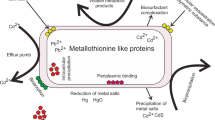
Abstract
Extremely acidophilic microorganisms have an optimal pH of <3 and are found in all three domains of life. As metals are more soluble at acid pH, acidophiles are often challenged by very high metal concentrations. Acidophiles are metal-tolerant by both intrinsic, passive mechanisms as well as active systems. Passive mechanisms include an internal positive membrane potential that creates a chemiosmotic gradient against which metal cations must move, as well as the formation of metal sulfate complexes reducing the concentration of the free metal ion. Active systems include efflux proteins that pump metals out of the cytoplasm and conversion of the metal to a less toxic form. Acidophiles are exploited in a number of biotechnologies including biomining for sulfide mineral dissolution, biosulfidogenesis to produce sulfide that can selectively precipitate metals from process streams, treatment of acid mine drainage, and bioremediation of acidic metal-contaminated milieux. This review describes how acidophilic microorganisms tolerate extremely high metal concentrations in biotechnological processes and identifies areas of future work that hold promise for improving the efficiency of these applications.


Similar content being viewed by others
References
Acuna LG, Cardenas JP, Covarrubias PC, Haristoy JJ, Flores R, Nunez H, Riadi G, Shmaryahu A, Valdes J, Dopson M, Rawlings DE, Banfield JF, Holmes DS, Quatrini R (2013) Architecture and gene repertoire of the flexible genome of the extreme acidophile Acidithiobacillus caldus. PLoS One 8:e78237
Amaral-Zettler LA, Zettler ER, Theroux SM, Palacios C, Aguilera A, Amils R (2011) Microbial community structure across the tree of life in the extreme Rio Tinto. ISME J 5:42–50
Arenas-Salinas M, Ortega S, Gonzales-Nilo D, Pohl E, Holmes DS, Quatrini R (2014) AFAL: a web service for profiling amino acids surrounding ligands in proteins Submitted
Auernik KS, Maezato Y, Blum PH, Kelly RM (2008) The genome sequence of the metal-mobilizing, extremely thermoacidophilic archaeon Metallosphaera sedula provides insights into bioleaching-associated metabolism. Appl Environ Microbiol 74:682–692
Baillet F, Magnin JP, Cheruy A, Ozil P (1998) Chromium precipitation by the acidophilic bacterium Thiobacillus ferrooxidans. Biotechnol Lett 20:95–99
Baker-Austin C, Dopson M (2007) Life in acid: pH homeostasis in acidophiles. Trends Microbiol 15:165–171
Baker-Austin C, Dopson M, Wexler M, Sawers G, Bond PL (2005) Molecular insight into extreme copper resistance in the extremophilic archaeon ‘Ferroplasma acidarmanus’ Fer1. Microbiology 151:2637–2646
Baker-Austin C, Dopson M, Wexler M, Sawers RG, Stemmler A, Rosen BP, Bond PL (2007) Extreme arsenic resistance by the acidophilic archaeon ‘Ferroplasma acidarmanus’ Fer1. Extremophiles 11:425–434
Bijmans MFM, van Helvoort PJ, Dar SA, Dopson M, Lens PNL, Buisman CJN (2009) Selective recovery of nickel over iron from a nickel-iron solution using microbial sulfate reduction in a gas-lift bioreactor. Water Res 43:853–861
Brierley CL, Brierley JA (2013) Progress in bioleaching: part B: applications of microbial processes by the minerals industries. Appl Microbiol Biotechnol 97:7543–7552
Bruins MR, Kapil S, Oehme FW (2000) Microbial resistance to metals in the environment. Ecotoxicol Environ Saf 45:198–207
