Abstract
Following the announcement of the pandemic of COVID-19 in December 2019, several studies focused on how to early predict the severity of the disease in symptomatic and asymptomatic patients. Many cytokines including interleukin-6, interleukin-8, and tumor necrotic factors have been concluded as strong indicators for COVID-19 infection. Additionally, miRNAs have been associated with dysregulation in the immune system. The aim of this study are the following: (1) to estimate the level of miRNA-16–2-3P, miRNA-618, IL-8, IL-1β as predictors for SARS-CoV-2 complications in PCR negative and positive patients; (2) to assess the biological role and effect of these miRNAs on SARS-CoV-2 pathogenicity. Our study showed that the level of IL-1β had been significantly associated with patient who need hospitalization, also the alteration of the level of miRNA-16–2-3P, miRNA-618 is positively correlated with the admission of these patients and influence the outcomes of SARS-cov-2 infection. Measurement of miRNA-16–2-3P, miRNA-618, IL-1β could be a good predictor of COVID-19 patient outcome. However the measurement of IL-8 levels during immune responses in the admitted and in ICU patients could have a prognostic value.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As of February 2021, the COVID-19 pandemic, caused by infection with severe acute respiratory syndrome-associated coronavirus-2 (SARS-CoV-2), has resulted in over 107 million cases and 2.35 million deaths worldwide (Farr et al. 2021). In Egypt, starting from the 3rd of January 2020 to the 21st of February 2022, 469,457 recorded cases of COVID-19 with 23,752 deaths have been detected (WHO 2022). The outcome of SARS-CoV-2 infection varies widely from asymptomatic to severe disease associated with acute respiratory distress syndrome (ARDS) and death (Wu et al. 2020). Several studies have established that host responses to infection play a critical role in determining disease outcomes in infected patients.
A significant decrease in lymphocytes were lower than 20% in severe cases has been detected (Del Valle et al. 2020), specifically, CD4 + T, CD8 + T, natural killer (NK) cells, and B cell number (Fara et al. 2020; Huang et al. 2020).
Many reviews have evaluated the associated inflammatory mediators with COVID-19. For example, hyper-inflammatory responses including high levels of circulating cytokines and chemokines (particularly interleukin (IL)-6, IL-8, and tumor necrosis factor (TNF)-α) (Xu et al. 2020), IL-10 and C-reactive protein (Del Valle et al. 2020), IL-17 (Darif et al. 2021), and IL-18 (Valizadeh et al. 2020); lymphopenia and immune cell infiltration in infected organs are considered major determinants of COVID-19 severity (Xu et al. 2020). Interleukin-8 (IL-8) is an effective pro-inflammatory cytokine that has been associated with the enrollment and activation of neutrophils during inflammation. Therefore, detected neutrophilia detected in COVID-19 patients can contribute to the pathophysiology of the disease (Feldmann et al. 2020).
The characterization of host factors associated with SARS-CoV-2 pathogenesis is critically important for the design of novel therapies.
MicroRNAs (miRNAs) are a class of non-coding RNAs that regulate endogenous gene expression at the post-transcriptional level. In most instances, miRNAs function by interacting with the 3′ untranslated region (3′ UTR) of target mRNAs to induce degradation and translational repression (Bartel 2018). There are currently over 2600 human miRNAs listed in the miRNA registry (miRBase, version 22), which are estimated to collectively regulate 60% of all human protein-coding genes (Farr et al. 2021).
Some studies have revealed that the viral miRNAs can modify some of the host inflammatory responses to constrain secure damage to susceptible organs including—lungs (Panda et al. 2022). On the other hand, the host miRNAs have been proved to inhibit viral replication via self-attachment at 3′UTR region of the viral genome or the cellular targeting receptor or blocking the structural and non-structural proteins of SARS-CoV-2 without disturbing the expression of the human genome (Chow and Salmena 2020). Therefore, miRNAs could modulate the immune response of the severely infected COVID-19 patients via different mechanisms.
