Abstract
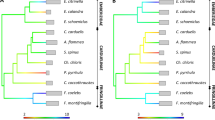
Major histocompatibility complex (MHC) class II DQB and DRA genes and class I gene of finless porpoises (Neophocaena phocaenoides) were investigated by single-strand conformation polymorphism and sequence analysis. The DRA, DQB, and MHC-I loci each contained 5, 14, and 34 unique sequences, respectively, and considerable sequence variation was found at the MHC-I and DQB loci. Gene duplication was manifested as three to five distinct sequences at each of the DQB and MHC-I loci from some individuals, and these sequences at each of the two loci separately clustered into four groups (cluster A, B, C, and D) based on the phylogenetic trees. Phylogenetic reconstruction revealed a trans-species pattern of evolution. Relatively high rates of non-synonymous (d N) vs synonymous (d S) substitution in the peptide-binding region (PBR) suggested balancing selection for maintaining polymorphisms at the MHC-I and DQB loci. In contrast, one single locus with little sequence variation was detected in the DRA gene, and no non-synonymous substitutions in the PBR indicated no balancing selection on this gene.




Similar content being viewed by others
References
Abbott KM, Wickings EJ, Knapp LA (2006) High levels of diversity characterize mandrill (Mandrillus sphinx) Mhc-DRB sequences. Immunogenetics 58:628–640
Aguilar AG, Roemer S, Debenham M, Binns D, Garcelon, Wayne RK (2004) High MHC diversity maintained by balancing selection in an otherwise genetically monomorphic mammal. Proc Natl Acad Sci USA 101:3490–3494
Amills M, Jiménez N, Jordana J, Riccardi A, Fernández-Arias A, Guiral J, Bouzat JL, Folch J, Sànchez A (2004) Low diversity in the major histocompatibility complex class II DRB1 gene of Spanish ibex, Capra pyrenaica. Heredity 93:266–272
Apanius V, Penn D, Slev PR, Ruff LR, Potts WK (1997) The nature of selection on the major histocompatibility complex. Crit Rev Immunol 17:179–224
Babik W, Durka W, Radwan J (2005) Sequence diversity of the MHC DRB gene in the Eurasian beaver (Castor fiber). Mol Ecol 14:4249–4257
Baker CS, Vant MD, Dalebout ML, Lento GM, O’Brien SJ, Yuhki N (2006) Diversity and duplication of DQB and DRB-like genes of the MHC in baleen whales (suborder: Mysticeti). Immunogenetics 58:283–296
Bandelt HJ, Forster P, Röhl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48
Bernatchez L, Landry C (2003) MHC studies in nonmodel vertebrates: what have we learned about natural selection in 15 years? J Evol Biol 16:363–377
Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC (1987) Structure of the human class I histocompatibility antigen, HLA-A2. Nature 329:506–512
Bowen L, Aldridge BM, Gulland F, Van Bonn W, DeLong R, Melin S, Lowenstine LJ, Sott JF, Johnson ML (2004) Class II multiformity generated by variable MHC-DRB region configurations in the California sea lion (Zalophus californianus). Immunogenetics 56:12–27
Bowen L, Aldridge BM, Delong R, Melin S, Godinez D, Zavala A, Gulland F, Lowenestine L, Stott JT, Johnson ML (2006) MHC gene configuration variation in geographically disparate populations of California sea lions (Zalophus californianus). Mol Ecol 15:529–533
Brown JH, Jardetzky TS, Gorga JC, Stern LJ, Urban RG, Strominger JL, Wiley DC (1993) 3-Dimensional structure of the human class-II histocompatibility antigen HLA-DR1. Nature 364:33–39
Cassens I, Vicario S, Waddell VG, Balchowsky H, Van Belle D, Ding W, Fan C, Lal Mohan RS, Simones-Lopes PC, Bastida R, Meyer A, Stanhope MJ, Milinkovitch MC (2000) Independent adaptation to riverine habitats allowed survival of ancient cetacean lineages. Proc Natl Acad Sci USA 97:11343–11347
Chu ZT, Carswell-Crumpton C, Cole BC, Jones PP (1994) The minimal polymorphism of class II E alpha chains is not due to the functional neutrality of mutations. Immunogenetics 40:9–20
De Muizon C (1988) Les relations phylogenetigues des Delphinida (Cetacea, Mammalia). Annales de Paleontol 74:159–227
De Swart RL, Ross PS, Vos JG, Osterhaus ADME (1996) Impaired immunity in harbour seals (Phoca vitulina) exposed to bioaccumulated environmental contaminants: review of a long-term feeding study. Environ Health Perspect 104:823–828
Dengjel J, Schoor O, Fischer R, Reich M, Kraus M, Muller M, Kreymborg K, Altenberend F, Brandenburg J, Kalbacher H, Brock R, Driessen C, Rammensee HG, Stevanovic S (2005) Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc Natl Acad Sci USA 102:7922–7927
Ellis SA, Ballingall KT (1999) Cattle MHC: evolution in action? Immunol Rev 167:159–168