Castagnetto JM, Hennessy SW, Roberts VA, Getzoff ED, Tainer JA, Pique ME (2002) MDB: the metalloprotein database and browser at The Scripps Research Institute. Nucleic Acids Res 30:379–382
Chakravarty R, Banerjee PC (2008) Morphological changes in an acidophilic bacterium induced by heavy metals. Extremophiles 12:279–284
Chi A, Valenzuela L, Beard S, Mackey AJ, Shabanowitz J, Hunt DF, Jerez CA (2007) Periplasmic proteins of the extremophile Acidithiobacillus ferrooxidans: a high throughput proteomics analysis. Mol Cell Proteomics 6:2239–2251
Cummings DE, Fendorf S, Singh NK, Sani RK, Peyton BM, Magnuson TS (2006) Reduction of Cr(VI) under acidic conditions by the facultative Fe(III)-reducing bacterium Acidiphilium cryptum. Environ Sci Technol 41:146–152
Dai Z, Guo X, Yin H, Liang Y, Cong J, Liu X (2014) Identification of nitrogen-fixing genes and gene clusters from metagenomic library of acid mine drainage. PLoS One 9:e87976
Dash HR, Das S (2012) Bioremediation of mercury and the importance of bacterial mer genes. Int Biodeterior Biodegrad 75:207–213
Degtyarenko KN, North ACT, Findlay JBC (1999) PROMISE: a database of bioinorganic motifs. Nucleic Acids Res 27:233–236
Demergasso C, Galleguillos F, Soto P, Serón M, Iturriaga V (2010) Microbial succession during a heap bioleaching cycle of low grade copper sulfides: does this knowledge mean a real input for industrial process design and control? Hydrometallurgy 104:382–390
Di Toro DM, Allen HE, Bergman HL, Meyer JS, Paquin PR, Santore RC (2001) Biotic ligand model of the acute toxicity of metals. 1. Technical basis. Environ Toxicol Chem 20:2383–2396
Diels L, Van Roy S, Taghavi S, Van Houdt R (2009) From industrial sites to environmental applications with Cupriavidus metallidurans. Antonie Van Leeuwenhoek 96:247–258
Dispiroto AA, Talnagi JW, Tuovinen OH (1983) Accumulation and cellular-distribution of uranium in Thiobacillus ferrooxidans. Arch Microbiol 135:250–253
Dopson M, Johnson DB (2012) Biodiversity, metabolism and applications of acidophilic sulfur- metabolizing micro-organisms. Environ Microbiol 14:2620–2631
Dopson M, Lindström EB (2004) Analysis of community composition during moderately thermophilic bioleaching of pyrite, arsenical pyrite and chalcopyrite. Microb Ecol 48:19–28
Dopson M, Lindström EB, Hallberg KB (2001) Chromosomally encoded arsenical resistance of the moderately thermophilic acidophile Acidithiobacillus caldus. Extremophiles 5:247–255
Dopson M, Baker-Austin C, Koppineedi PR, Bond PL (2003) Growth in sulfidic mineral environments: metal resistance mechanisms in acidophilic micro-organisms. Microbiology 149:1959–1970
Dopson M, Baker-Austin C, Bond PL (2005) Analysis of differential protein expression during growth states of Ferroplasma strains and insights into electron transport for iron oxidation. Microbiology 151:4127–4137
Dopson M, Ossandon F, Lövgren L, Holmes DS (2014) Metal resistance or tolerance? Acidophiles confront high metal loads via both abiotic and biotic mechanisms. Front Microbiol 5: doi: 10.3389/fmicb.2014.00157
Drewniak L, Sklodowska A (2013) Arsenic-transforming microbes and their role in biomining processes. Environ Sci Pollut Res 20:7728–7739
Goltsman DS, Denef VJ, Singer SW, VerBerkmoes NC, Lefsrud M, Mueller RS, Dick GJ, Sun CL, Wheeler KE, Zemla A, Baker BJ, Hauser L, Land M, Shah MB, Thelen MP, Hettich RL, Banfield JF (2009) Community genomic and proteomic analyses of chemoautotrophic iron-oxidizing “Leptospirillum rubarum” (Group II) and “Leptospirillum ferrodiazotrophum” (Group III) bacteria in acid mine drainage biofilms. Appl Environ Microbiol 75:4599–4615