Li et al. (2020) data analysis suggests that, 35 miRNAs were upregulated and 38 miRNAs were downregulated in the human patients with COVID‐19. The top genes were as follows: miR‐16‐2‐3p, miR‐6501‐5p, and miR‐618 were highly expressed in COVID‐19 patients and that miR‐183‐5p, miR‐627‐5p, and miR‐144‐3p were less expressed in COVID‐19 patients.
The scientific rationale for investigating miRNAs during viral infections is two-fold. Firstly, miRNA profiles offer unique insight into cellular pathways associated with virus replication and pathogenesis. For instance, the human coronavirus OC43 potentiates NF-kB activation during infection by binding and sequestering miR-9, a negative regulator of NF-kB (Lai et al. 2014). There is also evidence that coronaviruses co-opt the host miRNAs response to subvert antiviral immune responses. Infection by the, Alpha Corona Virus transmissible gastroenteritis virus (TGEV), downregulates miR-30a-5p expression, which disrupts the type I interferon response against TGEV (Ma et al. 2018).
Secondly, the characterization of host miRNAs responses to virus infection informs the development of biomarkers for improved disease detection and forecasting of disease outcome (Tribolet et al. 2020). Several pathogenic viruses, including SARS-CoV-1, induce changes to the circulating host miRNA profile (Tambyah et al. 2013).
Viral infection can regulate miRNA expression and that can cause other genes to regulate the host immune response to viral infection. MicroRNAs effect is on virus replication and they have recently emerged as important modulators of viral infections (Canatan and Sanctis 2020).
While it is recognized that the host response to infection plays a critical role in determining the severity and outcome of COVID-19, the host microRNA (miRNA) response to SARS-CoV-2 infection is poorly defined. The host response to SARS-CoV-2 infection provides insights into both viral pathogenesis and patient management (Farr et al. 2021).
Patient methods and subject study
Patients
This study was carried out in Luxor Fevers Hospital. It includes eighty patients aged from 18 to 70 years suffering from typical COVID-19 symptoms. Patients under the study have been divided into two groups according to the RT-PCR results:
-
Group 1: seventy cases with proved positivity for COVID-19 disease Sixty are not in need for ICU admission
-
◦ Ten of these cases are ICU admitted, and
-
◦ Sixty are not in need for ICU admission
-
-
Group 2: with proved negativity for COVID-19 disease using RT-PCR
Patient written informed consents were obtained from participated patients according to Helsinki Declaration. Also, approval from the Ministry of Health, Training and Research Sector has been obtained.
Methods
-
1.
Nasopharyngeal swab samples have been done for all patients under study for testing of COVID-19 by RT-PCR.
-
2.
Laboratory investigations
Blood samples collected: EDTA sample, Citrate samples and samples without anticoagulants (for collection of serum samples)
All groups under study have been subjected for the following:
-
Complete blood count (CBC) has been done on automated cell analyzer DXH 520 (Beckman Coulter).
-
Biochemical tests (including renal function tests (urea and creatinine) and liver function tests (bilirubin (T and D), proteins (total and albumin), AST, ALT, ALP, and GGt) have been done on automated chemistry analyzer AU480 (Beckman Coulter) and electrolytes (Na and K) on ion selective electrode analyzer.
