Abstract
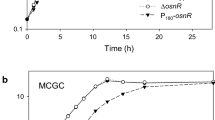
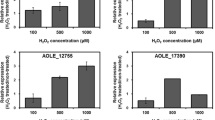
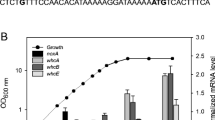
The gene whcE in Corynebacterium glutamicum positively responds to oxidative and heat stress. To search for proteins that interact with WhcE, we employed a two-hybrid system with WhcE as the bait. Sequencing analysis of the isolated clones revealed peptide sequences, one of which showed high sequence identity to a hydrophobe/amphiphile efflux-1 family transporter encoded by NCgl1497. The interaction of the NCgl1497-encoded protein with WhcE in vivo was verified using reporter gene expression by real-time quantitative PCR (RT-qPCR). The WhcE protein strongly interacted with the NCgl1497-encoded protein in the presence of oxidative and heat stress. Furthermore, purified WhcE and NCgl1497-encoded proteins interacted in vitro, especially in the presence of the oxidant diamide, and the protein–protein interaction was disrupted in the presence of the reductant dithiothreitol. In addition, the transcription of NCgl1497 was activated approximately twofold in diamide- or heat-treated cells. To elucidate the function of the NCgl497 gene, an NCgl1497-deleted mutant strain was constructed. The mutant showed decreased viability in the presence of diamide and heat stress. The mutant strain also exhibited reduced transcription of the thioredoxin reductase gene, which is known to be regulated by whcE. Based on the results, NCgl1497 was named spiE (stress protein interacting with WhcE). Collectively, our data suggest that spiE is involved in the whcE-mediated oxidative stress response pathway of C. glutamicum.






Similar content being viewed by others
References
Chater KF, Chandra G (2006) The evolution of development in Streptomyces analysed by genome comparisons. FEMS Microbiol Rev 30:651–672
Choi WW, Park SD, Lee SM, Kim HB, Kim Y, Lee HS (2009) The whcA gene plays a negative role in oxidative stress response of Corynebacterium glutamicum. FEMS Microbiol Lett 290:32–38
Crack JC, Munnoch J, Dodd EL, Knowles F, Al Bassam MM, Kamali S, Holland AA, Cramer SP, Hamilton CJ, Johnson MK, Thomson AJ, Hutchings MI, Le Brun NE (2015) NsrR from Streptomyces coelicolor is a nitric oxide-sensing [4Fe-4S] cluster protein with a specialized regulatory function. J Biol Chem 290:12689–12704
Crack JC, Smith LJ, Stapleton MR, Peck J, Watmough NJ, Buttner MJ, Buxton RS, Green J, Oganesyan VS, Thomson AJ (2010) Mechanistic insight into the nitrosylation of the [4Fe–4S] cluster of WhiB-like proteins. J Am Chem Soc 133:1112–1121
Eggeling L, Bott M (2015) A giant market and a powerful metabolism: L-lysine provided by Corynebacterium glutamicum. Appl Microbiol Biotechnol 99:3387–3394
Follettie MT, Sinskey AJ (1986) Recombinant DNA technology for Corynebacterium glutamicum. Food Technol 40:88–94
Follettie MT, Peoples OP, Agoropoulou C, Sinskey AJ (1993) Gene structure and expression of the Corynebacterium flavum N13 ask-asd operon. J Bacteriol 175:4096–4103
Gao B, Gupta RS (2012) Phylogenetic framework and molecular signatures for the main clades of the phylum Actinobacteria. Microbiol Mol Biol Rev 76:66–112
Green J, Paget MS (2004) Bacterial redox sensors. Nature Rev Microbiol 2:954–966
Hassan HM, Fridovich I (1979) Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch Biochem Biophys 196:385–395
den Hengst CD, Buttner MJ (2008) Redox control in Actinobacteria. Biochimica et Biophysica Acta (BBA)-General Sub 1780:1201–1216
Hwang BJ, Yeom HJ, Kim Y, Lee HS (2002) Corynebacterium glutamicum utilizes both transsulfuration and direct sulfhydrylation pathways for methionine biosynthesis. J Bacteriol 184:1277–1286
