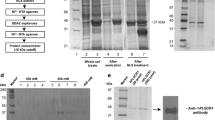
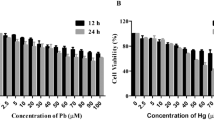
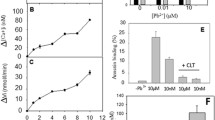
Abstract
Lead and mercury being common environmental pollutants are often associated with erythrocytes, where phosphatidylserine (PS) exposure-mediated procoagulant activation is induced. Human phospholipid scramblase 1 (hPLSCR1) identified in the erythrocyte membrane is a type II transmembrane protein involved in Ca2+-dependent bidirectional scrambling of phospholipids (PL) during blood coagulation, cell activation, and apoptosis. The prominent role of hPLSCR1 in Pb2+ and Hg2+ poisoning was demonstrated by a biochemical assay, where recombinant hPLSCR1 induced PL scrambling across bilayer with a higher binding affinity (Kd) towards Hg2+ (4.1 µM) and Pb2+ (5.8 µM) than Ca2+ (25.6 mM). The increased affinity could be the outcome of heavy metals interacting at auxiliary sites other than the calcium-binding motif of hPLSCR1. Similar to other metal-binding proteins, cysteine-based metal-binding motifs could be the potential additional binding sites in hPLSCR1. To explore the hypothesis, the cysteines were chemically modified, which significantly reduced only the Hg2+- and Pb2+-induced scrambling activity leaving Ca2+-induced activity unaltered. Recombinant constructs with deletion of prominent cysteine residues and point mutation in the calcium-binding motif including Δ100-hPLSCR1, Δ160-hPLSCR1, and D275A-hPLSCR1 were generated, purified, and assayed for scramblase activity. The cysteine-deleted constructs of hPLSCR1 showed reduced binding affinity (Kd) for Hg2+ and Pb2+ without altering the Ca2+-binding affinity whereas the point mutant had completely lost its affinity for Ca2+ and reduced affinities for Hg2+ and Pb2+. The results accentuated the significance of cysteine residues as additional binding sites for heavy metal ions in hPLSCR1.







Similar content being viewed by others
Abbreviations
- IPTG:
-
Isopropyl-β-d-thiogalactoside
- EGTA:
-
Ethylene glycol tetraacetic acid
- PMSF:
-
Phenylmethanesulfonyl fluoride
- PC:
-
Egg phosphatidylcholine
- PS:
-
Brain phosphatidyl serine
- NEM:
-
N-Ethylmaleimide
- DEPC:
-
Diethyl pyrocarbonate
- PG:
-
Phenyl glyoxal
- AEBSF:
-
4-(2-Aminoethyl) benzene sulfonyl fluoride
- NLS:
-
N-Lauroyl sarcosine
- NBD-PC:
-
1-Oleoyl-2-[6-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino] caproyl-sn-glycero-3-phosphocholine
- NBD-PS:
-
1-Oleoyl-2-[6-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]caproyl-sn-glycero-3-phosphoserine
References
Ahyayauch H, García-Arribas AB, Sot J et al (2018) Pb(II) induces scramblase activation and ceramide-domain generation in red blood cells. Sci Rep. https://doi.org/10.1038/s41598-018-25905-8
Assche F, Clijsters H (1990) Effects of metals on enzyme activity in plants. Plant Cell Environ 13:195–206. https://doi.org/10.1111/j.1365-3040.1990.tb01304.x
Bassé F, Stout JG, Sims PJ, Wiedmer T (1996) Isolation of an erythrocyte membrane protein that mediates Ca2+-dependent transbilayer movement of phospholipid. J Biol Chem 271:17205–17210
Chang Q, Gummadi SN, Menon AK (2004) Chemical modification identifies two populations of glycerophospholipid flippase in rat liver ER †. Biochemistry 43:10710–10718. https://doi.org/10.1021/bi049063a
Clarkson TW, Magos L, Myers GJ (2003) The toxicology of mercury—current exposures and clinical manifestations. N Engl J Med 349:1731–1737. https://doi.org/10.1056/NEJMra022471
Colín-González AL, Santana RA, Silva-Islas CA et al (2012) The antioxidant mechanisms underlying the aged garlic extract- and S-allylcysteine-induced protection. Oxid Med Cell Longev 2012:1–16. https://doi.org/10.1155/2012/907162
Dancis A, Yuan DS, Haile D et al (1994) Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell 76:393–402. https://doi.org/10.1016/0092-8674(94)90345-X
DeSilva TM, Veglia G, Porcelli F et al (2002) Selectivity in heavy metal- binding to peptides and proteins. Biopolymers 64:189–197. https://doi.org/10.1002/bip.10149
Flora G, Gupta D, Tiwari A (2012) Toxicity of lead: a review with recent updates. Interdiscip Toxicol 5:47–58. https://doi.org/10.2478/v10102-012-0009-2