-
Laboratory investigation to assess the severity of COVID-19
-
◦ Ferritin, by chemiluminescent assay cobas e411 (Roche)
-
◦ CRP, turbidmetric assay on automated chemistry analyzer AU480 (Beckman Coulter)
-
◦ D-Dimer, by chemiluminescent enzyme immunoassay for the quantitative measurement of D-Dimer concentration on PATHFAST instrument
-
-
Inflammatory cytokines (IL-1B and IL-8) using the Enzyme-Linked Immunosorbent Assay (ELISA) technique. Supplied kits from the Elabscience Biotechnology Inc. USA
-
Human IL-1β and IL-8 by ELISA technique:
-
Sample: Serum
-
Assay procedure
-
100 μL standard and sample added to each well. Incubated for 90 min at 37 ℃
-
Liquid removed. Aspirated and washed 5 times
-
100 μL Biotinylated Detection Ab has been added. Incubated for 1 h at 37 ℃
-
Aspirated and washed 5 times
-
100 μL HRP conjugate added. Intubated for 30 min at 37 ℃
-
Aspirated and washed 5 times
-
90 μL substrate reagent added. Incubated for 15 min at 37 ℃
-
50 μL stop solution added and read the standard and sample absorbance at 450 nm
-
Plotting the standard curve
-
Calculation of the results using standard curve
-
MicroRNAs (miRNA-16–2-3p and miRNA-618) were assessed using Real Time-PCR.
miRNA assay
RNA extraction and reverse transcription
-
Total RNA, containing miRNA, was extracted from plasma samples (200 µl plasma) using the Qiagen miRNeasy Mini Kit (Qiagen GmbH, Valencia, California, USA, catalog no. 217004). The RNA purity was confirmed by the relative absorbance at 260/280 nm. Then, the extracted RNA was stored at − 80 °C and prepared for usage. Ten microliter of the extracted RNA was used for cDNA synthesis using the miScript II reverse transcription (RT) Kits (Qiagen GmbH, Valencia, California, USA), under the reactive condition of 37 °C for 60 min, then 95 °C for 5 min. Then, the miScript SYBER Green PCR kits were used with the miScript Primer Assays according to the real-time PCR Amplifier manufacturer’s instructions (Qiagen GmbH, Valencia, California, USA, catalog no. 218073). The mature miRNA-16–2-3P and miRNA-618 sequences were (CCAAUAUUACUGUGCUGCUUUA) and (AAACUCUACUUGUCCUUCUGAGU) respectively with Housekeeping gene: miRNAU6 (RNU6) as an endogenous control. For qRT-PCR, 2 μL of diluted reverse transcription products was mixed with 12.5 μL SYBR®Green Real-time PCR Master Mix. Then we add 2.5 μL of forward primer, 2.5 μL reverse primers, and 5 μL RNase-free water in a final volume of 25 μL in each strip tube. The reactivity conditions: initial activation step at 95 °C for 15 min, followed by 3-step cycling: 1-denaturation 94 °C for 15 s, 2-annealing 55 °C for 30 s, 3-extension 70 °C for 34 s. The three steps cycle has been repeated for 40 cycles.. After reaction, the threshold cycle of fluorescence (Ct) was calculated to further analyze the expression level of miRNA in specimens utilizing endogenous control. A housekeeping gene was added to each sample as an internal negative control (Catalog no. 00033712). The relative expression was expressed by 2-ΔCT (ΔCT = CT target gene-CT reference gene). The relative quantification of each of miRNA-16–2-3P and miRNA-618 was performed using quantitative real-time (qRT)-PCR according to the manufacturer’s instructions. The ΔCt value was used to represent the relative level of expression of a single miRNA.
Statistical analysis
Data were verified, coded by the researcher, and analyzed using the IBM-SPSS 24.0 (IBM-SPSS Inc., Chicago, IL, USA). Descriptive statistics: means, standard deviations, median, interquartile range (IQR), frequency, and percentages were calculated. Test of significances: Fisher’s Exact test was calculated to compare the frequencies among groups. The Shapiro–Wilk test will be used to test for data normality. The Student t-test and Mann–Whitney U test were calculated to test the mean/median differences in continuous variables between groups (parametric and non-parametric). Multivariable logistic regression analysis was calculated to investigate the significant predictors of positive PCR (odds ratio (OR), 95% confidence interval − 95% CI, and likelihood ratio test (LRT)). The ROC curve was depicted to explore the diagnostic performance of the new biomarkers for prediction of ICU admission, analyzed as area under the curve (AUC), standard error (SE), and 95% CI. Validity statistics (sensitivity, specificity, positive, and negative predictive value (PPV and NPV)) were calculated. The Spearman rank correlation co-efficient was calculated for univariate correlations. A significant P value was considered when it is equal or less than 0.05 (References: * IBM_SPSS. Statistical Package for Social Science. Ver.24. Standard version. Copyright © SPSS Inc., 2012–2016. NY, USA. 2016).