Gonzalez-Contreras P, Weijma J, van der Weijden R, Buisman CJ (2010) Biogenic scorodite crystallization by Acidianus sulfidivorans for arsenic removal. Environ Sci Technol 44:675–680
Gonzalez-Contreras P, Weijma J, Buisman CJ (2012) Continuous bioscorodite crystallization in CSTRs for arsenic removal and disposal. Water Res 46:5883–5892
Gonzalez-Pastor JE, Mirete S (2010) Novel metal resistance genes from microorganisms: a functional metagenomic approach. Methods Mol Biol 668:273–285
Guo X, Yin H, Cong J, Dai Z, Liang Y, Liu X (2013) RubisCO gene clusters found in a metagenome microarray from acid mine drainage. Appl Environ Microbiol 79:2019–2026
Halinen A-K, Beecroft NJ, Määttä K, Nurmi P, Laukkanen K, Kaksonen AH, Riekkola-Vanhanen M, Puhakka JA (2012) Microbial community dynamics during a demonstration-scale bioheap leaching operation. Hydrometallurgy 125–126:34–41
Hallberg KB, Dopson M, Lindstrom EB (1996) Arsenic toxicity is not due to a direct effect on the oxidation of reduced inorganic sulfur compounds by Thiobacillus caldus. FEMS Microbiol Lett 145:409–414
Hallberg KB, Grail BM, du Plessis C, Johnson DB (2011) Reductive dissolution of ferric iron minerals: a new approach for bioprocessing nickel laterites. Miner Eng 24:620–624
Hao OJ, Chen JM, Huang L, Buglass RL (1996) Sulfate-reducing bacteria. Crit Rev Environ Sci Tech 26:155–187
Harrison JJ, Ceri H, Turner RJ (2007) Multimetal resistance and tolerance in microbial biofilms. Nat Rev Microbiol 5:928–938
Heijerick DG, De Schamphelaere KA, Janssen CR (2002) Biotic ligand model development predicting Zn toxicity to the alga Pseudokirchneriella subcapitata: possibilities and limitations. Comp Biochem Physiol C Toxicol Pharmacol 133:207–218
Imlay JA (2003) Pathways of oxidative damage. Annu Rev Microbiol 57:395–418
Jameson E, Rowe OF, Hallberg KB, Johnson DB (2010) Sulfidogenesis and selective precipitation of metals at low pH mediated by Acidithiobacillus spp. and acidophilic sulfate-reducing bacteria. Hydrometallurgy 104:488–493
Jones DS, Albrecht HL, Dawson KS, Schaperdoth I, Freeman KH, Pi Y, Pearson A, Macalady JL (2012) Community genomic analysis of an extremely acidophilic sulfur-oxidizing biofilm. ISME J 6:158–170
Kondrashov FA (2012) Gene duplication as a mechanism of genomic adaptation to a changing environment. Proc Biol Sci R Soc 279:5048–5057
Koschorreck M (2008) Microbial sulphate reduction at a low pH. FEMS Microbiol Ecol 64:329–342
Kotze AA, Tuffin IM, Deane SM, Rawlings DE (2006) Cloning and characterization of the chromosomal arsenic resistance genes from Acidithiobacillus caldus and enhanced arsenic resistance on conjugal transfer of ars genes located on transposon TnAtcArs. Microbiology 152:3551–3560
Li B, Lin J, Mi S, Lin J (2010) Arsenic resistance operon structure in Leptospirillum ferriphilum and proteomic response to arsenic stress. Bioresour Technol 101:9811–9814
Liljeqvist M, Ossandon F, Gonzalez C, Rajan S, Stell A, Valdes J, Holmes DS, Dopson M (2014) Metagenomic analysis reveals adaptations to a cold adapted lifestyle in a low temperature acid mine drainage stream. Manuscript
Liu XY, Chen BW, Wen JK (2008) Dominance of Acidithiobacillus at ore surface of Zijinshan commercial low-grade copper bioleaching heap. Trans Nonferrous Metals Soc China 18:1506–1512
Macomber L, Hausinger RP (2011) Mechanisms of nickel toxicity in microorganisms. Metallomics 3:1153–1162