Ellis SA, Staines KA, Holmes EC, Smith KB, Stear MJ, McKeever DJ, MacHugh ND, Morrison WI (1999) Variation in the number of expressed MHC genes in different cattle class I haplotypes. Immunogenetics 50:319–328
Flores-Ramirez S, Urban-Ramirez J, Miller RD (2000) Major histocompatibility complex class I loci from the gray whale (Eschrichtius robustus). J Heredity 91:279–282
Gao AL, Zhou KY (1995) Geographical variation of external measurements and three subspecies of Neophocaena phocaenoides in Chinese waters. Acta Theriol Sinica 15:81–92
Gutierrez-Espeleta GA, Hedrick PW, Kalinowski ST, Garrigan D, Boyce WM (2001) Is the decline of desert bighorn sheep from infectious disease the result of low MHC variation? Heredity 86:439–450
Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR, Grimes DJ, Hofmann EE, Lipp EK, Osterhaus ADME, Overstreet RM, Porter JW, Smith GW, Vasta GR (1999) Emerging marine diseases—climate links and anthropogenic factors. Science 285:1505–1510
Hayashi K, Nishida S, Yoshida H, Goto M, Pastene LA, Koike H (2003) Sequence variation of the DQB allele in the cetacean MHC. Mamm Study 28:89–96
Hedrick PW (2002) Pathogen resistance and genetic variation at MHC loci. Evolution 56:1902–1908
Hedrick PW, Parker KM, Miller EL, Miller PS (1999) Major histocompatibility complex variation in the endangered Przewalski’s horse. Genetics 152:1701–1710
Hedrick PW, Kim TJ, Parker KM (2001a) Parasite resistance and genetic variation in the endangered Gila topminnow. Anim Conserv 4:103–109
Hedrick PW, Parker KM, Lee RN (2001b) Using microsatellite and MHC variation to identify species, ESUs, and MUs in the endangered Sonoran topminnow. Mol Ecol 10:1399–1412
Hill AVS, Allsopp CEM, Kwiatkowski D, Anstey NM, Twumasi P, Rowe PA, Bennett S, Brewster D, McMichael AJ, Greenwood BM (1991) Common West African HLA antigens are associated with protection from severe malaria. Nature 352:595–600
Hoelzel AR, Stephens JC, O’Brien SJ (1999) Molecular genetic diversity and evolution at the MHC DQB locus in four species of pinnipeds. Mol Biol Evol 16:611–618
Hughes AL, Nei M (1989) Nucleotide substitution at major histocompatibility complex class II loci: evidence for overdominant selection. Proc Natl Acad Sci USA 86:958–962
Hughes AL, Yeager M (1998) Natural selection at major histocompatibility complex loci of vertebrates. Annu Rev Genet 32:415–435
Janitz M, Reiners-Schramm L, Lauster R (1998) Expression of the H2-Ea gene is modulated by a polymorphic transcriptional enhancer. Immunogenetics 48:266–272
Klein J (1986) The natural history of the major histocompatibility complex. Wiley, New York
Klein J (1987) Origin of major histocompatibility complex polymorphism-the transspecies hypothesis. Hum Immunol 19:155–162
Klein J, Takahata N (1990) The major histocompatibility complex and the quest for origins. Immunol Rev 113:5–25
Kriener K, O’hUigin C, Tichy H (2000) Convergent evolution of major histocompatibility complex molecules in humans and New World monkeys. Immunogenetics 51:169–178
Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5:150–163
Langefors A, Lohm J, Grahn M, Andersen O, von Schantz T (2001) Association between major histocompatibility complex class IIB alleles and resistance to Aeromonas salmonicida in Atlantic salmon. Proc R Soc Lond B 268:479–485
McClelland EE, Penn DJ, Potts WK (2003) Major histocompatibility complex heterozygote superiority during coinfection. Infect Immun 71:2079–2086
Murray BW, White BN (1998) Sequence variation at the major histocompatibility complex DRB loci in beluga (Delphinapterus leucas) and narwhal (Monodon monoceros). Immunogenetics 48:242–252
Murray BW, Malik S, White BN (1995) Sequence variation at the major histocompatibility complex locus DQB in beluga whales (Delphinapterus leucas). Mol Biol Evol 12:582–593
Nei M, Gojobori T (1986) Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol 3:418–426
Ohta T (1998) On the pattern of polymorphisms at major histocompatibility complex loci. J Mol Evol 46:633–638
Paterson S, Wilson K, Pemberton JM (1998) Major histocompatibility complex variation associated with juvenile survival and parasite resistance in a large unmanaged ungulate population (Ovis aries L.). Proc Natl Acad Sci USA 95:3714–3719
Penn DJ, Damjanovich K, Potts WK (2002) MHC heterozygocity confers a selective advantage against multiple-strain infections. Proc Natl Acad Sci USA 99:11260–11264
Pimtanothai N, Hurley CK, Leke R, Klitz W, Johnson AH (2001) HLA-DR and -DQ polymorphism in Cameroon. Tissue Antigens 58:1–8