Results
The demographic data of the two studied groups has been demonstrated in Table (1). There was no significant difference between the positive PCR COVID-19 group and negative group as regards the presence of the following diseases: asthma, chronic obstructive airway disease (COPD), hypertension (HTN), diabetes mellitus (DM), chronic kidney disease (CKD), ischemic heart disease (IHD), rheumatic heart disease (RHD), or cancer (Table 1).
Laboratory investigations of both groups revealed a significant difference regarding ALT, CRP, miRNA-618, and miRNA-16–2-3P in favor to positive PCR for COVID-19 group, as presented in Table 2.
A multivariable logistic regression model revealed that odd for detecting the COVID-19 infection by PCR increased to almost 3 times if the patient have COPD. Cyanosis showed 3.5 times increase in incidence for predicting the infection (P < 0.029), while presence of myalgia showed almost 4 times increase incidence for predicting COVID-19 infection positive PCR compared with the negative PCR cases (Table 3). Also, the incidence for prediction of COVID-19 infection increase 4 times with the level of miRNA-618 and 9 times with miRNA-16–2-3p. This may suggests that the presence of these signs or symptoms may be associated with increase viral load in the nasopharynex.
Regarding laboratory biomarkers and their association with ICU admission, ICU admitted cases show a significantly high IL-1β level in comparison to patients not in need for ICU admission. However, IL-8 level shows the reverse with a significantly high level in patients not in need for ICU admission in comparison to ICU admitted patients. Similarly, miRNA-618 level shows significant increase level in non ICU admitted patients in comparison to ICU admitted patients, while there was a marginal significant increase in the level of miRNA-16–2-3P in non ICU admitted patients. No significant difference was detected with serum iron or lead (Table 4). These biomarkers could have a prognostic value in COVID-19 infected patients.
Finally, the AUC for ROC curves was used for early prediction of the severity of COVID-19. The AUC for ROC curves showed bad predictability of IL-1β (0.518 ± 0.092, CI 0.261–0.623; P < 0.556) for the COVID-19 infection. The cut-off values for predicting the COVID-19 was 12 pg/ml with an accuracy of 50.5%, sensitivity of 80%, and a specificity of 21% (PPV%, 50%; NPV%, 51%). However, IL-8 showed a good predictability with AUC of 0.655 ± 0.077 (CI 0.583–0.884, P < 0.044). The cut-off value for IL-8 was 17 pg/ml with an accuracy of 74.5%, sensitivity of 70%, and specificity of 79% (PPV%, 77%; NPV%, 72.5%). miRNA-618 AUC was 0.625 ± 0.109 (CI 0.412–0.838, P < 0.254). The cut-off value was 1 with an accuracy reaches 60% (sensitivity of 75% and specificity of 44%) PPV% equals 57% and NPV% of 64%. The AUC for miRNA-16–2-3P was 0.743 ± 0.078 (CI 0.591–0.895, P < 0.041). The cut-off values for miRNA-16–2-3P were 3.5 with an accuracy of 72.5%, sensitivity of 84%, and specificity of 61%. PPV% was 68% and NPV% of 79% (Table 5) (Fig. 1).
Discussion
A negative COVID-19 test means the test did not detect the virus, but this does not rule out that you could have an infection. If you have symptoms you may have COVID-19, but tested before the virus was detectable, or you may have another illness.