Maezato Y, Johnson T, McCarthy S, Dana K, Blum P (2012) Metal resistance and lithoautotrophy in the extreme thermoacidophile Metallosphaera sedula. J Bacteriol 194:6856–6863
Magnuson TS, Swenson MW, Paszczynski AJ, Deobald LA, Kerk D, Cummings DE (2010) Proteogenomic and functional analysis of chromate reduction in Acidiphilium cryptum JF-5, an Fe(III)-respiring acidophile. Biometals 23:1129–1138
Mangold S (2012) Growth and survival of Acidithiobacilli in acidic, metal rich environments. PhD (Sweden, Umeå)
Mangold S, Potrykus J, Björn E, Lövgren L, Dopson M (2012) Extreme zinc tolerance in acidophilic microorganisms from the bacterial and archaeal domains. Extremophiles 17:75–85
Mangold S, Potrykus J, Björn E, Lövgren L, Dopson M (2013) Extreme zinc tolerance in acidophilic microorganisms from the bacterial and archaeal domains. Extremophiles 17:75–85
Martin PAW, Dugan PR, Tuovinen OH (1983) Uranium resistance of Thiobacillus ferrooxidans. Eur J Appl Microbiol Biotechnol 18:392–395
Mathema VB, Thakuri BC, Sillanpaa M (2011) Bacterial mer operon-mediated detoxification of mercurial compounds: a short review. Arch Microbiol 193:837–844
Mi S, Song J, Lin J, Che Y, Zheng H, Lin J (2011) Complete genome of Leptospirillum ferriphilum ML-04 provides insight into its physiology and environmental adaptation. J Microbiol 49:890–901
Mykytczuk NCS, Trevors JT, Ferroni GD, Leduc LG (2011) Cytoplasmic membrane response to copper and nickel in Acidithiobacillus ferrooxidans. Microbiol Res 166:186–206
Nancucheo I, Johnson DB (2010) Production of glycolic acid by chemolithotrophic iron- and sulfur-oxidizing bacteria and its role in delineating and sustaining acidophilic sulfide mineral-oxidizing consortia. Appl Environ Microbiol 76:461–467
Narayani M, Shetty KV (2013) Chromium-resistant bacteria and their environmental condition for hexavalent chromium removal: a review. Crit Rev Environ Sci Technol 43:955–1009
Navarro CA, Orellana LH, Mauriaca C, Jerez CA (2009) Transcriptional and functional studies of Acidithiobacillus ferrooxidans genes related to survival in the presence of copper. Appl Environ Microbiol 75:6102–6109
Navarro CA, von Bernath D, Jerez CA (2013) Heavy metal resistance strategies of acidophilic bacteria and their acquisition: importance for biomining and bioremediation. Biol Res 46:363–371
Nordstrom DK, Alpers CN, Ptacek CJ, Blowes DW (2000) Negative pH and extremely acidic mine waters from Iron Mountain, California. Environ Sci Technol 34:254–258
Okibe N, Gericke M, Hallberg KB, Johnson DB (2003) Enumeration and characterization of acidophilic microorganisms isolated from a pilot plant stirred-tank bioleaching operation. Appl Environ Microbiol 69:1936–1943
Orell A, Navarro CA, Arancibia R, Mobarec JC, Jerez CA (2010) Life in blue: copper resistance mechanisms of bacteria and Archaea used in industrial biomining of minerals. Biotechnol Adv 28:839–848
Orell A, Navarro CA, Rivero M, Aguilar JS, Jerez CA (2012) Inorganic polyphosphates in extremophiles and their possible functions. Extremophiles 16:573–583
Orellana LH, Jerez CA (2011) A genomic island provides Acidithiobacillus ferrooxidans ATCC 53993 additional copper resistance: a possible competitive advantage. Appl Microbiol Biotechnol 92:761–767
Osorio H, Martinez V, Nieto PA, Holmes DS, Quatrini R (2008) Microbial iron management mechanisms in extremely acidic environments: comparative genomics evidence for diversity and versatility. BMC Microbiology 8:doi:10.1186/1471-2180-1188-1203
