Abstract
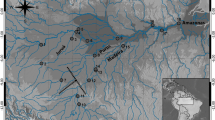
Understanding the population genetic structure is a prerequisite for conservation of a species. The degree of genetic variability characteristic of the mitochondrial DNA control region has been widely exploited in studies of population genetic structure and can be useful in identifying meaningful population subdivisions. To estimate the genetic profile of the Yangtze finless porpoise (Neophocaena phocaenoides asiaeorientalis), an endangered freshwater population endemic to China, the complete mtDNA control region was examined in 39 individuals belonging to seven different stocks inhabiting the middle and lower reaches of the Yangtze River. Very low genetic diversity was found (nucleotide diversity 0.0011± 0.0002 and haplotypic diversity 0.65± 0.05). The mtDNA genetic pattern of the Yangtze population appears to indicate a founder event in its evolutionary history and to support the marine origin for this population. Analyses by Fst and Φst yielded statistically significant population genetic structure (Fst = 0.44, P < 0.05; Φst = 0.36, P < 0.05). These results may have significant implications for the management and conservation of the Yangtze finless porpoise in the future.
Similar content being viewed by others
References
Árnason, Ú., Gullberg, A., and Widegren, B. (1991). The complete nucleotide sequence of the mitochondrial DNA of the fin whale Balaenoptera physalus. J. Mol. Evol. 33:556–568.
Barret, S. C. H., and Kohn, J. D. (1991). Genetic and evolutionary consequences of small population size in plants: Implications for conservation. In Falk, D. A., and Holsinger, K. E. (eds.), Genetics and Conservation of Rare Plants, Oxford University Press, New York, pp. 3–30.
Caughley, G. (1994). Directions in conservation biology. J. Anim. Ecol. 63:215–244.
Crandall, K. A., and Templeton, A. R. (1993). Empirical tests of some predictions from coalescent theory with application to intraspecific phylogeny reconstruction. Genetics 134:959–969.
Excoffier, L., Smouse, P. E., and Quattro, J. M. (1992). Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics 131:479–491.
Felsenstein, J. (1985). Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39:783–791.
Filatov, D. A. (2002). ProSeq: A software for preparation and evolutionary analysis of DNA sequence data sets. Mol. Ecol. Notes 2:621–624.
Frankel, O. H., and Soule, M. E. (1981). Conservation and Evolution, Cambridge University Press, Cambridge.
Frankham, R. (1995). Conservation genetics. Ann. Rev. Genet. 29:305–327.
Frankham, R. (1998). Inbreeding and extinction: Island populations. Conserv. Biol. 12:665–675.
Franklin, L. A. (1980). Evolutionary changes in small populations. In Soulé, M. E., and Wilcon, B. A. (eds.), Conservation Biology: An Evolutionary-Ecological Perspective, Sinauer Associates, Sunderland, pp. 135–150.
Galtier, N., Gouy, M., and Gautier, C. (1996). SeaView and Phylo win, two graphic tools for sequence alignment and molecular phylogeny. Comput. Appl. Biosci. 12:543–548.
Gao, A. L., and Zhou, K. Y. (1995). Geographical variation of external measurements and three subspecies of Neophocaena phocaenoides in Chinese waters. Acta Theriol. Sin. 15:81–92.
Hilton-Taylor, C. (2000). 2000 IUCN Red List of Threatened Species, IUCN-The World Conservation Union, Gland, Switzerland.
Hoelzel, A. R., Hancock, J. M., and Dover, G. A. (1991). Evolution of the cetacean mitochondrial D-loop region. Mol. Biol. Evol. 8:475–493.
Jeanmougin, F., Thompson, J. D., Gouy, M., Higgins, D. G., and Gibson, T. J. (1998). Multiple sequence alignment with Clustal X. Comput. Corner 23:78–80.
Kasuya, T. (1999). Finless porpoise Neophocaena phocaenoides (G. Guvier, 1829). In Ridgwag, S. H., and Harrison, R. (eds.), Handbook of Marine Mammals, Volume 6: The Second Book of Dolphins and the Porpoises, Academic Press, San Diego, California, pp. 411–442.
Krauss, S. L., Dixon, B., and Dixon, K. W. (2001). Rapid genetic decline in a transloated population of the endangered Plant. Grevillea scapigera. Conserv. Biol. 16:986–994.
Kumar, S., Tamura, K., Jakobsen, I. B., and Nei, M. (2001). MEGA2: Molecular Evolutionary Genetics Analysis Software, Arizona State University, Tempe, Arizona, USA.
Lacy, R. C. (1997). The importance of genetic variation to the viability of mammalian populations. J. Mammal. 78:320–335.
Li, Q. M., Xu, Z. F., and He, T. H. (2002). Ex situ genetic conservation of endangered Vatica guangxiensis (Dipterocarpaceae) in China. Biol. Conserv. 106:151–156.
Liu, R. J., and Wang, D. (1996). Studies on population size and activities alteration regularities of Lipotes vexillifer and Neophocaena phocaenoides in the Yangtze River. IBI Reports No. 6, pp. 1–8.
Liu, R. J., Wang, D., Yang, J., and Zhang, X. F. (1997). Some new considerations for the conservation of Lipotes vexillifer and Neophocaena phocaenoides in China. IBI Reports No. 7, pp. 39–44.