Our study includes both symptomatic COVID-19 positive PCR and symptomatic negative PCR patients for COVID-19 and compare the tested cytokines (IL-1β and IL-8), and miRNA (miRNA-618 and miRNA-16–2-3p) in both groups.
In our study, positive PCR patients for COVID-19 showed no significant difference regarding clinical symptoms as sore throat, cough, fatigue, vomiting, nausea, and chills when compared to the negative PCR group for COVID-19. Although most of the cases suffered these symptoms, only myalgia and anosmia show a mild significant increase in the positive PCR group for COVID-19 in comparison to the negative PCR one; 28% of the positive PCR group had anosmia compared to non in the negative PCR group. Additionally, 32.5% of the positive PCR group had myalgia when compared to 10% of the negative PCR group (P < 0.05).
Early studied meta-analyses assessing the impact of comorbidities on the course and clinical outcome of COVID-19 found that about 31% of adult patients had comorbidities. Hypertension being the most prevalent condition (20.93%) followed by heart failure (10.5%), diabetes mellitus (10.4%), and coronary heart disease (8.5%) (Jutzeler et al. 2020). These pre-existing comorbidities were found to be linked with the severity of the COVID-19.
Comparison between different laboratory tests revealed a significant difference regarding only ALT and CRP in favor to cases group with no differences regarding comorbidities. In addition, the presence of COPD (OR: 3, P < 0.04), cyanosis (OR: 3.5, P < 0.029), epigastric pain had 9% chance of predicting the COVID-19 infection (P < 0.047) while myalgia increased the odd for prediction to almost 4 times compared with the negative cases.
Using the multivariable logistic regression model of analysis, it has been found that presence of the clinical symptoms and signs, COPD, cyanosis, epigastric pain, and myalgia, is a good predictor for COVID-19 (P < 0.05).
Farr et al. (2021) study stated that the host response to SARS-CoV-2 infection provides insights into both viral pathogenesis and patient management. Cytokine storm is an activation cascade of auto-amplifying cytokine production and leads to excessive activation of immune cells and the generation of pro-inflammatory cytokines (Tisoncik et al. 2012).
To lower mortality due to cytokine storm, we have to discover and find good predictors for this aggressive inflammatory response, even before its development. Several clinical studies report the infiltration of monocytes and macrophages into the lungs of the COVID-19 patients contribute to the production of pro-inflammatory cytokines and chemokines that result in cytokine storm leading to tissue damage, organ system dysfunction, and progression to ARDS as well as mortality (Costela-Ruiz et al. 2020; Gómez-Rial et al. 2020). It has been proved that the higher the level of cytokines the poor the prognosis (Chen et al. 2020, and Guohua et al. 2020). Narożna and Rubiś (2021) suggesting that COVID-2 inhibits the IFN receptor’s activation, which reduces the antiviral effect while allowing cytokine storm syndrome (i.e., IL-1, IL-6, IL-8) release. Additional study reveals a significant difference between the disease stages and different levels of IL-8 and IL-6. Also, a significant negative correlation was detected between IL-6 and IL-8 levels and SpO2, PaO2, which indicate respiratory failure. Furthermore, positive correlation between IL-8 and IL-6 and CRP was detected. The study has concluded that IL-6 and IL-8 can act as predictive biomarkers for the COVID-19 infection and severity (Li et al. 2021).
Evidence suggests that excessive inflammatory process activation leads to abundant interleukin-1β (IL-1β) release and subsequent aggravation of pulmonary injury and induce hypercoagulability, favoring progression to respiratory failure and widespread thrombosis eventually leading to multi-organ failure and death (Nicola et al. 2022).
Our data reveals that IL-1β level was significantly increase in the COVID-19 positive patients, and this level significantly increases in ICU admitted patients; this makes IL-1β a good predictor of the disease severity and patient’s outcome. In the same line with IL-1β the level of IL-8, miRNA-618 and miRNA-16–2-3p show a significantly increase level in the COVID-19 positive patients. However, the level of these parameters, IL-8, miRNA-618, and miRNA-16–2-3p, are significantly decrease in the COVID-19 ICU admitted patients in comparison to non ICU admitted one.