Francis VG, Majeed MA, Gummadi SN (2012) Recovery of functionally active recombinant human phospholipid scramblase 1 from inclusion bodies using N-lauroyl sarcosine. J Ind Microbiol Biotechnol 39:1041–1048. https://doi.org/10.1007/s10295-012-1105-1
Fullmer CS, Edelstein S, Wasserman RH (1985) Lead-binding properties of intestinal calcium-binding proteins. J Biol Chem 260:6816–6819
Garza A, Vega R, Soto E (2006) Cellular mechanisms of lead neurotoxicity. Med Sci Monit 12:RA57-65
Hamer DH (1986) Metallothionein. Annu Rev Biochem 55:913–951. https://doi.org/10.1146/annurev.bi.55.070186.004405
Hobman JL, Brown NL (1996) Overexpression of MerT, the mercuric ion transport protein of transposon Tn501, and genetic selection of mercury hypersensitivity mutations. Molec Gen Genet 250:129–134. https://doi.org/10.1007/BF02191833
Jan A, Azam M, Siddiqui K et al (2015) Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. IJMS 16:29592–29630. https://doi.org/10.3390/ijms161226183
Kaegi JHR, Schaeffer A (1988) Biochemistry of metallothionein. Biochemistry 27:8509–8515. https://doi.org/10.1021/bi00423a001
Kelly SM, Jess TJ, Price NC (2005) How to study proteins by circular dichroism. Biochim et Biophys Acta BBA Proteins Proteomics 1751:119–139. https://doi.org/10.1016/j.bbapap.2005.06.005
Kielland J (1937) Individual activity coefficients of ions in aqueous solutions. J Am Chem Soc 59:1675–1678. https://doi.org/10.1021/ja01288a032
Lim K-M, Kim S, Noh J-Y et al (2010) Low-level mercury can enhance procoagulant activity of erythrocytes: a new contributing factor for mercury-related thrombotic disease. Environ Health Perspect 118:928–935. https://doi.org/10.1289/ehp.0901473
Mah V, Jalilehvand F (2010) Glutathione complex formation with mercury(II) in aqueous solution at physiological pH. Chem Res Toxicol 23:1815–1823. https://doi.org/10.1021/tx100260e
Mejáre M, Bülow L (2001) Metal-binding proteins and peptides in bioremediation and phytoremediation of heavy metals. Trends Biotechnol 19:67–73. https://doi.org/10.1016/S0167-7799(00)01534-1
Moore MR, Meredith PA (1978) The carcinogenicity of lead. Arch Toxicol 42:87–94. https://doi.org/10.1007/BF00316488
Opella SJ, DeSilva TM, Veglia G (2002) Structural biology of metal-binding sequences. Curr Opin Chem Biol 6:217–223. https://doi.org/10.1016/s1367-5931(02)00314-9
Palanirajan SK, Gummadi SN (2020) Heavy-metals-mediated phospholipids scrambling by human phospholipid scramblase 3: a probable role in mitochondrial apoptosis. Chem Res Toxicol 33:553–564. https://doi.org/10.1021/acs.chemrestox.9b00406
Papanikolaou NC, Hatzidaki EG, Belivanis S et al (2005) Lead toxicity update. A brief review. Med Sci Monit 11:329–336
Perez RR, Sousa CA, Vankeersbilck T et al (2013) Evaluation of the role of glutathione in the lead-induced toxicity in Saccharomyces cerevisiae. Curr Microbiol 67:300–305. https://doi.org/10.1007/s00284-013-0364-z
Rajasekharan A, Gummadi SN (2011) Flip-flop of phospholipids in proteoliposomes reconstituted from detergent extract of chloroplast membranes: kinetics and phospholipid specificity. PLoS ONE 6:e28401. https://doi.org/10.1371/journal.pone.0028401
Rayala S, Francis VG, Sivagnanam U, Gummadi SN (2014) N-terminal proline-rich domain is required for scrambling activity of human phospholipid scramblases. J Biol Chem 289:13206–13218. https://doi.org/10.1074/jbc.M113.522953
Sahu SK, Gummadi SN (2008) Flippase activity in proteoliposomes reconstituted with spinacea oleracea endoplasmic reticulum membrane proteins: evidence of biogenic membrane flippase in plants. Biochemistry 47:10481–10490. https://doi.org/10.1021/bi8014339
Sahu SK, Gummadi SN, Manoj N, Aradhyam GK (2007) Phospholipid scramblases: an overview. Arch Biochem Biophys 462:103–114. https://doi.org/10.1016/j.abb.2007.04.002
Sahu SK, Aradhyam GK, Gummadi SN (2009) Calcium binding studies of peptides of human phospholipid scramblases 1 to 4 suggest that scramblases are new class of calcium binding proteins in the cell. Biochim Biophys Acta 1790:1274–1281. https://doi.org/10.1016/j.bbagen.2009.06.008
Shettihalli AK, Gummadi SN (2013) Biochemical evidence for lead and mercury induced transbilayer movement of phospholipids mediated by human phospholipid scramblase 1. Chem Res Toxicol 26:918–925. https://doi.org/10.1021/tx400090h