Paez-Espino D, Tamames J, de Lorenzo V, Canovas D (2009) Microbial responses to environmental arsenic. Biometals 22:117–130
Pal C, Bengtsson-Palme J, Rensing C, Kristiansson E, Larsson DGJ (2014) BacMet: antibacterial biocide and metal resistance genes database. Nucleic Acids Res 42:D737–D743
Potrykus J, Rao Jonna V, Dopson M (2011) Iron homeostasis and responses to iron limitation in extreme acidophiles from the Ferroplasma genus. Proteomics 11:52–63
Quatrini R, Lefimil C, Veloso FA, Pedroso I, Holmes DS, Jedlicki E (2007) Bioinformatic prediction and experimental verification of Fur-regulated genes in the extreme acidophile Acidithiobacillus ferrooxidans. Nucleic Acids Res 35:2153–2166
Rawlings DE, Johnson DB (2007) The microbiology of biomining: development and optimization of mineral-oxidizing microbial consortia. Microbiology 153:315–324
Rawlings DE, Tributsch H, Hansford GS (1999) Reasons why “Leptospirillum”-like species rather than Thiobacillus ferrooxidans are the dominant iron-oxidizing bacteria in many commercial processes for the biooxidation of pyrite and related ores. Microbiology 145:5–13
Remonsellez F, Orell A, Jerez CA (2006) Copper tolerance of the thermoacidophilic archaeon Sulfolobus metallicus: possible role of polyphosphate metabolism. Microbiology 152:59–66
Rhee SK, Liu X, Wu L, Chong SC, Wan X, Zhou J (2004) Detection of genes involved in biodegradation and biotransformation in microbial communities by using 50-mer oligonucleotide microarrays. Appl Environ Microbiol 70:4303–4317
Riekkola-Vanhanen M (2013) Talvivaara mining company—from a project to a mine. Miner Eng 48:2–9
Rzhepishevska OI, Lindström EB, Tuovinen OH, Dopson M (2005) Bioleaching of sulfidic tailing samples with a novel, vacuum-positive pressure driven bioreactor. Biotechnol Bioeng 92:559–567
San Martin-Uriz P, Mirete S, Alcolea PJ, Gomez MJ, Amils R, Gonzalez-Pastor JE (2014) Nickel-resistance determinants in Acidiphilium sp. PM identified by genome-wide functional screening. PLoS ONE 9:e95041
Schelert J, Dixit V, Hoang V, Simbahan J, Drozda M, Blum P (2004) Occurrence and characterization of mercury resistance in the hyperthermophilic archaeon Sulfolobus solfataricus by use of gene disruption. J Bacteriol 186:427–437
Schelert J, Rudrappa D, Johnson T, Blum P (2013) Role of MerH in mercury resistance in the archaeon Sulfolobus solfataricus. Microbiology 159:1198–1208
Schönknecht G, Chen W-H, Ternes CM, Barbier GG, Shrestha RP, Stanke M, Bräutigam A, Baker BJ, Banfield JF, Garavito RM, Carr K, Wilkerson C, Rensing SA, Gagneul D, Dickenson NE, Oesterhelt C, Lercher MJ, Weber APM (2013) Gene transfer from bacteria and archaea facilitated evolution of an extremophilic eukaryote. Science 339:1207–1210
Slonczewski JL, Fujisawa M, Dopson M, Krulwich TA (2009) Cytoplasmic pH measurement and homeostasis in bacteria and archaea. Adv Microb Physiol 55:1–79
Takeuchi F, Iwahori K, Kamimura K, Negishi A, Maeda T, Sugio T (2001) Volatilization of mercury under acidic conditions from mercury-polluted soil by a mercury-resistant Acidithiobacillus ferrooxidans SUG 2-2. Biosci Biotechnol Biochem 65:1981–1986
Teitzel GM, Parsek MR (2003) Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa. Appl Environ Microbiol 69:2313–2320
Tian J, Wu N, Li J, Liu Y, Guo J, Yao B, Fan Y (2007) Nickel-resistant determinant from Leptospirillum ferriphilum. Appl Environ Microbiol 73:2364–2368
