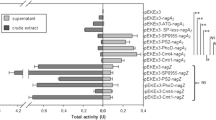
Abstract
Corynebacterium glutamicum grows with a variety of carbohydrates and carbohydrate derivatives as sole carbon sources; however, growth with glucosamine has not yet been reported. We isolated a spontaneous mutant (M4) which is able to grow as fast with glucosamine as with glucose as sole carbon source. Glucosamine also served as a combined source of carbon, energy and nitrogen for the mutant strain. Characterisation of the M4 mutant revealed a significantly increased expression of the nagB gene encoding the glucosamine-6P deaminase NagB involved in degradation of glucosamine, as a consequence of a single mutation in the promoter region of the nagAB-scrB operon. Ectopic nagB overexpression verified that the activity of the NagB enzyme is in fact the growth limiting factor under these conditions. In addition, glucosamine uptake was studied, which proved to be unchanged in the wild-type and M4 mutant strains. Using specific deletion strains, we identified the PTSGlc transport system to be responsible for glucosamine uptake in C. glutamicum. The affinity of this uptake system for glucosamine was about 40-fold lower than that for its major substrate glucose. Because of this difference in affinity, glucosamine is efficiently taken up only if external glucose is absent or present at low concentrations. C. glutamicum was also examined for its suitability to use glucosamine as substrate for biotechnological purposes. Upon overexpression of the nagB gene in suitable C. glutamicum producer strains, efficient production of both the amino acid l-lysine and the diamine putrescine from glucosamine was demonstrated.





Similar content being viewed by others
References
Alvarez-Anorve LI, Calcagno ML, Plumbridge J (2005) Why does Escherichia coli grow more slowly on glucosamine than on N-acetylglucosamine? Effects of enzyme levels and allosteric activation of GlcN6P deaminase (NagB) on growth rates. J Bacteriol 187:2974–2982
Alvarez-Anorve LI, Bustos-Jaimes I, Calcagno ML, Plumbridge J (2009) Allosteric regulation of glucosamine-6-phosphate deaminase (NagB) and growth of Escherichia coli on glucosamine. J Bacteriol 191:6401–6407
Appuhn A, Joergensen RG, Raubuch M, Scheller E, Wilke B (2004) The automated determination of glucosamine, galactosamine, muramic acid, and mannosamine in soil and root hydrolysates by HPLC. J Plan Nutr Soil Sci 167:17–21
Arndt A, Auchter M, Ishige T, Wendisch VF, Eikmanns BJ (2007) Ethanol catabolism in Corynebacterium glutamicum. J Mol Microbiol Biotechnol 15:222–233
Chaudhry MT, Huang Y, Shen XH, Poetsch A, Jiang CY, Liu SJ (2007) Genome-wide investigation of aromatic acid transporters in Corynebacterium glutamicum. Microbiology 153:857–865
Claes WA, Puhler A, Kalinowski J (2002) Identification of two prpDBC gene clusters in Corynebacterium glutamicum and their involvement in propionate degradation via the 2-methylcitrate cycle. J Bacteriol 184:2728–2739
Cocaigne M, Monnet C, Lindley ND (1993) Batch kinetics of Corynebacterium glutamicum during growth on various carbon substrates: use of substrate mixtures to localise metabolic bottlenecks. Appl Microbiol Biotechnol 40:526–530
Eggeling L (2005) Export of amino acids and other solutes. In: Eggeling L, Bott M (eds) Handbook of Corynebacterium glutamicum. Taylor & Francis, Boca Raton, pp 187–214
Engels V, Wendisch VF (2007) The DeoR-type regulator SugR represses expression of ptsG in Corynebacterium glutamicum. J Bacteriol 189:2955–2966
Engels V, Georgi T, Wendisch VF (2008) ScrB (Cg2927) is a sucrose-6-phosphate hydrolase essential for sucrose utilization by Corynebacterium glutamicum. FEMS Microbiol Lett 289:80–89