Shin J-H, Lim K-M, Noh J-Y et al (2007) Lead-induced procoagulant activation of erythrocytes through phosphatidylserine exposure may lead to thrombotic diseases. Chem Res Toxicol 20:38–43. https://doi.org/10.1021/tx060114+
Solioz M, Vulpe C (1996) CPx-type ATPases: a class of P-type ATPases that pump heavy metals. Trends Biochem Sci 21:237–241
Stout JG, Zhou Q, Wiedmer T, Sims PJ (1998) Change in conformation of plasma membrane phospholipid scramblase induced by occupancy of Its Ca 2+ binding site †. Biochemistry 37:14860–14866. https://doi.org/10.1021/bi9812930
Summers AO (1986) Organization, expression, and evolution of genes for mercury resistance. Annu Rev Microbiol 40:607–634. https://doi.org/10.1146/annurev.mi.40.100186.003135
Vas J, Monestier M (2008) Immunology of mercury. Ann N Y Acad Sci 1143:240–267. https://doi.org/10.1196/annals.1443.022
Waldron HA (1966) The anaemia of lead poisoning: a review. Occup Environ Med 23:83–100. https://doi.org/10.1136/oem.23.2.83
Wernimont AK, Huffman DL, Lamb AL et al (2000) Structural basis for copper transfer by the metallochaperone for the Menkes/Wilson disease proteins. Nat Struct Biol 7:766–771. https://doi.org/10.1038/78999
Wiemann M, Schirrmacher K, Büsselberg D (1999) Interference of lead with the calcium release activated calcium flux of osteoblast-like cells. Calcif Tissue Int 65:479–485. https://doi.org/10.1007/s002239900736
Williamson P, Schlegel RA (2002) Transbilayer phospholipid movement and the clearance of apoptotic cells. Biochim et Biophys Acta Mol Cell Biol Lipids 1585:53–63. https://doi.org/10.1016/S1388-1981(02)00324-4
Williamson P, Kulick A, Zachowski A et al (1992) Calcium induces transbilayer redistribution of all major phospholipids in human erythrocytes. Biochemistry 31:6355–6360. https://doi.org/10.1021/bi00142a027
Wilson MA, Brunger AT (2003) Domain flexibility in the 1.75 Å resolution structure of Pb 2+ -calmodulin. Acta Crystallogr D Biol Crystallogr 59:1782–1792. https://doi.org/10.1107/S0907444903016846
Wilson JR, Leang C, Morby AP et al (2000) MerF is a mercury transport protein: different structures but a common mechanism for mercuric ion transporters? FEBS Lett 472:78–82. https://doi.org/10.1016/s0014-5793(00)01430-7
Zalups RK (2000) Molecular interactions with mercury in the kidney. Pharmacol Rev 52:113–143
Zhou Q, Sims PJ, Wiedmer T (1998) Identity of a conserved motif in phospholipid scramblase that is required for Ca 2+-accelerated transbilayer movement of membrane phospholipids †. Biochemistry 37:2356–2360. https://doi.org/10.1021/bi972625o
Zwaal RFA, Schroit AJ (1997) Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood 89:1121–1132. https://doi.org/10.1182/blood.V89.4.1121
Acknowledgements
The authors acknowledge the Indian Institute of Technology Madras for facilities. The authors thank the Department of Science and Technology, Government of India, and Indian Institute of Technology Madras for Circular Dichroism studies. AKS acknowledges BMSCE, Bengaluru and AICTE, Government of India, New Delhi for providing the fellowship. S.K.P. wishes to thank the Ministry of Human Resource Development (MHRD), Government of India, and Indian Institute of Technology Madras for fellowship.
Funding
No funding for this project.
Author information
Authors and Affiliations
Contributions
Conceptualization: SNG; Methodology: AKS; Formal analysis and investigation: AKS, SKP; Writing—original draft preparation: SKP, AKS; Writing—review and editing: SKP, SNG; Resources and Supervision: SNG.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
249_2021_1521_MOESM1_ESM.doc
Details of expression vector construction, overexpression, and purification of (His)6-hPLSCR1, (His)6-Δ100-hPLSCR1 (His)6- Δ160-hPLSCR1, and (His)6- D275A-hPLSCR1 along with a schematic representation of deletion constructs of hPLSCR1 and the principle of scramblase assay are provided in the supplementary information (DOC 1256 KB)
Rights and permissions
About this article
Cite this article
Shettihalli, A.K., Palanirajan, S.K. & Gummadi, S.N. Are cysteine residues of human phospholipid scramblase 1 essential for Pb2+ and Hg2+ binding-induced scrambling of phospholipids?. Eur Biophys J 50, 745–757 (2021). https://doi.org/10.1007/s00249-021-01521-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-021-01521-9