Potts WK, Wakeland EK (1993) Evolution of MHC genetic diversity: a tale of incest, pestilence and sexual preference. Trends Genet 9:408–412
Reeves RR, Wang JY, Leatherwood S (1997) The finless porpoise, Neophocaena phocaenoides (G. Cuvier, 1829): a summary of current knowledge and recommendations for conservation action. Asian Mar Biol 14:111–143
Rice DW (1998) Marine mammals of the world: systematics and distribution. Marine Mammal Society, Special Publishion No. 4. Society for Marine Mammalogy, Lawrence, KS, USA
Richardson DS, Westerdahl H (2003) MHC diversity in two Acrocephalus species: the outbred great reed warbler and the inbred Seychelles warbler. Mol Ecol 12:3523–3529
Sambrook J, Russell DW (eds) (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Schad J, Ganzhorn JU, Sommer S (2005) Parasite burden and constitution of major histocompatibility complex in the Malagasy mouse lemur, Microcebus murinus. Evolution 59:439–450
Sena L, Schneider MPC, Brenig B, Honeycutt RL, Womack JE, Skow LC (2003) Polymorphisms in MHC-DRA and -DRB alleles of water buffalo (Bubalus bubalis) reveal different features from cattle DR alleles. Anim Genet 34:1–10
Shirakihara M, Shirakihara K, Takemura A (1994) Distribution and seasonal density of the finless porpoise, Neophocaena phocaenoides, in the coastal waters of western Kyushu. Japan Fisheries Science 60:82–85
Stern LJ, Brown JH, Jardetzky TS, Gorga JC, Urban RG, Strominger JL, Wiley DC (1994) Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide. Nature 368:215–221
Takada T, Kikkawa Y, Yonekawa H, Amano Y (1998) Analysis of goat MHC class II DRA and DRB genes: identification of the expressed gene and new DRB alleles. Immunogenetics 48:408–412
Trowsdale J, Gorves V, Arnason A (1989) Limited MHC polymorphism in whales. Immunogenetics 29:19–24
Weber DS, Stewart BS, Schienman J, Lehman N (2004) Major histocompatibility complex variation at three class II loci in the northern elephant seal. Mol Ecol 13:711–718
Xia JH, Zheng JH, Wang D (2005) Individual identification of the Yangtze finless porpoises Neophocaena phocaenoides asiaeorientalis inhabiting the Tian-e-Zhou Natural Reserve based on microsatellite fingerprints. Acta Zool Sinica 51:142–148
Yang G, Zhou KY, Ren WH, Ji GQ, Liu S, Bastida R, Rivero L (2002a) Molecular systematics of river dolphins inferred from complete mitochondrial cytochrome b gene sequences. Mar Mamm Sci 18:20–29
Yang G, Ren WH, Zhou KY, Liu S, Ji GQ, Yan J, Wang L (2002b) Population genetic structure of finless porpoises Neophocaena phocaenoides in Chinese waters, inferred from mitochondrial control region sequences. Mar Mamm Sci 18:336–347
Yang G, Liu S, Ren WH, Zhou KY, Wei FW (2003) Mitochondrial control region variability of baiji and the Yangtze finless porpoises, two sympatric small cetaceans in the Yangtze river. Acta Theriol 48:469–483
Yang G, Yan J, Zhou KY, Wei FW (2005) Sequence variation and gene duplication at MHC DQB loci of baiji (Lipotes vexillifer), a Chinese river dolphin. J Heredity 96:310–317
Yang G, Guo L, Bruford M, Wei FW, Zhou KY (2007) Mitochondrial phylogeography and population history of finless porpoises in Sino-Japanese waters. Biol J Linn Soc (in press)
Yoshida H, Shirakihara K, Shirakihara M, Takemura A (1995) Geographic variation in the skull morphology of the finless porpoise Neophocaena phocaenoides in Japanese waters. Fish Sci (Tokyo) 61:555–558
Yoshida H, Yoshioka M, Shirakihara M, Chow S (2001) Population structure of finless porpoises (Neophocaena phocaenoides) in coastal waters of Japan based on mitochondrial DNA sequences. J Mammal 82:123–130
Zheng JS, Xia JH, He SP, Wang D (2005) Population genetic structure of the Yangtze finless porpoise (Neophocaena phocaenoides asiaeorientalis): implications for management and conservation. Biochem Genet 43:307–320
Acknowledgments
This study was supported through the National Natural Science Foundation Commission of China Grants, No.30270212, No.30470253, and No.30670294 and “Qinglan Project” of Jiangsu Province awarded to Dr. Guang Yang. The authors thank Anli Gao, Xin-Rong Xu, Hua Chen, and Qing Chang for collecting samples during the years, and members of the Institute of Genetic Resources, Nanjing Normal University, for their contributions to this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, S., Sun, P., Zhou, K. et al. Sequence variability at three MHC loci of finless porpoises (Neophocaena phocaenoides). Immunogenetics 59, 581–592 (2007). https://doi.org/10.1007/s00251-007-0223-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00251-007-0223-9