Tuffin M, de Groot P, Deane SM, Rawlings DE (2004) Multiple sets of arsenic resistance genes are present within highly arsenic-resistant industrial strains of the biomining bacterium, Acidithiobacillus caldus. 3rd International Conference of Comparative Physiology and Biochemistry, eds Morris S, Vosloo A, pp 165-172
Tuffin IM, de Groot P, Deane SM, Rawlings DE (2005) An unusual Tn21-like transposon containing an ars operon is present in highly arsenic-resistant strains of the biomining bacterium Acidithiobacillus caldus. Microbiology 151:3027–3039
Tuovinen OH, Bhatti TM (1999) Microbiological leaching of uranium ores. Min Metall Proc 16:51–60
Tyson GW, Chapman J, Hugenholtz P, Allen EE, Ram RJ, Richardson PM, Solovyev VV, Rubin EM, Rokhsar DS, Banfield JF (2004) Community structure and metabolism through reconstruction of microbial genomes from the environment. Nature 428:37–43
Valdes J, Holmes DS (2009) Genomic lessons from biomining organisms: case study of the Acidithiobacillus genus. Adv Mat Res 71–73:215–218
Valdes J, Pedroso I, Quatrini R, Dodson RJ, Tettelin H, Blake R, Eisen JA, Holmes DS (2008) Acidithiobacillus ferrooxidans metabolism: from genome sequence to industrial applications. BMC Genomics 9:597
Valdes J, Quatrini R, Hallberg K, Dopson M, Valenzuela PD, Holmes DS (2009) Draft genome sequence of the extremely acidophilic bacterium Acidithiobacillus caldus ATCC 51756 reveals metabolic versatility in the genus Acidithiobacillus. J Bacteriol 191:5877–5878
Vera M, Guiliani N, Jerez CA (2003) Proteomic and genomic analysis of the phosphate starvation response of Acidithiobacillus ferrooxidans. Hydrometallurgy 71:125–132
Vera M, Schippers A, Sand W (2013) Progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfide oxidation—part A. Appl Microbiol Biotechnol 97:7529–7541
Villafane A, Voskoboynik Y, Ruhl I, Sannino D, Maezato Y, Blum P, Bini E (2011) CopR of Sulfolobus solfataricus represents a novel class of archaeal-specific copper-responsive activators of transcription. Microbiology:2808-2817
Vollmecke C, Drees SL, Reimann J, Albers SV, Lubben M (2012) Both ATPases CopA and CopB contribute to copper resistance of the thermoacidophilic archaeon Sulfolobus solfataricus. Microbiology 158:1622–1633
von Rozycki T, Nies D (2009) Cupriavidus metallidurans: evolution of a metal-resistant bacterium. Antonie Van Leeuwenhoek 96:115–139
Wang Y, Zeng W, Qiu G, Chen X, Zhou H (2014) A moderately thermophilic mixed microbial culture for bioleaching of chalcopyrite concentrate at high pulp density. Appl Environ Microbiol 80:741–750
Wu X, Wong ZL, Sten P, Engblom S, Österholm P, Dopson M (2013) Microbial community potentially responsible for acid and metal release from an Ostrobothnian acid sulfate soil. FEMS Microbiol Ecol 84:555–563
Xia L, Yin C, Cai L, Qiu G, Qin W, Peng B, Liu J (2010) Metabolic changes of Acidithiobacillus caldus under Cu2+ stress. J Basic Microbiol 50:591–598
Acknowledgments
MD would like to thank Cost Action CM0902 for funding research included in this review, and DH would like to thank Fondecyt 1130683.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dopson, M., Holmes, D.S. Metal resistance in acidophilic microorganisms and its significance for biotechnologies. Appl Microbiol Biotechnol 98, 8133–8144 (2014). https://doi.org/10.1007/s00253-014-5982-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5982-2