Jakimowicz P, Cheesman MR, Bishai WR, Chater KF, Thomson AJ, Buttner MJ (2005) Evidence that the Streptomyces developmental protein WhiD, a member of the WhiB family, binds a [4Fe-4S] cluster. J Biol Chem 280:8309–8315
Kim TH, Park JS, Kim HJ, Kim Y, Kim P, Lee HS (2005) The whcE gene of Corynebacterium glutamicum is important for survival following heat and oxidative stress. Biochem Biophys Res Commun 337:757–764
Kosower NS, Kosower EM (1995) Diamide: an oxidant probe for thiols. Methods Enzymol 251:123–133
Lee JY, Kim HJ, Kim ES, Kim P, Kim Y, Lee HS (2013) Regulatory interaction of the Corynebacterium glutamicum whc genes in oxidative stress responses. J Biotechnol 168:149–154
Lee JY, Park JS, Kim HJ, Kim Y, Lee HS (2012) Corynebacterium glutamicum whcB, a stationary phase-specific regulatory gene. FEMS Microbiol Lett 327:103–109
Ma D, Cook DN, Alberti M, Pon NG, Nikaido H, Hearst JE (1995) Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol Microbiol 16:45–55
MacNeil DJ, Gewain KM, Ruby CL, Dezeny G, Gibbons PH, MacNeil T (1992) Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene 111:61–68
Nikaido E, Shirosaka I, Yamaguchi A, Nishino K (2011) Regulation of the AcrAB multidrug efflux pump in Salmonella enterica serovar typhimurium in response to indole and paraquat. Microbiol 157:648–655
von der Osten CH, Gioannetti C, Sinskey AJ (1989) Design of a defined medium for growth of Corynebacterium glutamicum in which citrate facilitates iron uptake. Biotechnol Lett 11:11–16
Park JC, Kim Y, Lee HS (2015) Involvement of the NADH oxidase-encoding noxA gene in oxidative stress responses in Corynebacterium glutamicum. Appl Microbiol Biotechnol 99:1363–1374
Park JS, Lee JY, Kim HJ, Kim ES, Kim P, Kim Y, Lee HS (2012) The role of Corynebacterium glutamicum spiA gene in whcA-mediated oxidative stress gene regulation. FEMS Microbiol Lett 331:63–69
Park JS, Shin S, Kim ES, Kim P, Kim Y, Lee HS (2011) Identification of SpiA that interacts with Corynebacterium glutamicum WhcA using a two-hybrid system. FEMS Microbiol Lett 322:8–14
Park SD, Lee SN, Park IH, Choi JS, Jeong WK, Kim Y, Lee HS (2004) Isolation and characterization of transcriptional elements from Corynebacterium glutamicum. J Microbiol Biotechnol 14:789–795
Park SD, Youn JW, Kim YJ, Lee SM, Kim Y, Lee HS (2008) Corynebacterium glutamicum σE is involved in responses to cell surface stresses and its activity is controlled by the anti-σ factor CseE. Microbiol 154:915–923
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. Cold Spring Harbor Laboratory Press New York
Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73
Smith L, Stapleton M, Fullstone G, Crack J, Thomson A, Le Brun N, Hunt D, Harvey E, Adinolfi S, Buxton R (2010) Mycobacterium tuberculosis WhiB1 is an essential DNA-binding protein with a nitric oxide-sensitive iron-sulfur cluster. Biochem J 432:417–427
Yoshihama M, Higashiro K, Rao EA, Akedo M, Shanabruch WG, Follettie MT, Walker GC, Sinskey AJ (1985) Cloning vector system for Corynebacterium glutamicum. J Bacteriol 162:591–597
Zheng F, Long Q, Xie J (2012) The function and regulatory network of WhiB and WhiB-like protein from comparative genomics and systems biology perspectives. Cell Biochem Biophys 63:103–108
Acknowledgments
This work was supported by a Korea University Grant to H.-S. Lee.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 152 kb)
Rights and permissions
About this article
Cite this article
Park, J.C., Park, JS., Kim, Y. et al. SpiE interacts with Corynebacterium glutamicum WhcE and is involved in heat and oxidative stress responses. Appl Microbiol Biotechnol 100, 4063–4072 (2016). https://doi.org/10.1007/s00253-016-7440-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7440-9