Our data demonstrates that the level of IL-8 significantly increase in the Covid-19 infected patients, but this level was found to decrease in ICU admitted patient in comparison to non ICU admitted patients, and these results against the recorded results by many authors who demonstrates a positive correlation between the level of IL-8 and the severity of the disease (Nagant et al. 2020; Li et al. 2021). Also, Del Valle et al. (2020) found an association between the level of IL-8 and decrease or worse survival. Our controversies results may be due to the included ICU admitted patients who are under treatment that may improve the condition and causes decrease in IL-8 level, we did not include any type of treatment in our study. So, further study includes the type of treatment and correlates this with IL-8 level may help in prediction and follow up response of the patients.
Our statistical data analysis reveals that IL-1β has a bad predictability for the COVID-19 infection. However IL-8 has a sensitivity of 70% with more specificity 79% than IL-1β with 77% PPV and 72.5% NPV. This makes IL-8 has a more diagnostic predictor for the COVID-19 patients, with an accuracy of 74.5% at a cut off value of 17 pg/ml.miRNAs are known to regulate numerous physiological pathways and biological processes including cell development, maturation, differentiation, and activation. miRNAs are vital for the regulation and elimination of undesired or malformed mRNA. miRNAs can act as anti-viral tool within the host cell as an entry for the diffusion of the virus (Abu-Izneid et al. 2021).
In our study, we compare the level of miRNA-618 and miRNA-16–2-3p between the two symptomatic groups, one with proved Covid-19 by PCR and the other group is negative for COVID-19 by PCR.
Our data analysis of the miRNA-618 and miRNA-16–2-3p levels shows a significant increase in its level in the positive PCR group in comparison to the negative PCR group (P < 0.05).
In one study, COVID-19 patients displayed 200 significant differentially expressed (SDE) miRNAs. Around 75 miRNAs were detected in asymptomatic patients compared to symptomatic patients who showed platelet aggregation and cytokine pathways. Moreover, the study has found that miRNAs were significantly correlated with inflammatory cytokines (Fernández-Pato et al. 2022).
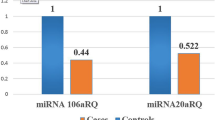
One study has revealed that miRNA-618 highly expressed in patients with COVID-19 and it increased to 1.5 fold in symptomatic patients with COVID-19 compared to healthy volunteers (Li et al. 2020).
Statistical analysis of our laboratory data reveals that miRNA-618 and miRNA-16–2-3p level shows a significant increase in its level in the positive PCR for the Covid-19 group in comparison to negative PCR for the COVID-19 group (P < 0.05). In agreement with our results, Li et al. (2020) study reveals that miR-16–2-3p, miR-6501-5p, and miR-618 were more highly expressed in COVID-19 patients than in healthy controls.
Our study reveals that miRNA-618 and miRNA-16–2-3p level was low in ICU admitted patients in comparison to non-ICU admitted patients; however, this low level were statistically significant in miRNA-618 only (P = 0.042) not with miRNA-16–2-3p (P = 0.051). That means there is a down regulation of the miRNA-618 and miRNA-16–2-3p, and this downregulation increase with the severity of the disease and the need for ICU.
However, statistical analysis of miRNA-618 and miRNA-16–2-3p level shows it has a positive predictive value in positive COVID-19 patients. Moreover, miRNA-16–2-3p has a more sensitivity (84%) and more specificity (61%) at a cut off value 3.5 than miRNA-618 (sensitivity of 75% and specificity of 44%) at a cut off 1.0 in diagnostic prediction of COVID-19.