Frunzke J, Bott M (2008) Regulation of iron homeostasis in Corynebacterium glutamicum. In: Burkovski A (ed) Corynebacteria—genomics and molecular biology. Caister Academic Press, Norfolk, pp 241–266
Gopinath V, Meiswinkel TM, Wendisch VF, Nampoothiri KM (2011) Amino acid production from rice straw and wheat bran hydrolysates by recombinant pentose-utilizing Corynebacterium glutamicum. Appl Microbiol Biotechnol 92:985–996
Gosset G (2005) Improvement of Escherichia coli production strains by modification of the phosphoenolpyruvate:sugar phosphotransferase system. Microb Cell Fact 4:14
Jolkver E, Emer D, Ballan S, Krämer R, Eikmanns BJ, Marin K (2009) Identification and characterization of a bacterial transport system for the uptake of pyruvate, propionate, and acetate in Corynebacterium glutamicum. J Bacteriol 191:940–948
Kalinowski J, Bathe B, Bartels D, Bischoff N, Bott M, Burkovski A, Dusch N, Eggeling L, Eikmanns BJ, Gaigalat L, Goesmann A, Hartmann M, Huthmacher K, Kramer R, Linke B, McHardy AC, Meyer F, Mockel B, Pfefferle W, Puhler A, Rey DA, Ruckert C, Rupp O, Sahm H, Wendisch VF, Wiegrabe I, Tauch A (2003) The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of l-aspartate-derived amino acids and vitamins. J Biotechnol 104:5–25
Kawaguchi H, Vertès AA, Okino S, Inui M, Yukawa H (2006) Engineering of a xylose metabolic pathway in Corynebacterium glutamicum. Appl Environ Microbiol 72:3418–3428
Kurita K (2006) Chitin and chitosan: functional biopolymers from marine crustaceans. Mar Biotechnol (NY) 8:203–226
Lambert C, Erdmann A, Eikmanns M, Krämer R (1995) Triggering glutamate excretion in Corynebacterium glutamicum by modulating the membrane state with local anesthetics and osmotic gradients. Appl Environ Microbiol 61:4334–4342
Lindner SN, Seibold GM, Henrich A, Kramer R, Wendisch VF (2011) Phosphotransferase system-independent glucose utilization in Corynebacterium glutamicum by inositol permeases and glucokinases. Appl Environ Microbiol 77:3571–3581
Merkens H, Beckers G, Wirtz A, Burkovski A (2005) Vanillate metabolism in Corynebacterium glutamicum. Curr Microbiol 51:59–69
Moon MW, Kim HJ, Oh TK, Shin CS, Lee JS, Kim SJ, Lee JK (2005) Analyses of enzyme II gene mutants for sugar transport and heterologous expression of fructokinase gene in Corynebacterium glutamicum ATCC 13032. FEMS Microbiol Lett 244:259–266
Nakayama K, Sagamihara K, Araki K (1973) Process for producing l-lysine. In. US patent no. 3, 395 (ed). pp
Netzer R, Peters-Wendisch P, Eggeling L, Sahm H (2004) Cometabolism of a nongrowth substrate: l-serine utilization by Corynebacterium glutamicum. Appl Environ Microbiol 70:7148–7155
Parche S, Burkovski A, Sprenger GA, Weil B, Kramer R, Titgemeyer F (2001a) Corynebacterium glutamicum: a dissection of the PTS. J Mol Microbiol Biotechnol 3:423–428
Parche S, Thomae AW, Schlicht M, Titgemeyer F (2001b) Corynebacterium diphtheriae: a PTS view to the genome. J Mol Microbiol Biotechnol 3:415–422
Parsons JW (1981) Chemistry and distribution of amino sugars in soils and soils organisms. In: Paul EA, Ladd JN (eds) Soil biochemistry. Marcel Dekker, New York, pp 197–227
Patek M, Nesvera J (2011) Sigma factors and promoters in Corynebacterium glutamicum. J Biotechnol 154:101–113
Plumbridge JA (1991) Repression and induction of the nag regulon of Escherichia coli K-12: the roles of nagC and nagA in maintenance of the uninduced state. Mol Microbiol 5:2053–2062
Plumbridge J (1998) Control of the expression of the manXYZ operon in Escherichia coli: Mlc is a negative regulator of the mannose PTS. Mol Microbiol 27:369–380