Liu, R. J., Zhang, X. F., Wang, D., and Yang, J. (1996). Once again studies on the conservation of Lipotes vexillifer and Neophocaena phocaenoides. Resour. Environ. Yangtze Basin 5:220–225.
Marshall, D. R., and Brown, A. H. D. (1975). Optimum sampling strategies in genetic conservation. In Frankel, O. H., and Hawkes, J. G. (eds.), Crop Genetic Resource for Today and Tomorrow, Cambridge University Press, Cambridge, pp. 53–80.
Nei, M. (1987). Molecular Evolutionary Genetics, Columbia University Press, NewYork, NY.
Nei, M., and Tajima, F. (1981). DNA polymorphism detectable by restriction endonucleases. Genetics 97:145–163.
O'Corry-Crowe, G. M., Suydam, R. S., Rosenberg, A., Frost, K. J., and Dizon, A. E. (1997). Phylogeography, population structure and dispersal patterns of the beluga whale Delphinapterus leucas in the western Nearctic revealed by mitochondrial DNA. Mol. Ecol. 6:955–970.
Parsons, E. C. M., and Wang, J. Y. (1998). A review of finless porpoise Neophocaena phocaenoides from the South China Sea. In Morton, B. (ed.), The Marine Biology of the South China Sea, Hong Kong University Press, Hong Kong, pp. 287–306.
Reeves, R. R., Wang, J. Y., and Leatherwood, S. (1997). The finless porpoise, Neophocaena phocaenoides (G. Cuvier, 1829): A summary of current knowledge and recommendations for conservation. Asian Mar. Biol. 14:111–143.
Rosel, P. E., and Rojas-Bracho, L. (1999). Mitochondrial DNA variation in the critically endangered Vaquita Phocoena sinus Norris and Macfarland, 1958. Mar. Mammal Sci. 15:990–1003.
Rozas, J., and Rozas, R. (1999). DnaSP 3: An integrated program for molecular population genetics and molecular evolution analysis. Bioinformatics 15:174–175.
Schneider, S., Roessli, D., and Excoffier, L. (2000). Arlequin Ver. 2.000: A software for population genetics data analysis. Genetics and Biometry Laboratory, University of Geneva, Geneva, Switzerland.
Slatkin, M. (1985). Rare alleles as indicators of gene flow. Evolution 39:53–65.
Slatkin, M. (1987). Gene flow and the geographic structure of natural populations. Science 236:787–792.
Takahata, N. (1988). The coalescent in two partially isolated diffusion populations. Genet. Res. 52:213–222.
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., and Higgins, D. G. (1997). The Clustal X windows interface: Flexible strategies for multiple sequences alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876–4882.
Wang, D., Liu, R. J., Zhang, X. F., Yang, J., Wei, Z., Zhao, Q. Z., and Wang, X. Q. (2000). Population status and conservation of the Yangtze finless porpoise. Occasional Papers of the IUCN/SSC 23:81–85.
Wei, Z., Wang, D., Zhang, X. F., Zhao, Q. Z., Wang, K. X., and Kuang, X. A. (2002a). Population size, behavior, movement pattern and protection of Yangtze finless porpoise at Balijiang section of the Yangtze River. Resour. Environ. Yangtze Basin 11:427–432.
Wei, Z., Wang, D., Kuang, X. A., Wang, K. X., Wang, X. Q., Xiao, J. Q., Zhao, Q. Z., and Zhang, X. F. (2002b). Observations on behavior and ecology of the Yangtze finless porpoise Neophocaena phocaenoides asiaeorientalls group at Tian-e-Zhou Oxbow of the Yangtze River. Raffles B. Zool. 10:97–103.
Wright, S. (1931). Evolution in mendelian populations. Genetics 16:97–159.
Wright, S. (1965). The interpretation of population structure by F-statistics with special regard to systems of mating. Evolution 19:395–420.
Yang, G., and Zhou, K. Y. (1997). Variability of the mitochondrial control region in population of finless porpoise, Neophocaena phocaenoides, in Chinese waters. Acta Zool. Sin. 43:411–419.
Yang, G., Ren, W. H., Zhou, K. Y., Liu, S., Ji, G. Q., Yan, J., and Wang, L. M. (2002). Population genetic structure of finless porpoises Neophocaena phocaenoides in Chinese waters, inferred from mitochondrial control region sequences. Mar. Mammal Sci. 18:336–347.
Yang, G., Liu, S., Ren, W. H., Zhou, K. Y., and Wei, F. W. (2003). Mitochondrial control region variability of baiji and the Yangtze finless porpoises, two sympatric small cetaceans in the Yangtze River. Acta Theriol. 48:469–483.
Zhang, X. F., Liu, R. J., Zhao, Q. Z., Zhang, G. C., Wei, Z., Wang, X. Q., and Yang, J. (1993). The population of finless porpoise in the middle and lower reaches of the Yangtze River. Acta Theriol. Sin. 13:260–270.
Zhang, X. F., and Wang, K. X. (1999). Population viability analysis for the Yangtze finless porpoise. Acta Ecol. Sin. 19:529–533.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, JS., Xia, JH., He, SP. et al. Population Genetic Structure of the Yangtze Finless Porpoise (Neophocaena phocaenoides asiaeorientalis): Implications for Management and Conservation. Biochem Genet 43, 307–320 (2005). https://doi.org/10.1007/s10528-005-5222-7
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10528-005-5222-7