Our data analysis shows a negative correlation between lymphocytic percent and the level of IL-1β (-0.213 (= 0.028)). Also, this negative correlation has been statistically observed between lymphocytic differential count and miRNA-16–2-3p (-0.208 (= 0.049)). Also, there was a negative correlation between monocyte percent and miRNA-618 and miRNA-16–2-3p (-0.341 (= 0.003), -0.292 (= 0.009) respectively).
From our study we suggest that measurement of the IL-1β, IL-8, and host miRNA response could improve COVID-19 detection, outcome prediction and this potentially useful tool for early management of patients with severe forms.
Nevertheless, our study has several limitations. First, our negative PCR patients not tested for other viruses that could be the cause of symptoms. Also the number of symptomatic negative PCR cases included is low relative to positive PCR cases. Further studies would be needed to explore how the duration of illness influences the Interleukins or microRNAs level.
Change history
04 July 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00251-023-01312-w
References
Abu-Izneid T, AlHajri N, Ibrahim AM, Javed MdN, Salem KM, Pottoo FH et al (2021) Micro-RNAs in the regulation of immune response against SARS CoV-2 and other viral infections. J Adv Res 30:133–45
Bartel DP (2018) Metazoan MicroRNAs. Cell 173(1):20–51
Canatan D, De Sanctis V (2020) The impact of MicroRNAs (miRNAs) on the genotype of coronaviruses. Acta Biomed 91:195–198
Chen L, Liu HG, Liu W, Liu J, Liu K, Shang J et al (2020) Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi 43:203–208. https://doi.org/10.3760/cma.j.issn.1001-0939.2020.0005
Chow JTS, Salmena L (2020) Prediction and analysis of SARS-CoV-2-targeting MicroRNA in human lung epithelium. Genes 11(9):1002
Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L (2020) SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev 54:62–75
Darif D, Hammi I, Kihel A, El Idrissi SI, Guessous F, Akarid K (2021) The pro-inflammatory cytokines in COVID-19 pathogenesis: what goes wrong? Microb Pathog 153:104799
Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B et al (2020) An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med 26(10):1636–1643
Fara A, Mitrev Z, Rosalia RA, Assas BM (2020) Cytokine storm and COVID-19: a chronicle of pro-inflammatory cytokines. Open Biol 10(9):200160
Farr RJ, Rootes CL, Rowntree LC, Nguyen THO, Hensen L, Kedzierski L, Cheng AC, Kedzierska K, Au GG, Marsh GA, Vasan SS, Foo CH, Cowled C, Stewart CR (2021) Altered microRNA expression in COVID-19 patients enables identification of SARS-CoV-2 infection. PLoS Pathog 17(7):e1009759. https://doi.org/10.1371/journal.ppat.1009759. PMID: 34320031; PMCID: PMC8318295
Feldmann M, Maini RN, Woody JN, Holgate ST, Winter G, Rowland M et al (2020) Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. The Lancet 395(10234):1407–1409
Fernández-Pato A, Virseda-Berdices A, Resino S, Ryan P, Martínez-González O, Pérez-García F et al (2022) Plasma miRNA profile at COVID-19 onset predicts severity status and mortality. Emerging Microbes & Infections 11(1):676–688
Gómez-Rial J, Rivero-Calle I, Salas A, Martinón-Torres F (2020) Role of monocytes/macrophages in Covid-19 pathogenesis: implications for therapy. Infect Drug Resist 13:2485–2493
Guohua L, Ling L, Min H, Haibiao L, Peifeng K, Zishao Z et al (2020) Value of various inflammatory markers combined with lymphocyte subsets on clinical diagnosis of different clinical types of COVID-19. J Chong Med Univ. https://doi.org/10.13406/j.cnki.cyxb.002465
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China the Lancet 395(10223):497–506