Plumbridge J (2001) DNA binding sites for the Mlc and NagC proteins: regulation of nagE, encoding the N-acetylglucosamine-specific transporter in Escherichia coli. Nucleic Acids Res 29:506–514
Polen T, Schluesener D, Poetsch A, Bott M, Wendisch VF (2007) Characterization of citrate utilization in Corynebacterium glutamicum by transcriptome and proteome analysis. FEMS Microbiol Lett 273:109–119
Postma PW, Lengeler JW, Jacobson GR (1993) Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol Rev 57:543–594
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbour Laboratory Press, Cold Spring Harbour
Sasaki M, Jojima T, Inui M, Yukawa H (2008) Simultaneous utilization of d-cellobiose, d-glucose, and d-xylose by recombinant Corynebacterium glutamicum under oxygen-deprived conditions. Appl Microbiol Biotechnol 81:691–699
Schneider J, Wendisch VF (2010) Putrescine production by engineered Corynebacterium glutamicum. Appl Microbiol Biotechnol 88:859–868
Schneider J, Wendisch VF (2011) Biotechnological production of polyamines by bacteria: recent achievements and future perspectives. Appl Microbiol Biotechnol 91:17–30
Schneider J, Eberhardt D, Wendisch VF (2012) Improving putrescine production by Corynebacterium glutamicum by fine-tuning ornithine transcarbamoylase activity using a plasmid addiction system. Appl Microbiol Biotechnol 95:169–178
Stevenson FJ (1982) Organic forms of soil nitrogen. In: Stevenson FJ (ed) Nitrogen in agricultural soils. American Society of Agronomy, Madison, pp 101–104
Tanaka Y, Teramoto H, Inui M, Yukawa H (2011) Translation efficiency of antiterminator proteins is a determinant for the difference in glucose repression of two beta-glucoside phosphotransferase system gene clusters in Corynebacterium glutamicum R. J Bacteriol 193:349–357
Titgemeyer F, Reizer J, Reizer A, Saier MH Jr (1994) Evolutionary relationships between sugar kinases and transcriptional repressors in bacteria. Microbiology 140:2349–2354
Vogler AP, Trentmann S, Lengeler JW (1989) Alternative route for biosynthesis of amino sugars in Escherichia coli K-12 mutants by means of a catabolic isomerase. J Bacteriol 171:6586–6592
Wendisch VF (2003) Genome-wide expression analysis in Corynebacterium glutamicum using DNA microarrays. J Biotechnol 104:273–285
Wendisch VF, de Graaf AA, Sahm H, Eikmanns BJ (2000) Quantitative determination of metabolic fluxes during coutilization of two carbon sources: comparative analyses with Corynebacterium glutamicum during growth on acetate and/or glucose. J Bacteriol 182:3088–3096
Youn JW, Jolkver E, Kramer R, Marin K, Wendisch VF (2008) Identification and characterization of the dicarboxylate uptake system DccT in Corynebacterium glutamicum. J Bacteriol 190:6458–6466
Youn JW, Jolkver E, Kramer R, Marin K, Wendisch VF (2009) Characterization of the dicarboxylate transporter DctA in Corynebacterium glutamicum. J Bacteriol 191:5480–5488
Acknowledgments
Work in the labs of the authors was funded in part by grants 0315589 G and 0315589 F from BMBF in the CRP ‘Corynebacterium: improving flexibility and fitness for industrial production’.
Author information
Authors and Affiliations
Corresponding author
Additional information
Andreas Uhde and Jung-Won Youn contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 63 kb)
Rights and permissions
About this article
Cite this article
Uhde, A., Youn, JW., Maeda, T. et al. Glucosamine as carbon source for amino acid-producing Corynebacterium glutamicum . Appl Microbiol Biotechnol 97, 1679–1687 (2013). https://doi.org/10.1007/s00253-012-4313-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4313-8