H, Squier TC, Zachara JM, Fredrickson JK (2011) Purification and characterization of the [NiFe]-hydrogenase of Shewanella oneidensis MR-1. Appl Environ Microbiol 77(16):5584–5590
Sieber JR, Le HM, McInerney MJ (2014) The importance of hydrogen and formate transfer for syntrophic fatty, aromatic and alicyclic metabolism. Environ Microbiol 16(1):177–188
Skibinski DA, Golby P, Chang YS, Sargent F, Hoffman R, Harper R, Guest JR, Attwood MM, Berks BC, Andrews SC (2002) Regulation of the hydrogenase-4 operon of Escherichia coli by the sigma(54)-dependent transcriptional activators FhlA and HyfR. J Bacteriol 184(23):6642–6653
Stams AJM, de Bok FAM, Plugge CM, van Eekert MHA, Dolfing J, Schraa G (2006) Exocellular electron transfer in anaerobic microbial communities. Environ Microbiol 8(3):371–382
Suppmann B, Sawers G (1994) Isolation and characterization of hypophosphite--resistant mutants of Escherichia coli: identification of the FocA protein, encoded by the pfl operon, as a putative formate transporter. Mol Microbiol 11(5):965–982
Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, Lepore R, Schwede T (2018) SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46(1):296–303
Wu C, Cheng YY, Yin H, Song XN, Li WW, Zhou XX, Zhao LP, Tian LJ, Han JC, Yu HQ (2013) Oxygen promotes biofilm formation of Shewanella putrefaciens CN32 through a diguanylate cyclase and an adhesin. Sci Rep 3:1945
Funding
This work was supported by the National Natural Science of Foundation of China (31670126).
Author information
Authors and Affiliations
Contributions
CW and JX conceived of and designed study and analyzed data. JX, DC, FC, and MC performed most of experiments. QW and XS helped to analyze chemicals. XG performed sequence analysis. CW and XG wrote the paper. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 396 kb)
Rights and permissions
About this article
Cite this article
Xiong, J., Chan, D., Guo, X. et al. Hydrogen production driven by formate oxidation in Shewanella oneidensis MR-1. Appl Microbiol Biotechnol 104, 5579–5591 (2020). https://doi.org/10.1007/s00253-020-10608-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10608-w