Jutzeler CR, Bourguignon L, Weis CV, Tong B, Wong C, Rieck B et al (2020) Comorbidities, clinical signs and symptoms, laboratory findings, imaging features, treatment strategies, and outcomes in adult and pediatric patients with COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis 37:101825
Lai FW, Stephenson KB, Mahony J, Lichty BD (2014) Human coronavirus OC43 nucleocapsid protein binds microRNA 9 and potentiates NF-kappaB activation. J Virol 88(1):54–65
Li L, Li J, Gao M, Fan H, Wang Y, Xu X et al (2021) Interleukin-8 as a biomarker for disease prognosis of coronavirus disease-2019 patients. Front Immunol 8(11):602395
Li C, Hu X, Li L, Li JH (2020) Differential microRNA expression in the peripheral blood from human patients with COVID-19. J Clin Lab 34, e23590. https://doi.org/10.1002/jcla.23590
Li J, Rong L, Cui R, Feng J, Jin Y, Chen X et al (2021) Dynamic changes in serum IL-6, IL-8, and IL-10 Predict the outcome of ICU patients with severe COVID-19. Ann Palliat Med 10(4):3706–3714
Ma Y, Wang C, Xue M, Fu F, Zhang X, Li L, Yin L, Xu W, Feng L, Liu P (2018) The coronavirus transmissible gastroenteritis virus evades the type I interferon response through IRE1α-mediated manipulation of the MicroRNA miR-30a-5p/SOCS1/3 axis. J Virol 92(22). https://doi.org/10.1128/JVI.00728-18
Nagant C, Ponthieux F, Smet J, Dauby N, Doyen V, Besse-Hammer T et al (2020) A score combining early detection of cytokines accurately predicts COVID-19 severity and intensive care unit transfer. Int J Infect Dis 101:342–345
Narożna M, Rubiś B (2021) Anti-SARS-CoV-2 strategies and the potential role of miRNA in the assessment of COVID-19 morbidity, recurrence, and therapy. IJMS 22(16):8663
Potere N, Del Buono MG, Caricchio R, Cremer PC, Vecchié A, Porreca E, Dalla Gasperina D, Dentali F, Abbate A, Bonaventura A (2022) Interleukin-1 and the NLRP3 inflammasome in COVID-19: Pathogenetic and therapeutic implications. EBioMedicine 85:104299
Panda M, Kalita E, Singh S, Kumar K, Rao A, Prajapati VK (2022) MiRNA-SARS-CoV-2 dialogue and prospective anti-COVID-19 therapies. Life Sci 305:120761
Tambyah PA, Sepramaniam S, Mohamed Ali J, Chai SC, Swaminathan P, Armugam A et al (2013) microRNAs in circulation are altered in response to influenza A virus infection in humans. PLoS ONE 8(10):e76811
Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG (2012) Into the eye of the cytokine storm. Microbiol Mol Biol Rev 76:16–32. https://doi.org/10.1128/MMBR.05015-11
Tribolet L, Kerr E, Cowled C, Bean AGD, Stewart CR, Dearnley M et al (2020) MicroRNA biomarkers for infectious diseases: from basic research to biosensing. Front Microbiol 11:1197 (Epub 2020/06/26)
Valizadeh H, Abdolmohammadi-vahid S, Danshina S, Ziya Gencer M, Ammari A, Sadeghi A et al (2020) Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int Immunopharmacol 89:107088
WHO (2022) Egypt COVID 19 updated cases [Internet]. Egypt. Available from: https://covid19.who.int/region/emro/country/eg
Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S et al (2020) Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus Disease 2019 pneumonia in Wuhan. China JAMA Intern Med 180(7):934–943
Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C et al (2020) Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8(4):420–422
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to additional affiliation of the first and second authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hassan, N.E., Moselhy, W.A., Eldomany, E.B. et al. Evaluation of miRNA-16–2-3P, miRNA-618 levels and their diagnostic and prognostic value in the regulation of immune response during SARS Cov-2 infection. Immunogenetics 75, 403–410 (2023). https://doi.org/10.1007/s00251-023-01308-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00251-023-01308-6